-
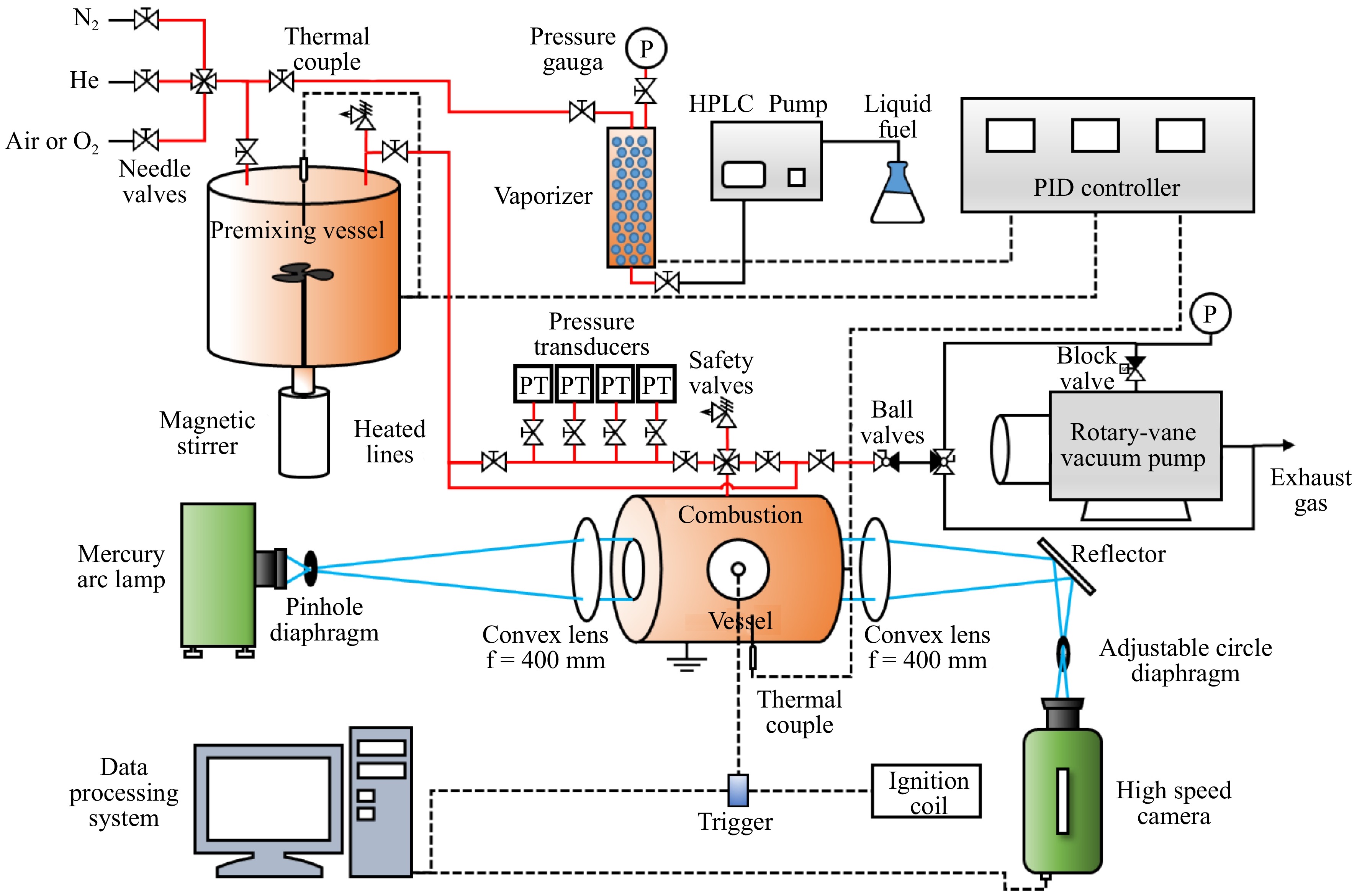
Figure 1.
A schematic constant-volume combustion vessel[10].
-
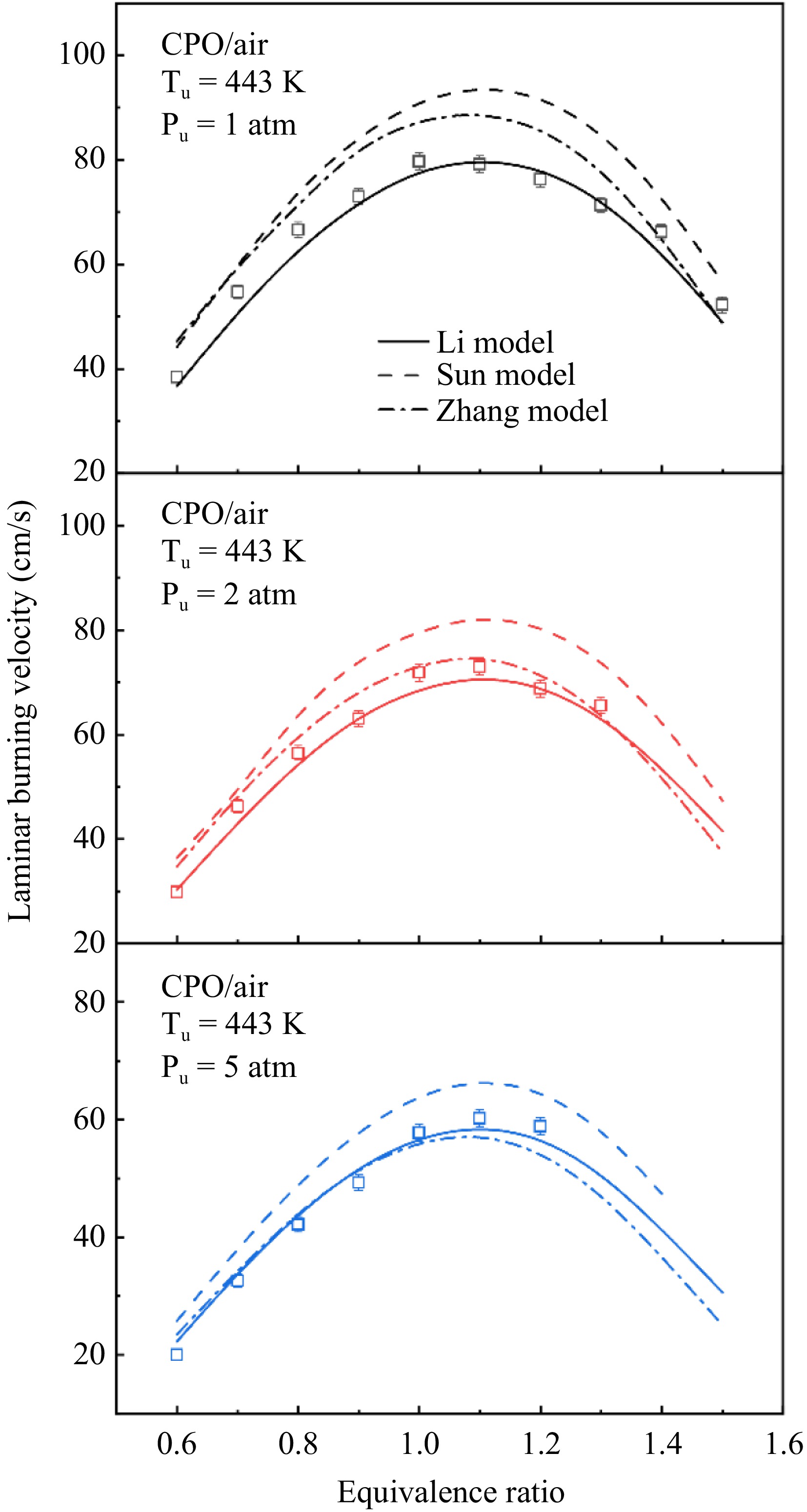
Figure 2.
