-
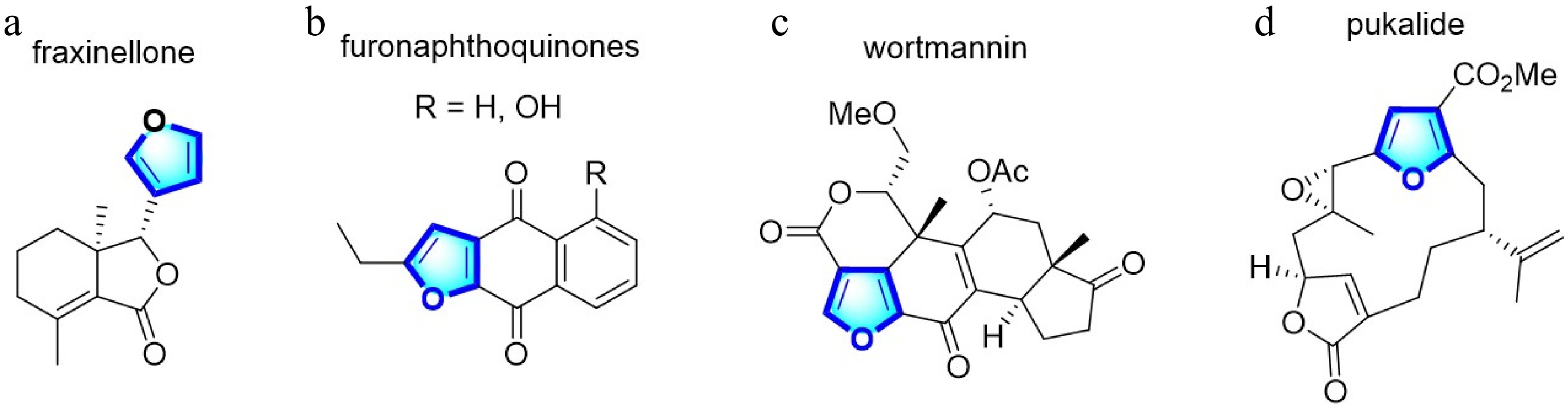
Figure 1.
Natural medicinal and agricultural products of furan derivatives.
-
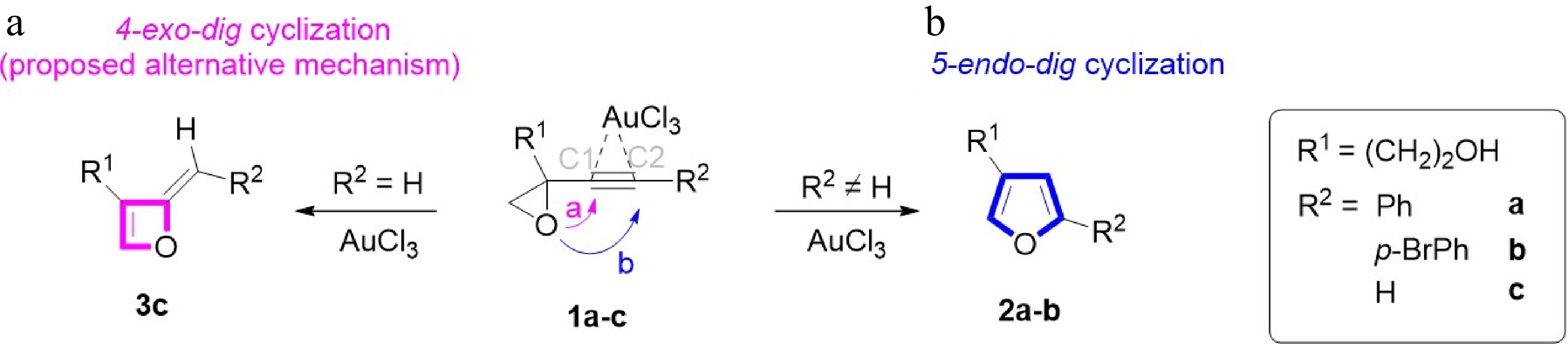
Figure 2.
