-
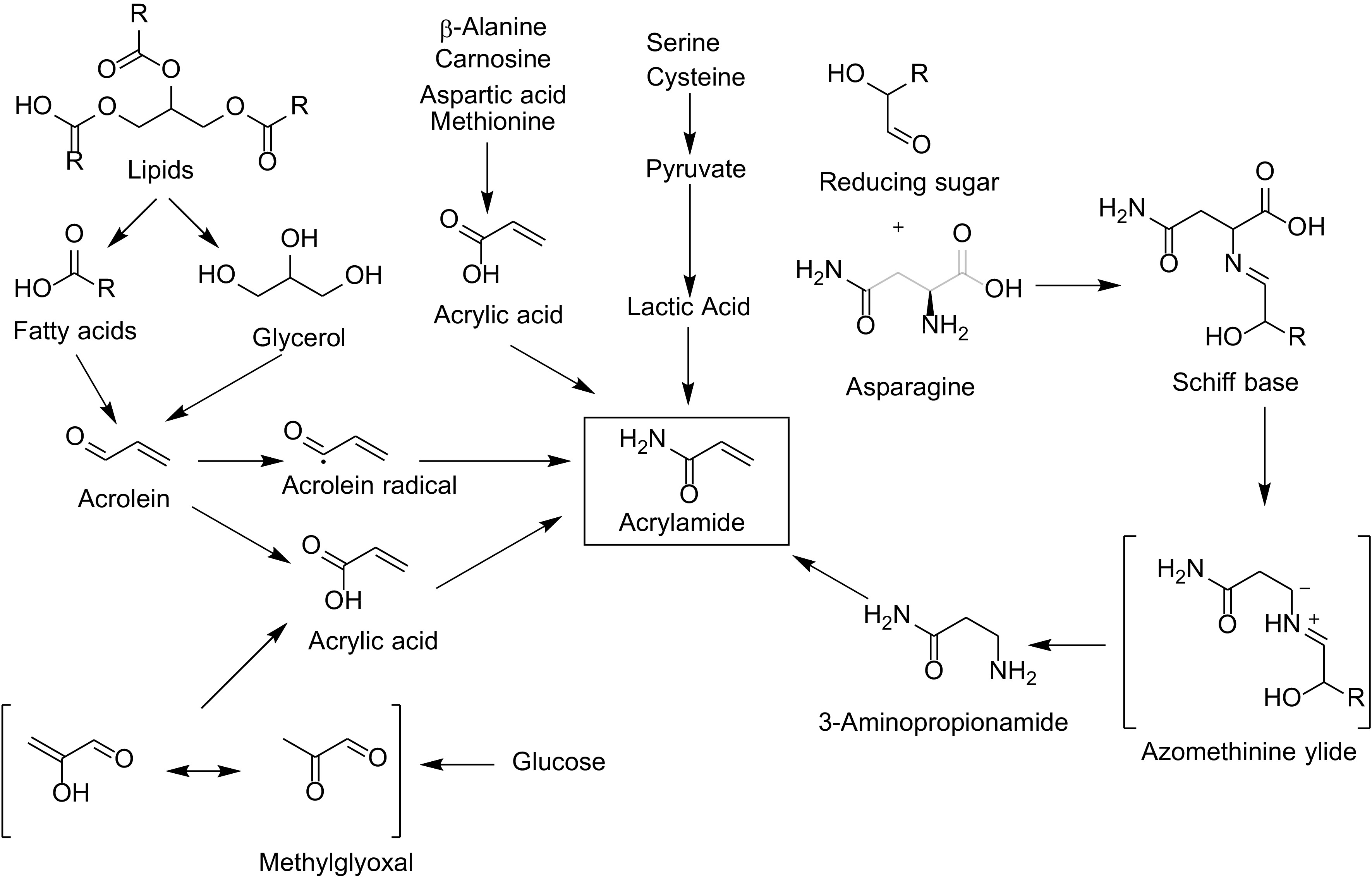
-
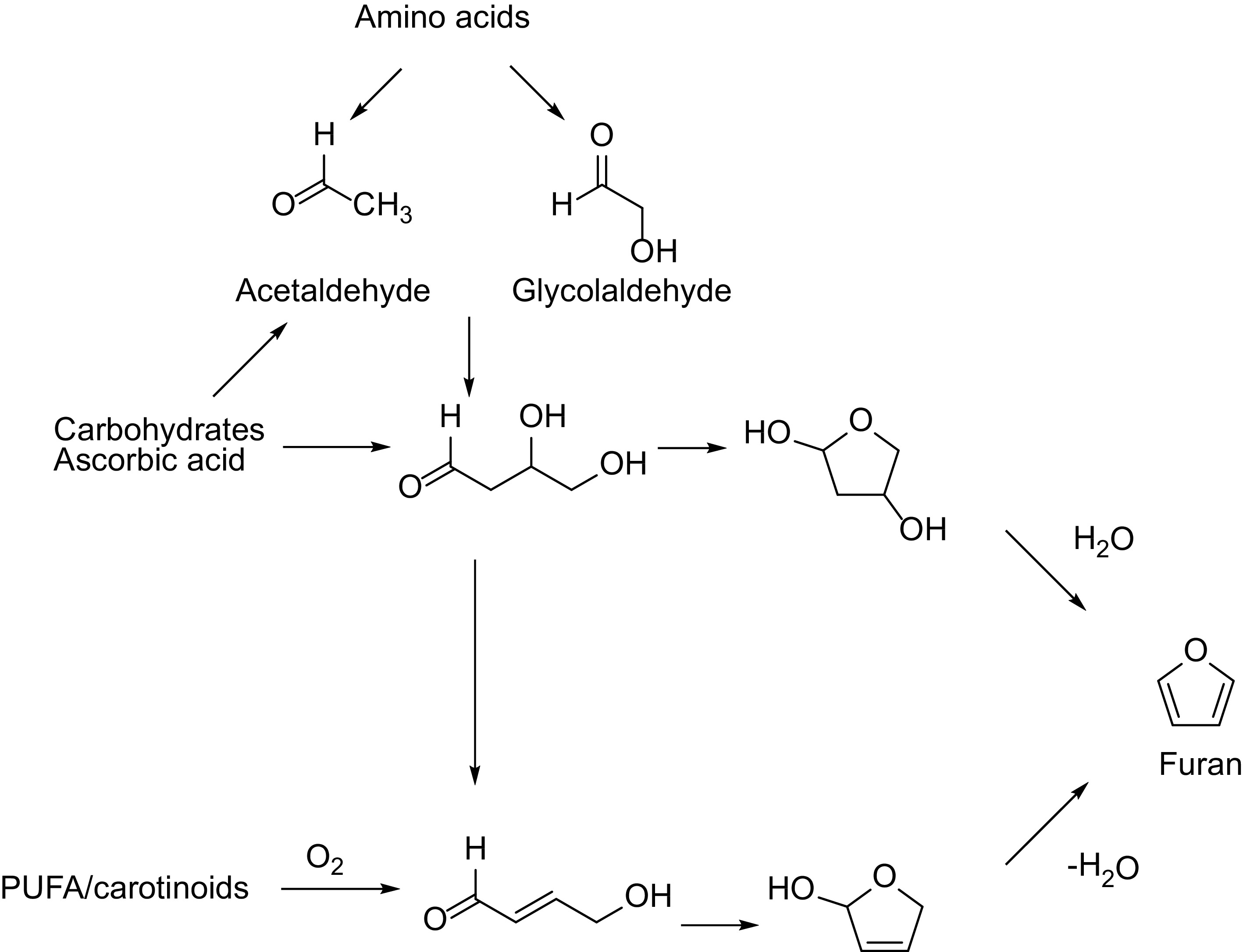
Figure 2.
Possible formation pathways of furan.