Experimental and simulated LBVs of cyclopentanone/air mixtures at 443 K and 1−5 atm.
-
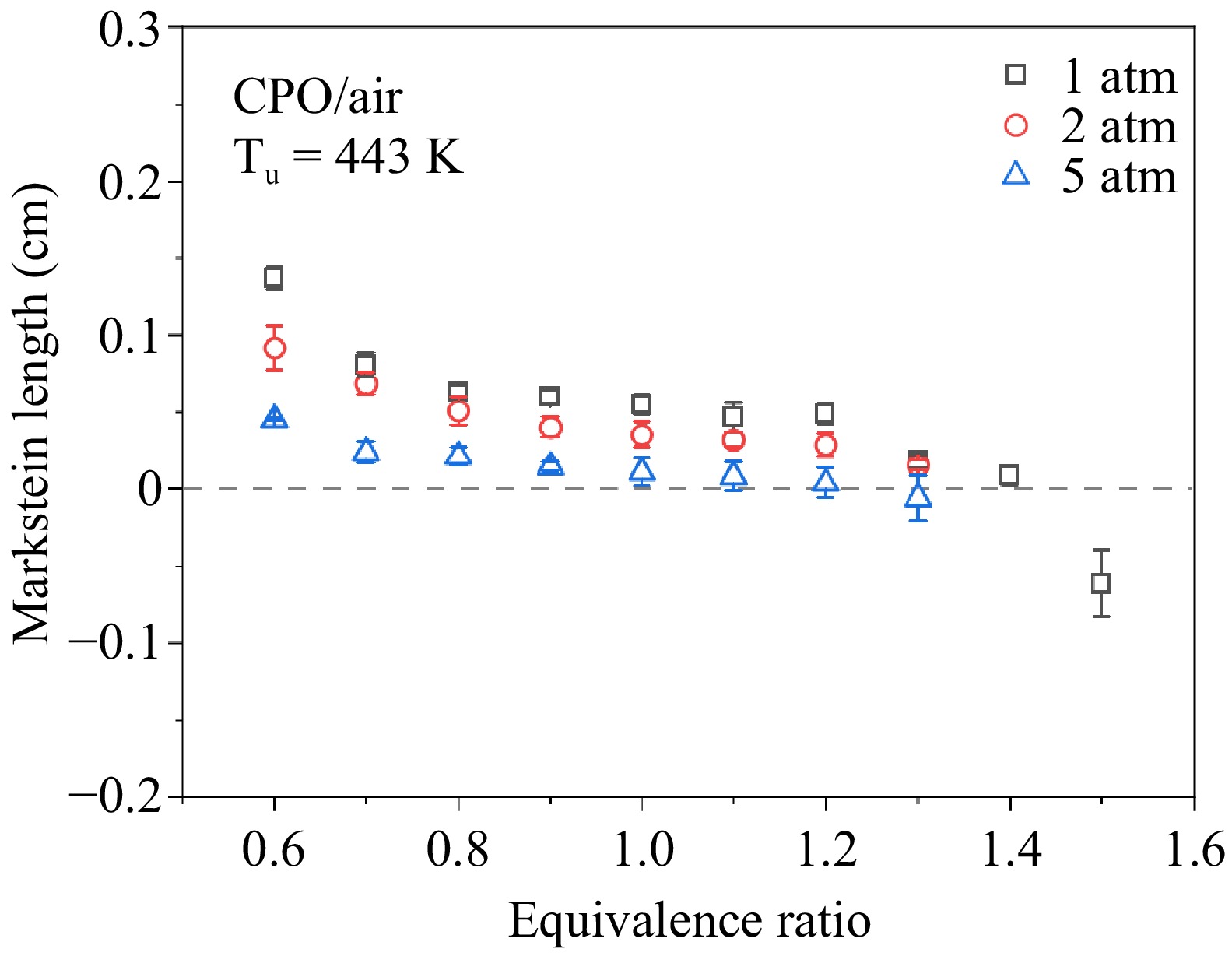
Figure 3.
Measured Markstein lengths of cyclopentanone/air flames at 443 K and 1–5 atm.