Regioselective gold-catalyzed isomerization of 1 to 2 or 3.
-
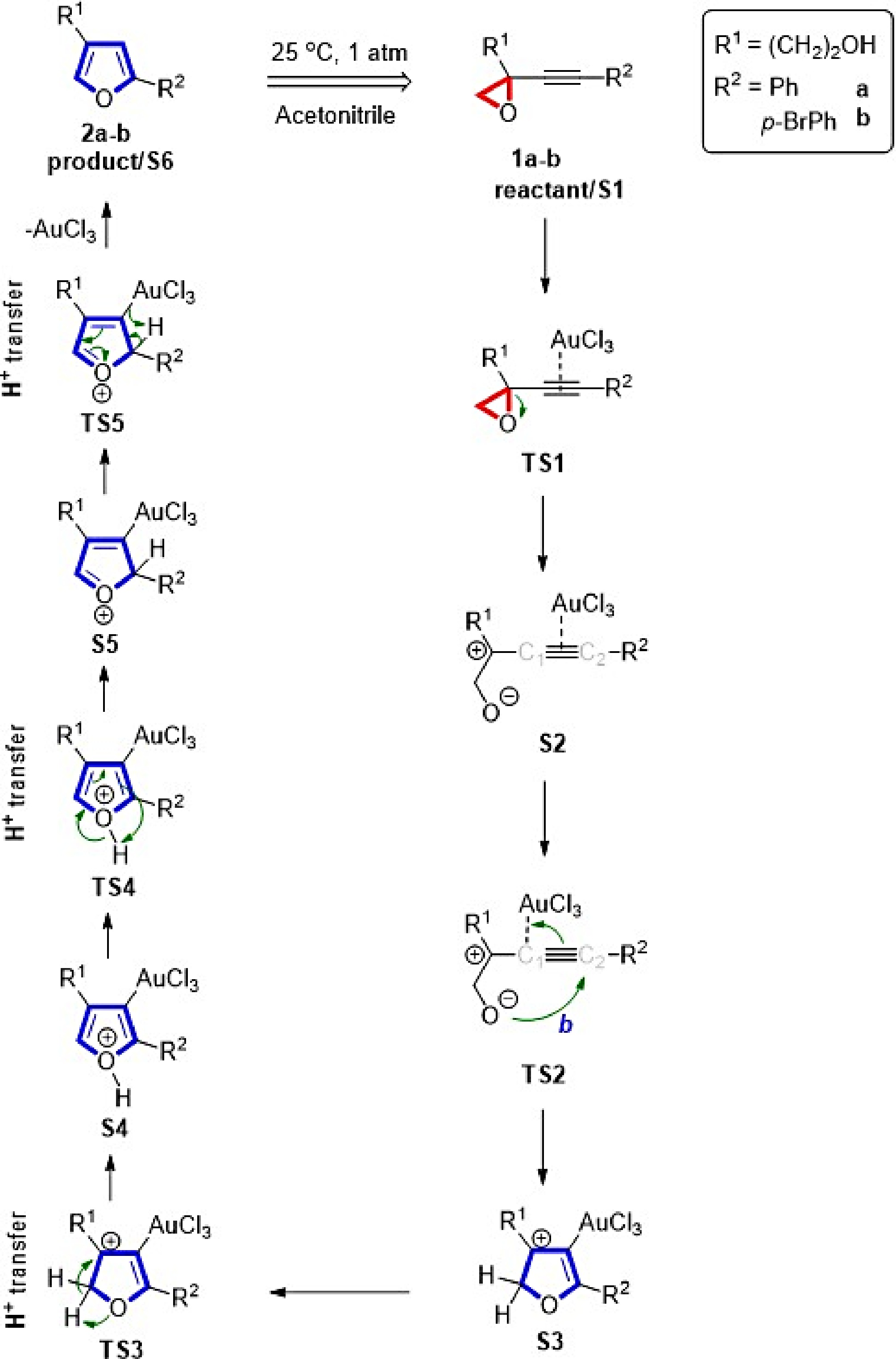
Figure 1.