-
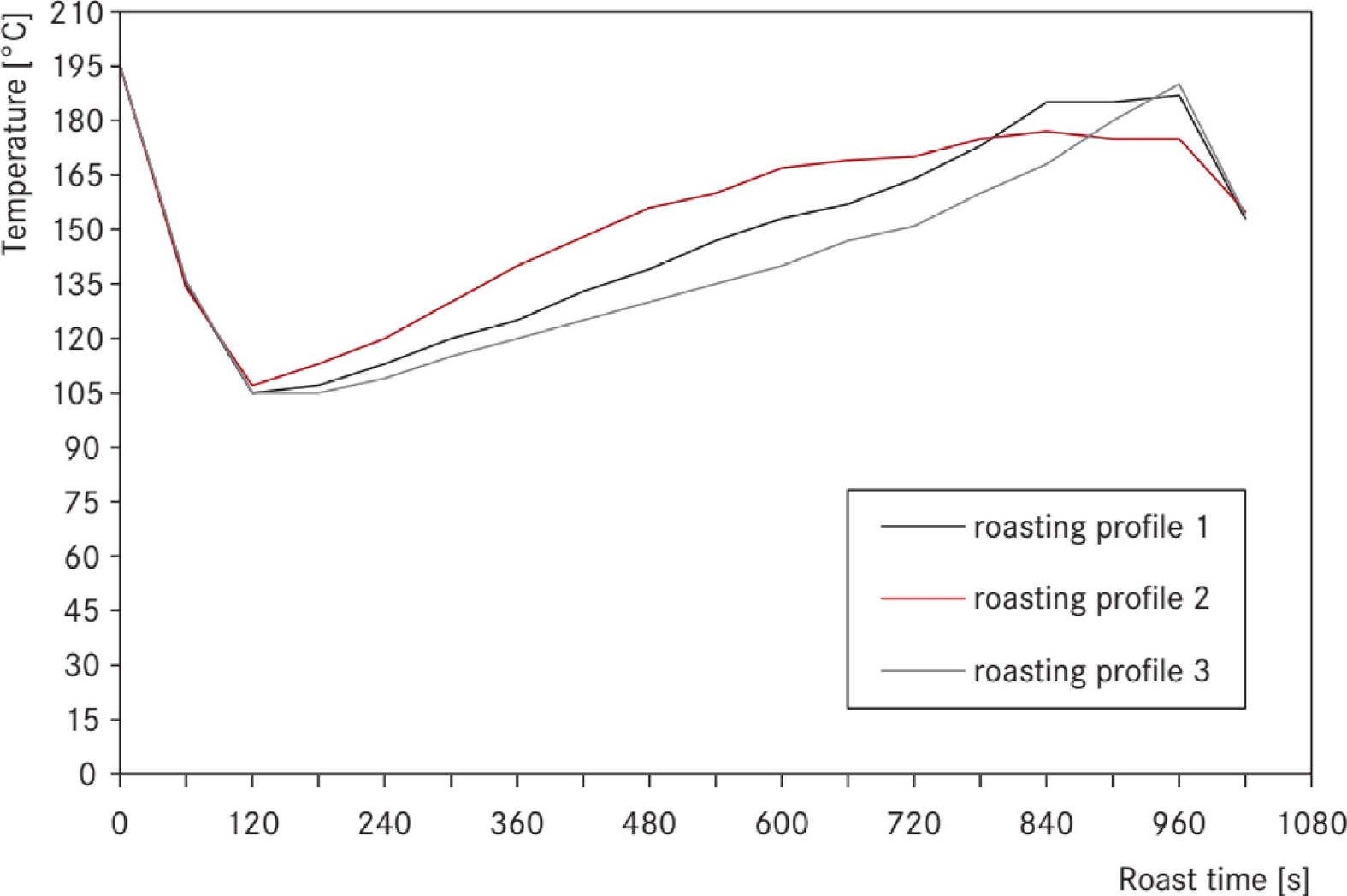
Figure 3.
The roasting profiles of the first series[[20]].