-
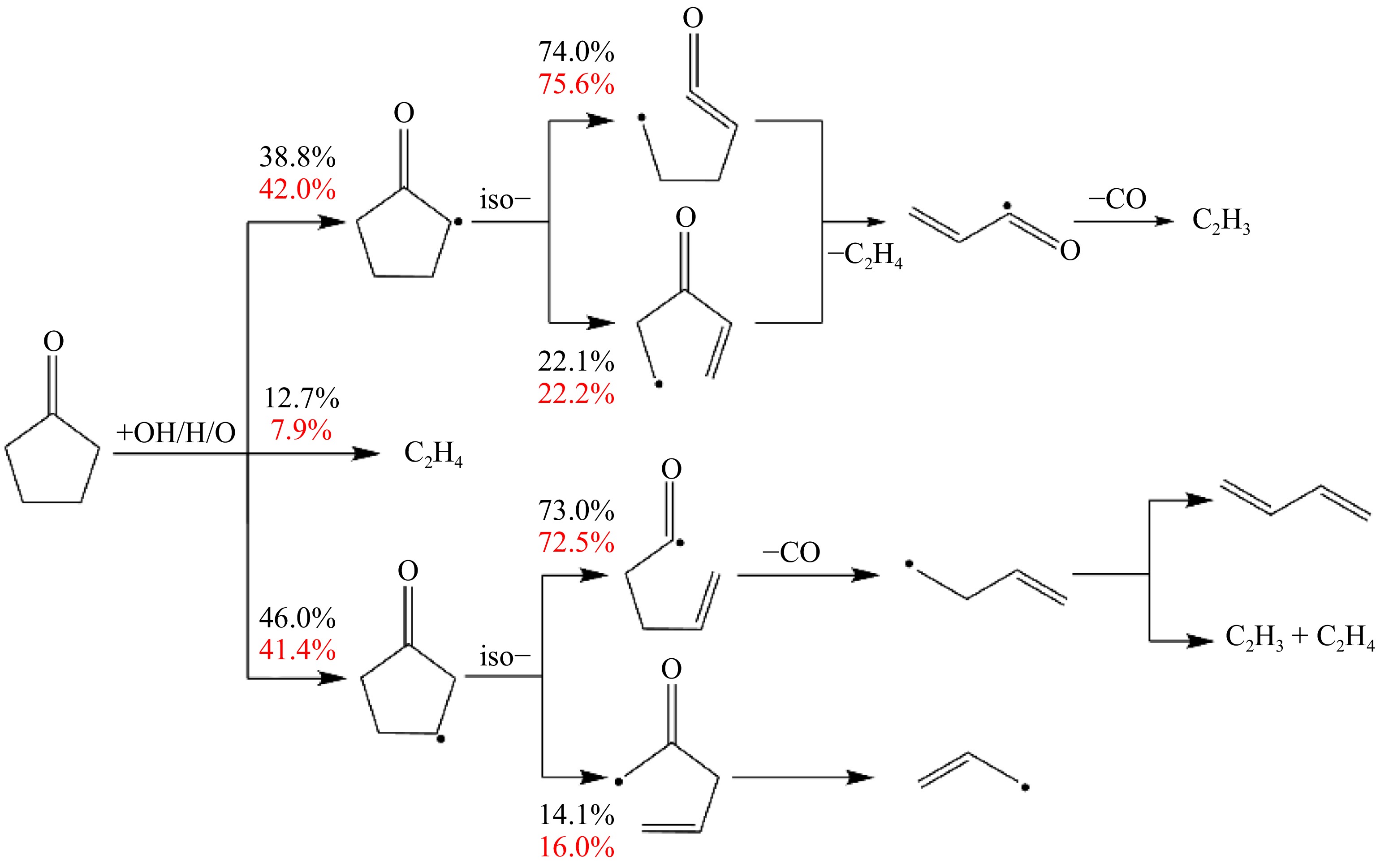
Figure 4.
Main reaction networks in cyclopentanone flame at 1 atm (black: ϕ = 0.7, red: ϕ = 1.4).
-
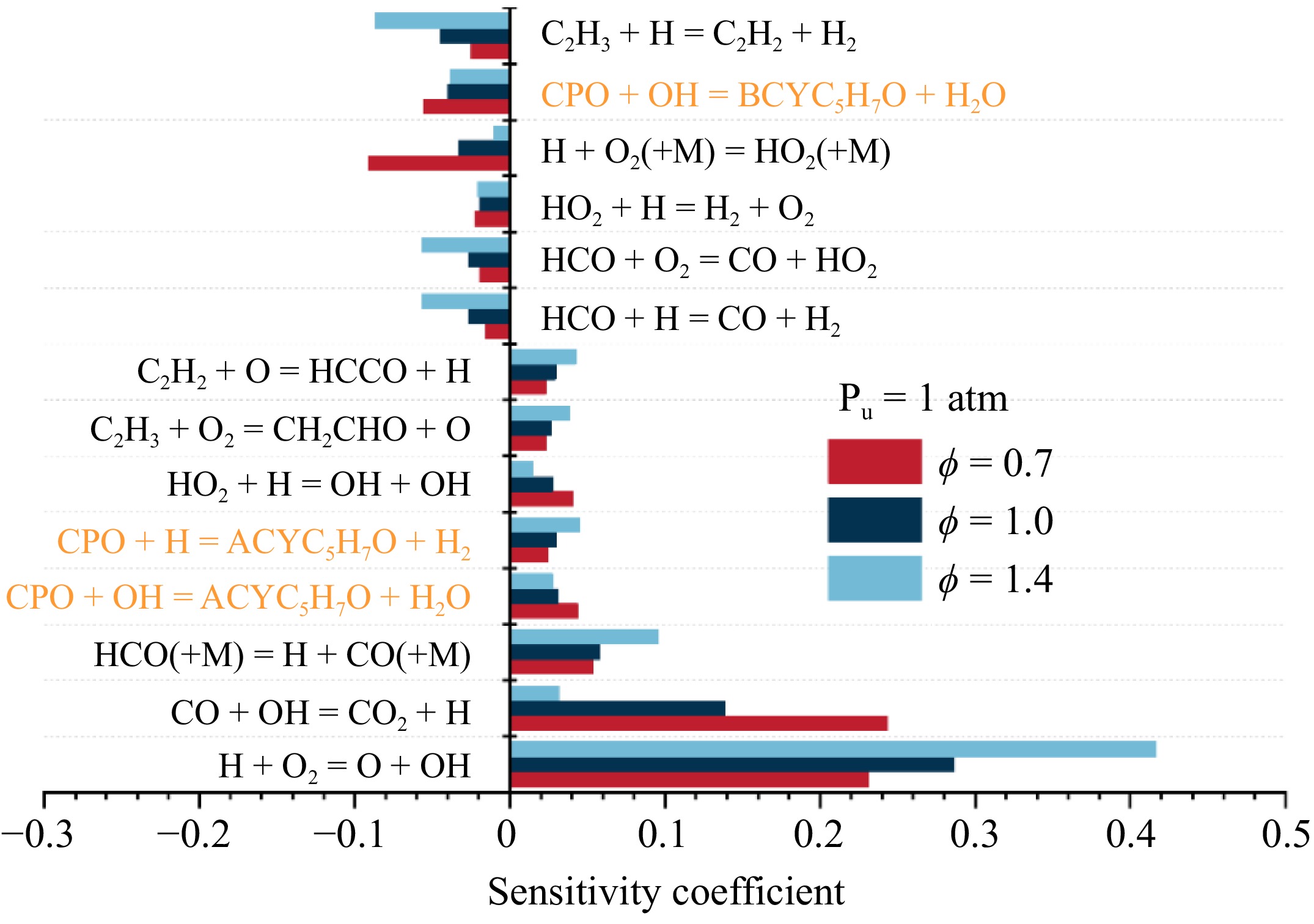
Figure 5.