Proposed creation of furans from alkynyl epoxides.
-
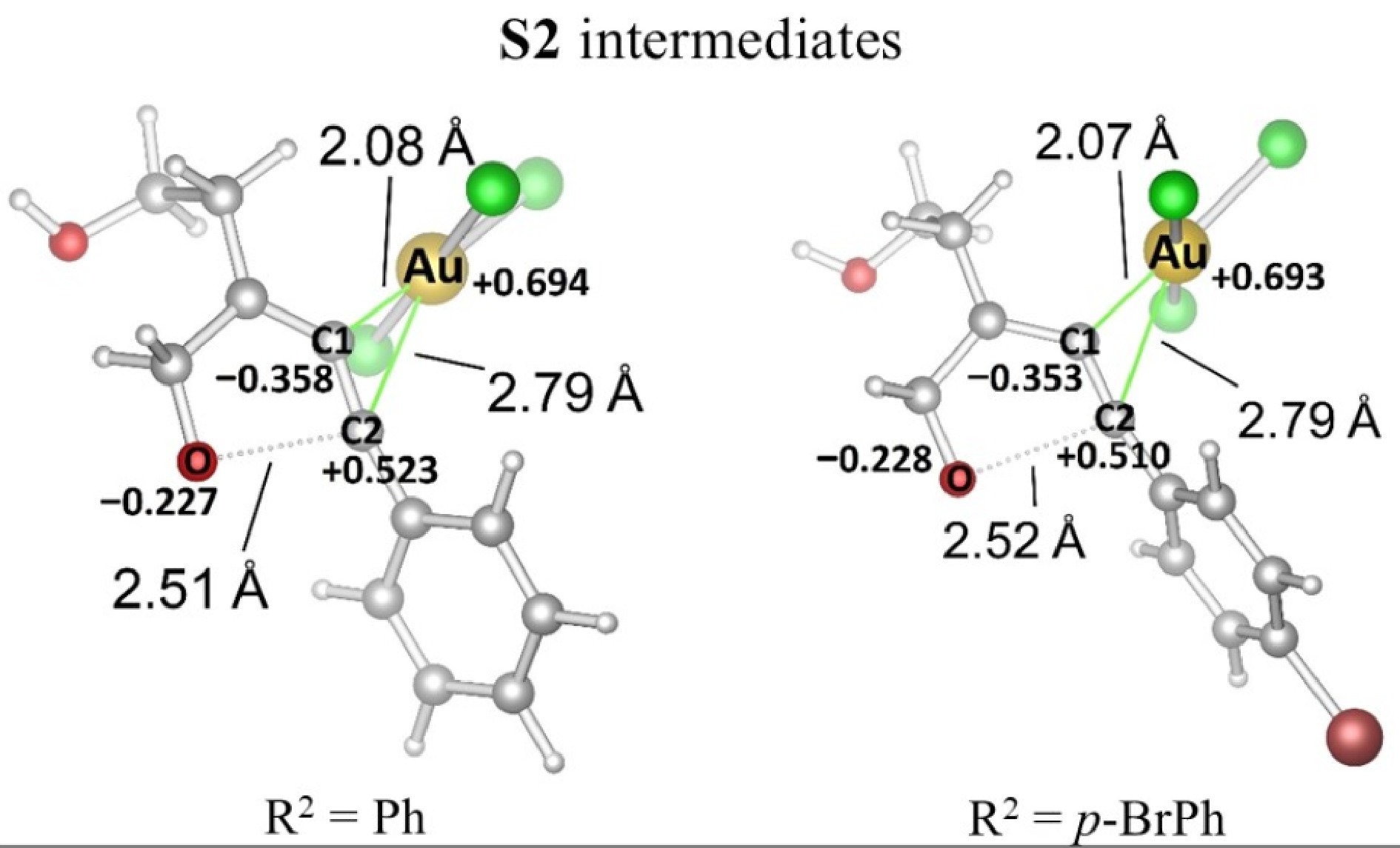
Figure 3.