-
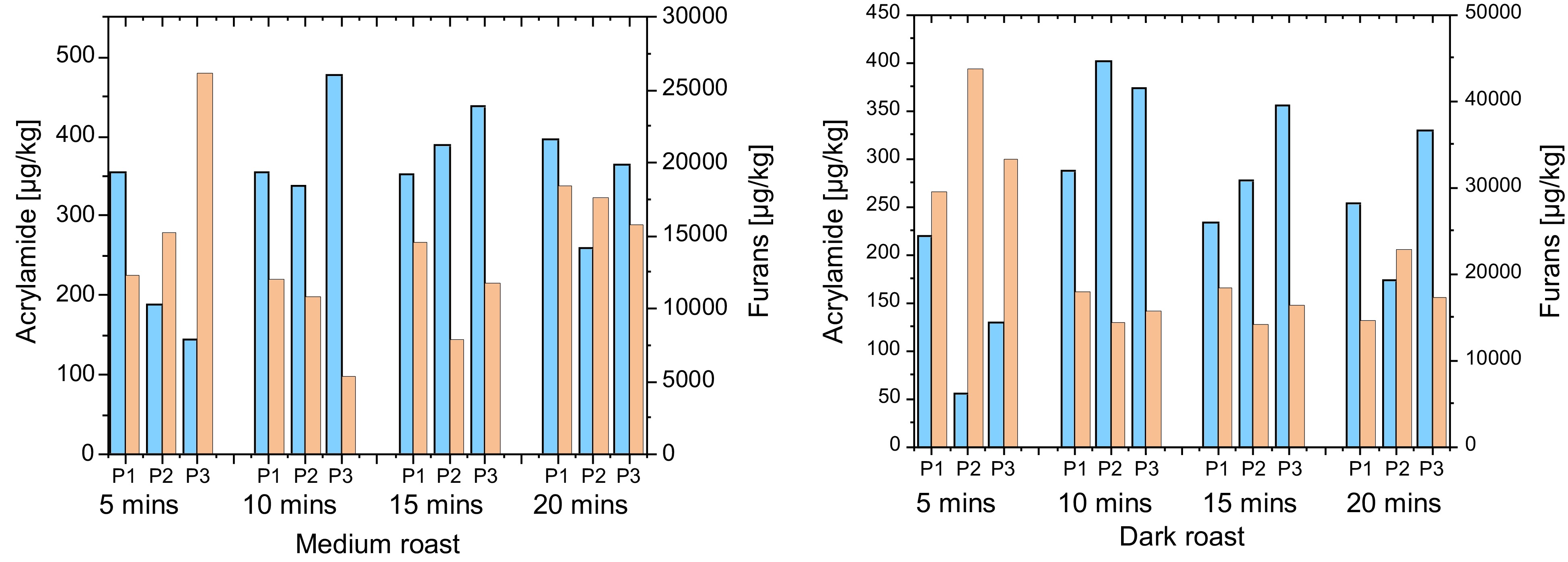
Figure 4.
Acrylamide and furan content of Brazil Arabica (drum roast – medium and dark).
-
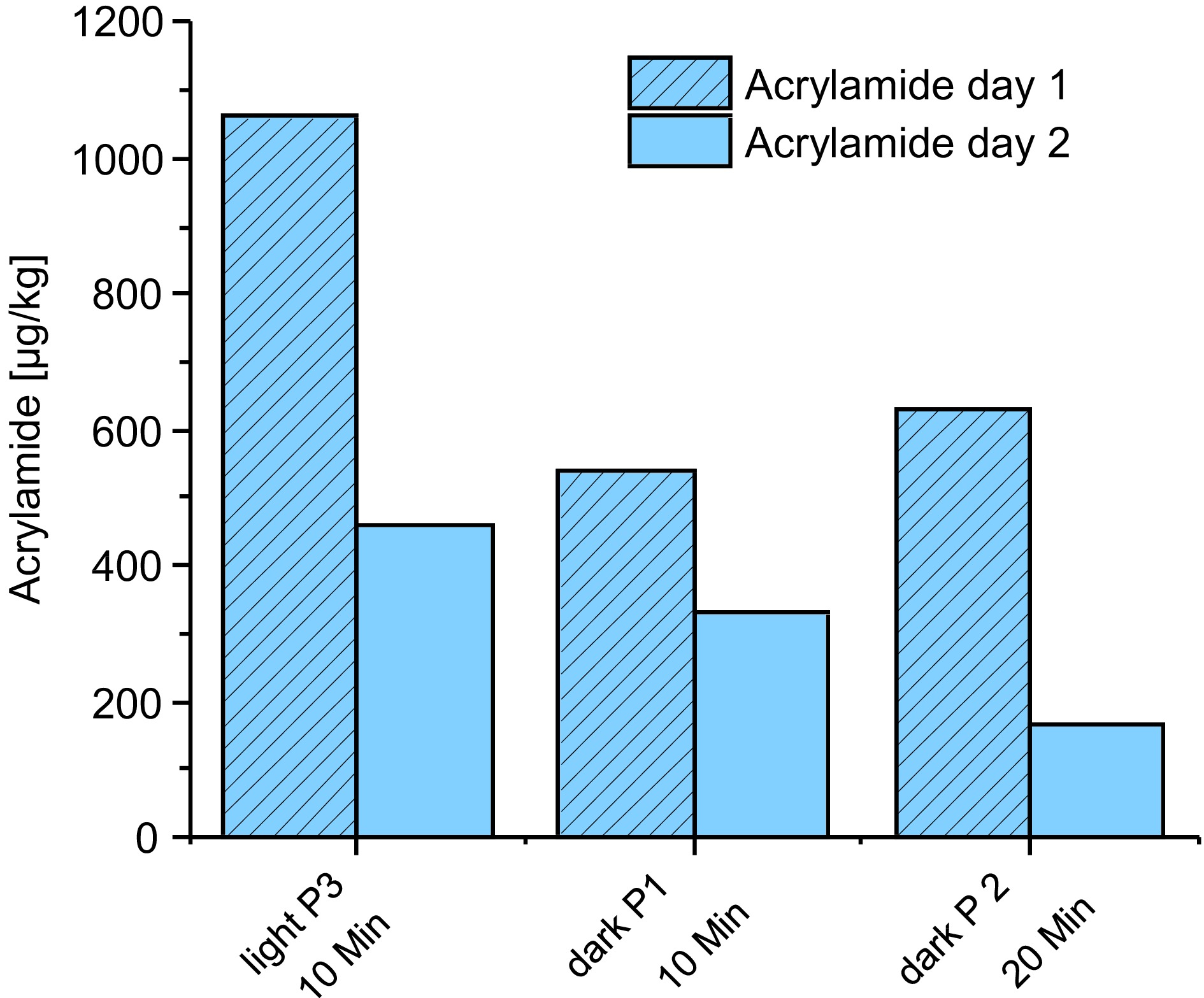
Figure 5.
Comparison of acrylamide content of Brazil Arabica after day 1 and 2.
-
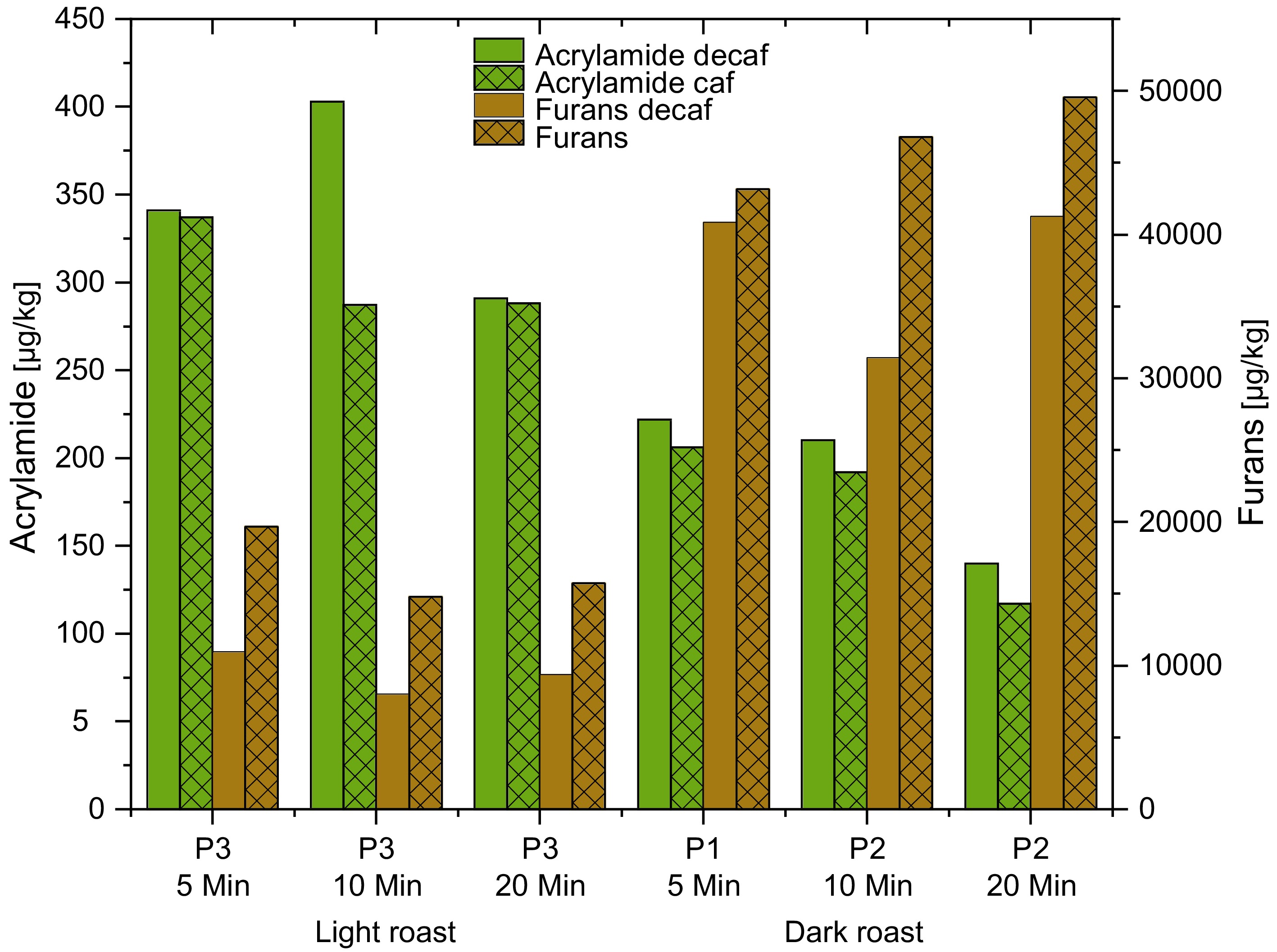
Figure 6.
Water decaffeinated samples vs non decaffeinated samples.
-

Figure 7.
Steam treated unroasted sample (left), and untreated sample (right).
-
GC Trace 1300 GC (Thermo Fisher Scientific, Dreieich, Germany) PTV mode CT split Inlet temperature 240 °C Column VF-WAXms, 30 m, ID 0.25 mm, 0.25 μm (Agilent J & W Columns, Waldbronn, Germany) Carrier Helium 5.0 Mass spectrometer TSQ DUO (Thermo Fisher Scientific, Dreieich, Germany) Data processing Thermo TraceFinder