Sensitivity analysis of LBV for cyclopentanone/air mixtures at 473 K, 1 atm, and various equivalence ratios.
-
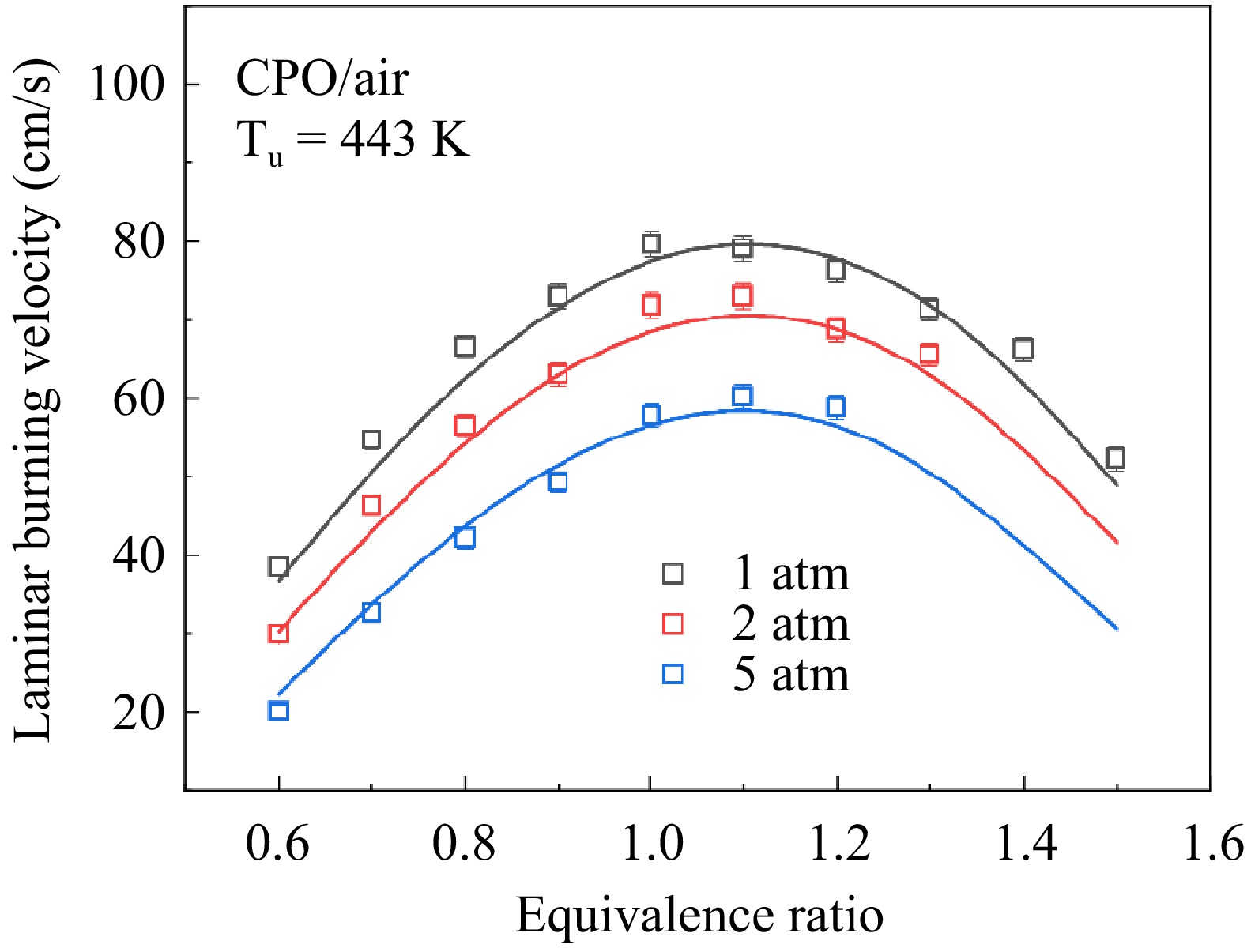
Figure 6.
Measured and simulated LBVs for cyclopentanone/air mixtures.
-
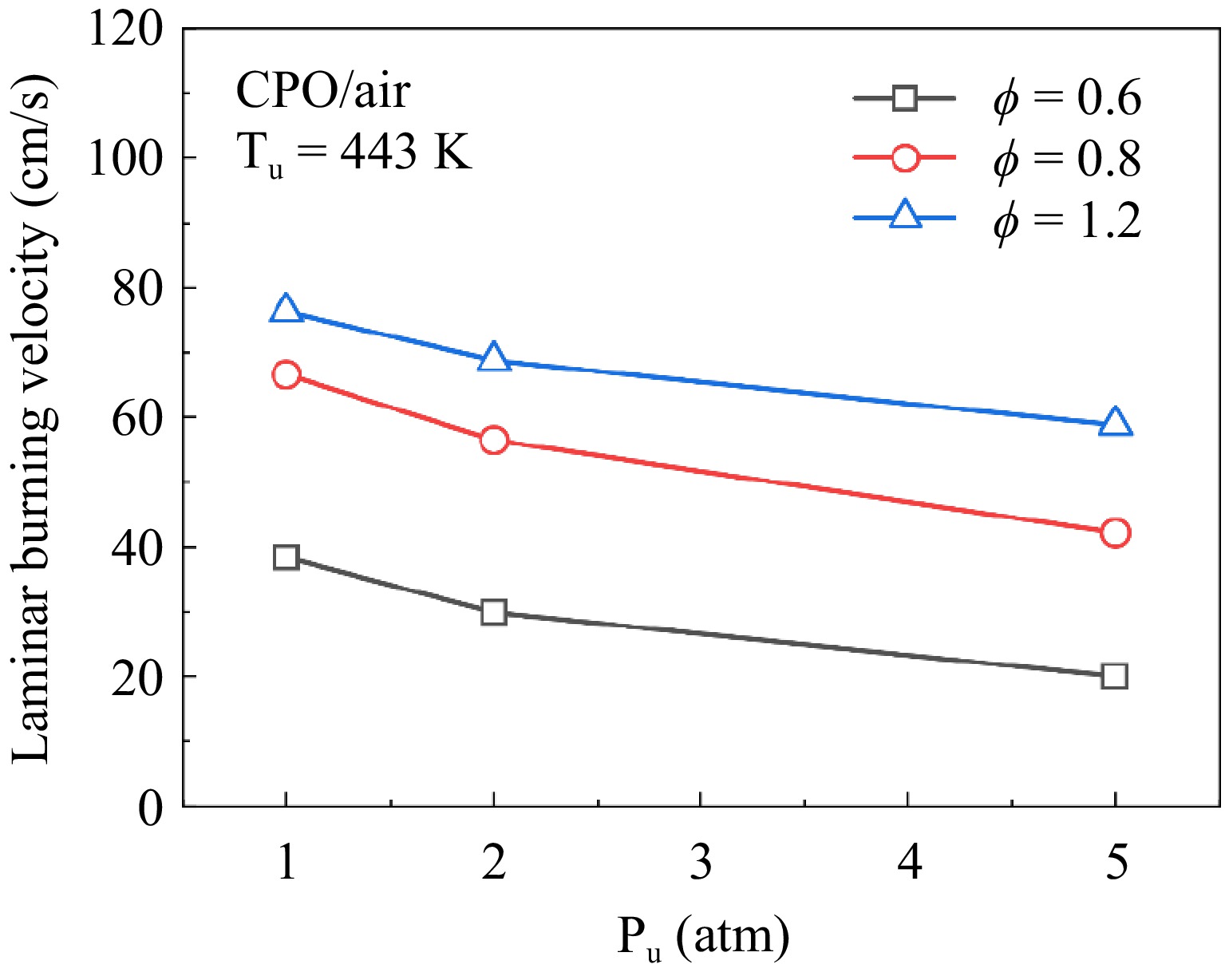
Figure 7.
Measured LBVs of cyclopentanone/air mixtures at 443 K, ϕ = 0.6, 0.8, and 1.2.
-
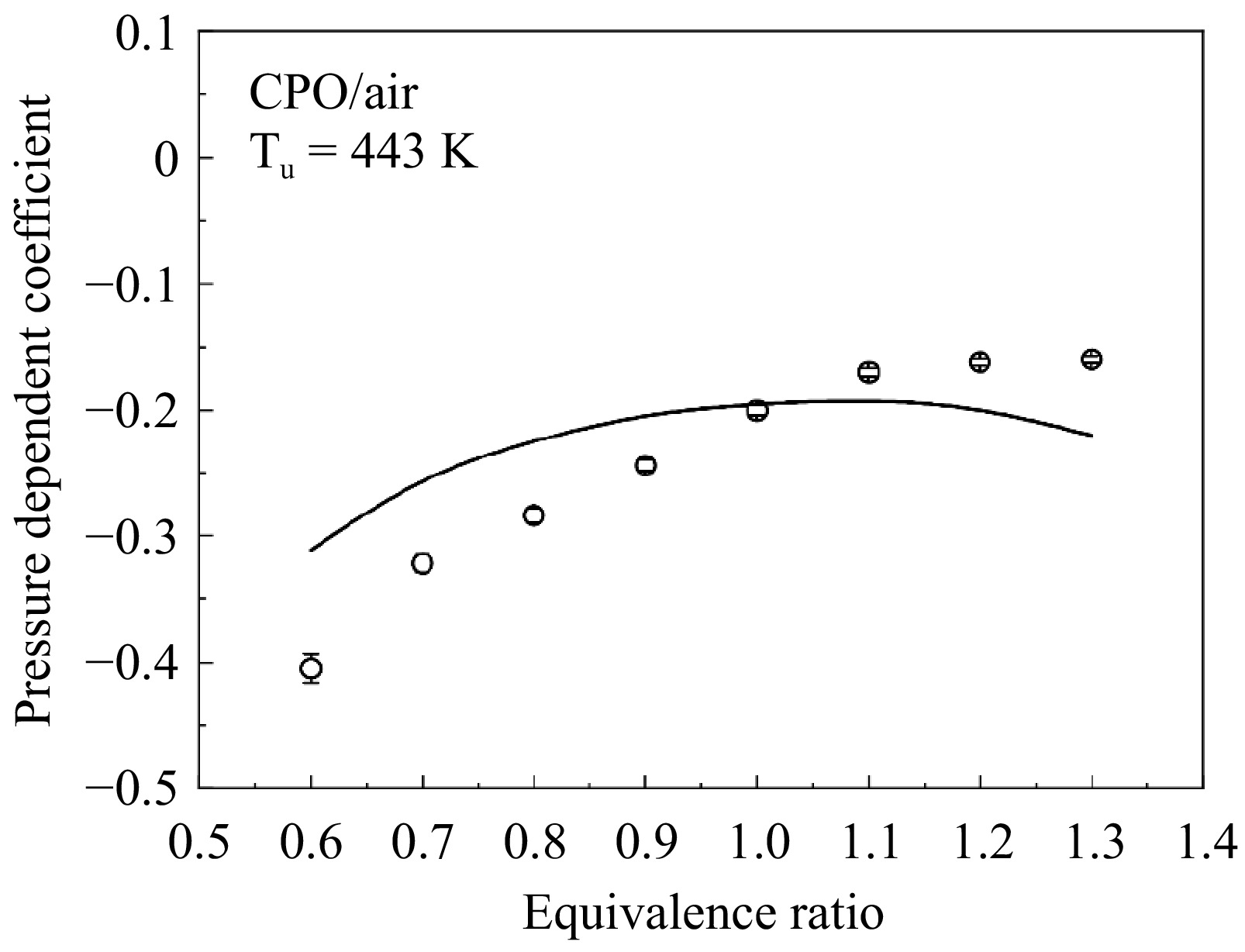
Figure 8.