GC 3.2, Thermo Xcalibur 3.0 (Thermo Fisher Scientific, Dreieich, Germany) Ionisation mode EI+, 70 eV MS transfer line/ion source temperature 250 °C Collision gas Argon Mode SRM (acrylamide) and SIM (furans) Autosampler Acrylamide: TRIPLUS RSH with 10 μl Syringe,
57 mm (Thermo Fisher Scientific, Dreieich, Germany)
Furans: TRIPLUS RSH, Temperature
70 °C, with 5 mL syringe, 65 mm; (Thermo Fisher Scientific, Dreieich, Germany)Split flow 12.0 ml/min (acrylamide) and splitless (furans) Flow 1.2 ml/min (acrylamide) and 1.0 mL/min (furans) Carrier mode Constant flow Table 1.
GC-MS settings for both acrylamide and furans determination.
-
2-Bromopropenamide Identification + quantification: m/z 149 [C3H479BrON]+
→ 106 [C2H379Br]+ (identification)D2-Bromopropenamide Identification and quantification: m/z 153 [C32H21H381BrON]+
→ 110 [C22H21H181Br]+ (identification)Table 2.
Ions used for the identification and quantification of acrylamide.
-
Analyte Ions [m/z] Furan 68 [C4H4O]+ (identification and quantification) 2-methylfuran/
3-methylfuran82 [C5H6O]+ (identification and quantification)
81 (identification)2,5-dimethylfuran 95 [C6H7O]+ (identification and quantification)
96 (identification)D4-furan 72 [C4D4O]+ (identification and quantification) D3-2-methylfuran/
D3-3-methylfuran85 [C5H3D3O]+ (identification and quantification)
84 (identification)D3-2,5-dimethylfuran 98 [C6H4D3O]+ (identification and quantification)
99 (identification)Table 3.
Ions used for the identification and quantification of furans.
Figures
(7)
Tables
(3)