Measured (symbols) and simulated (lines) pressure dependent coefficient β for cyclopentanone/air flame.
-
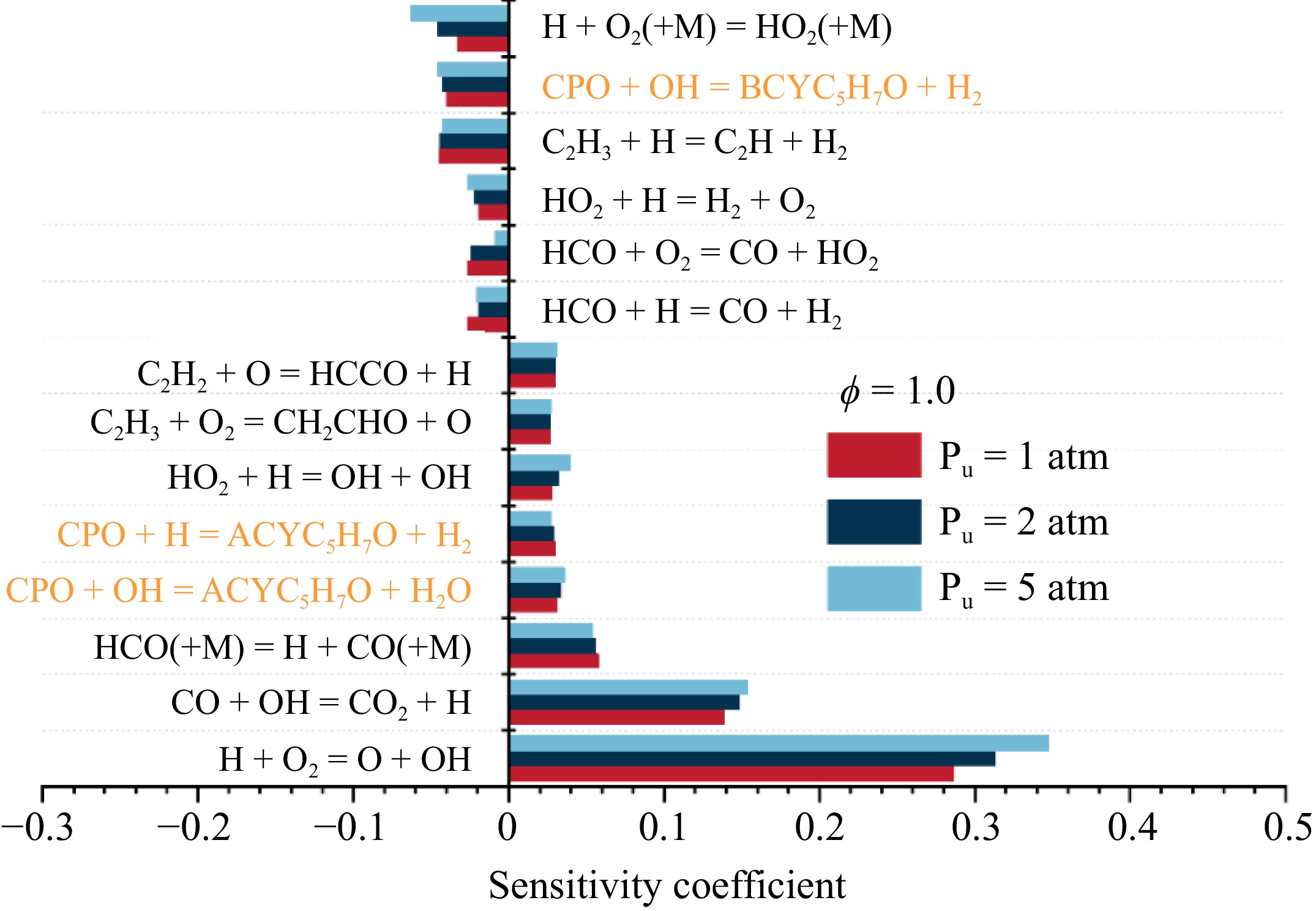
Figure 9.
Sensitivity analysis of LBV for cyclopentanone/air mixtures at ϕ = 1.0 and various pressures.
-
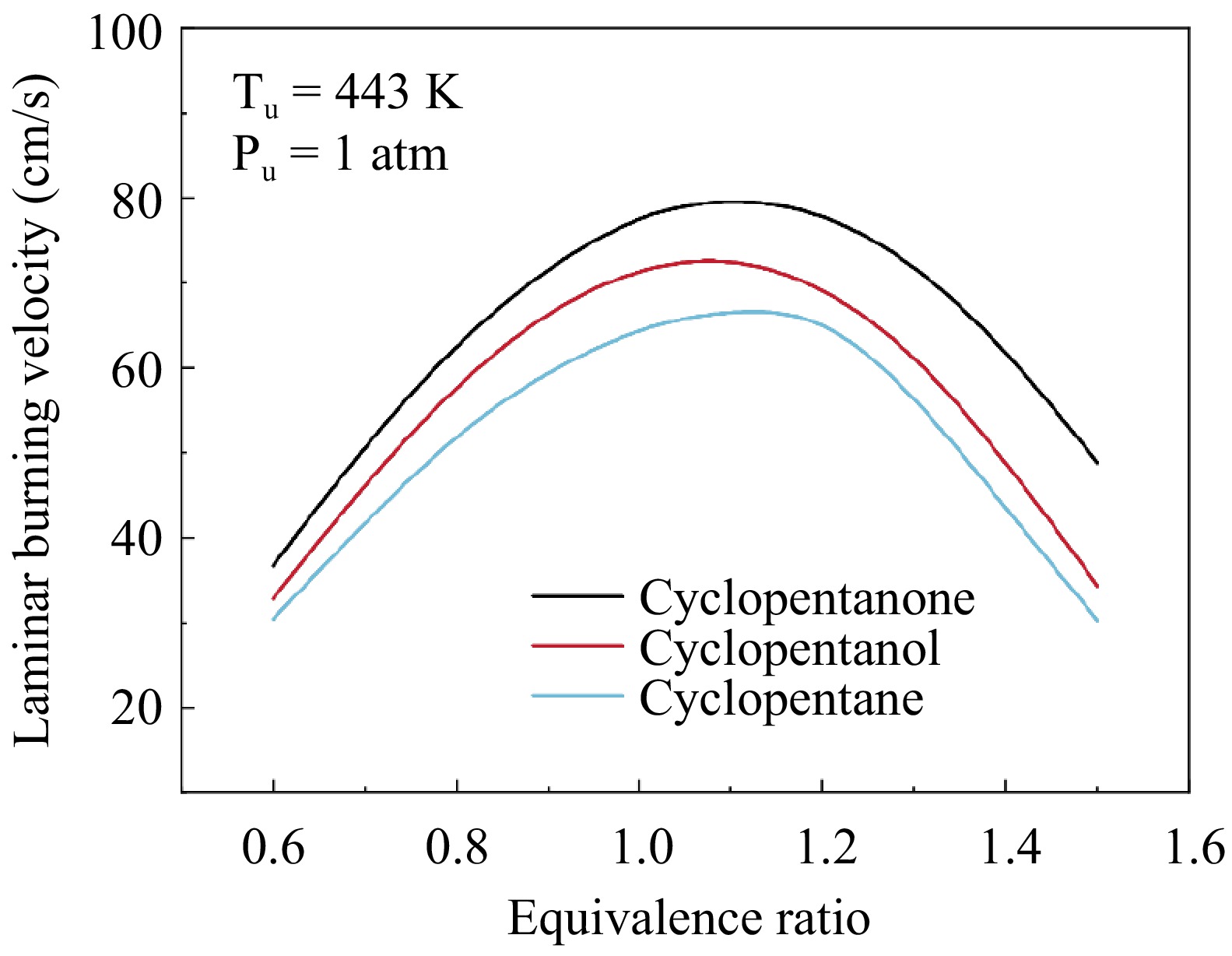
-
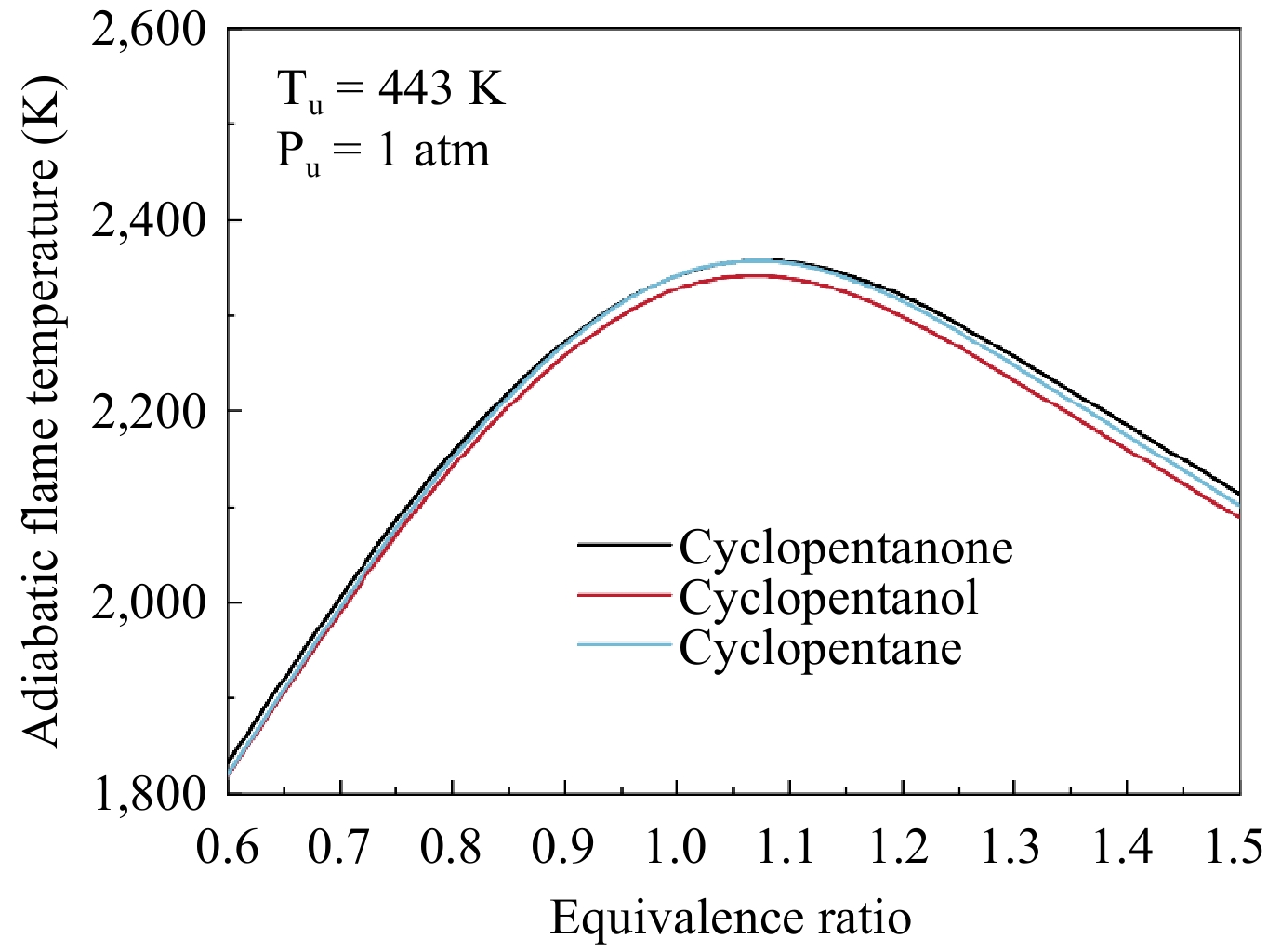
Figure 11.
Calculated adiabatic flame temperatures for cyclopentanone, cyclopentanol, and cyclopentane at 1 atm and 443 K.
-
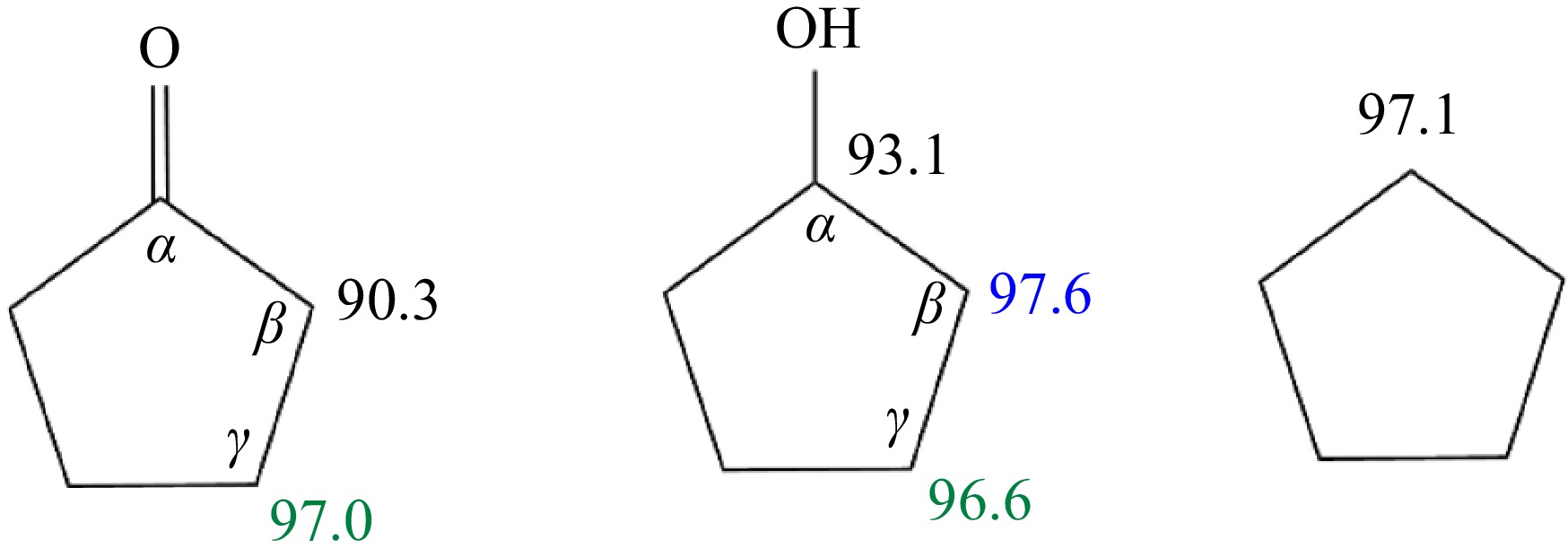
-
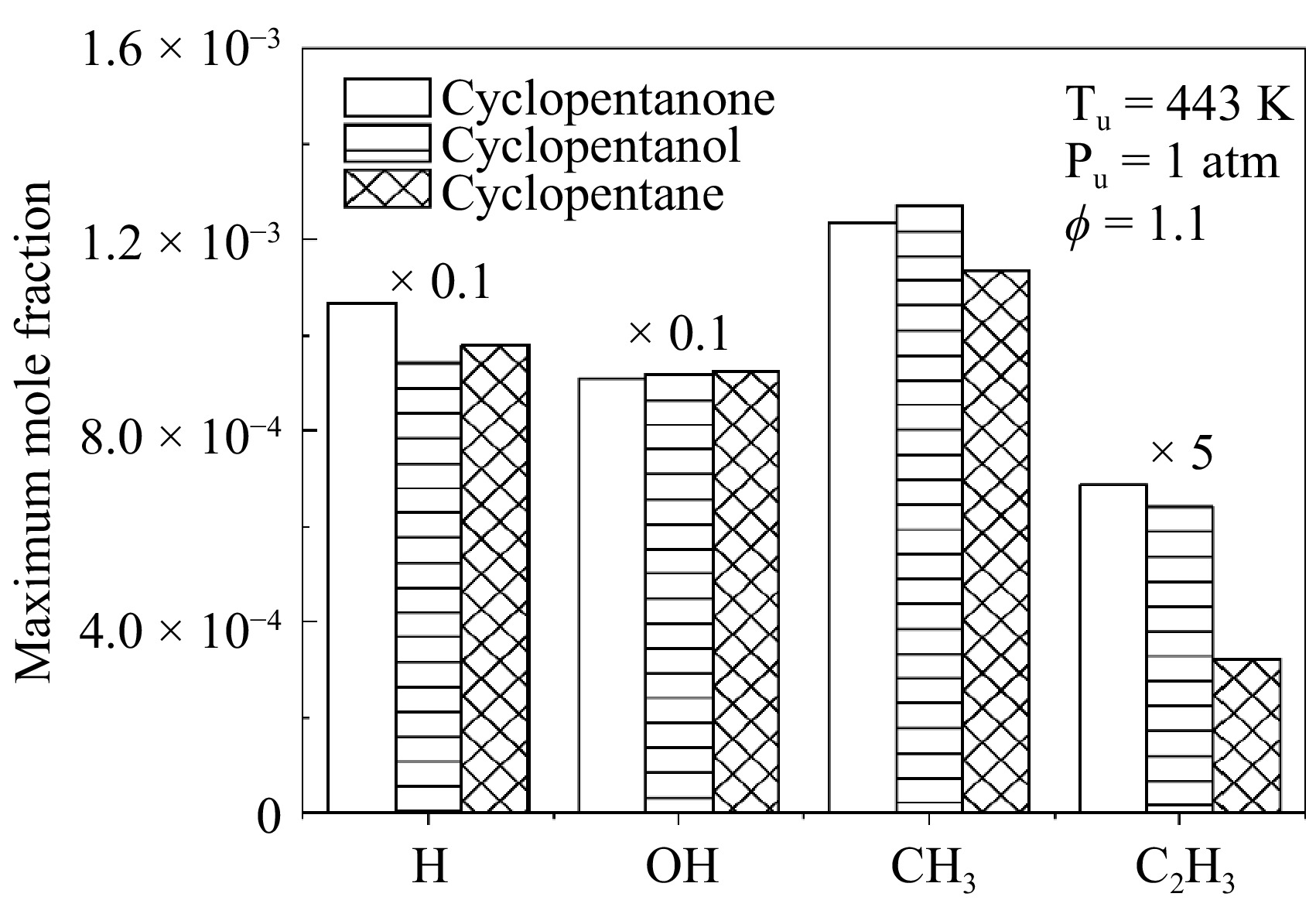
Figure 13.
Simulated maximum mole fraction of key radicals in cyclopentanone, cyclopentane, and cyclopentanol flames
-
Model Year Number of species Number of reactions Sun model 2018 515 2,840 Zhang model 2020 239 1,660 Li model 2021 220 1,671 Table 1.
Details of the chemical kinetic models published in literature.
Figures
(13)
Tables
(1)