Optimization of R2 = Ph and p-BrPh substitutes S2/AuCl3 complexes.
-
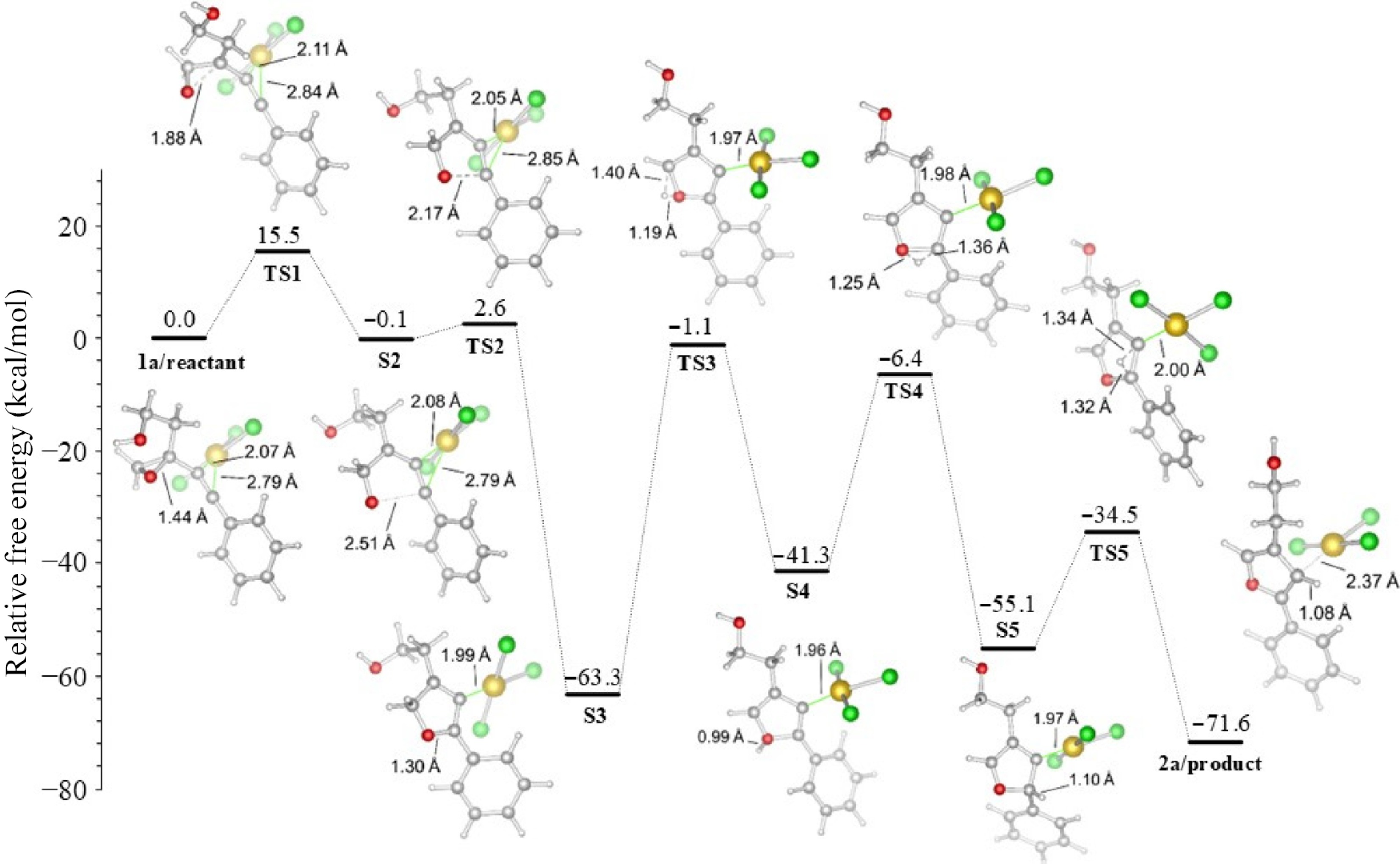
Figure 4.
R2 = Ph derived furan formation in acetonitrile.
-
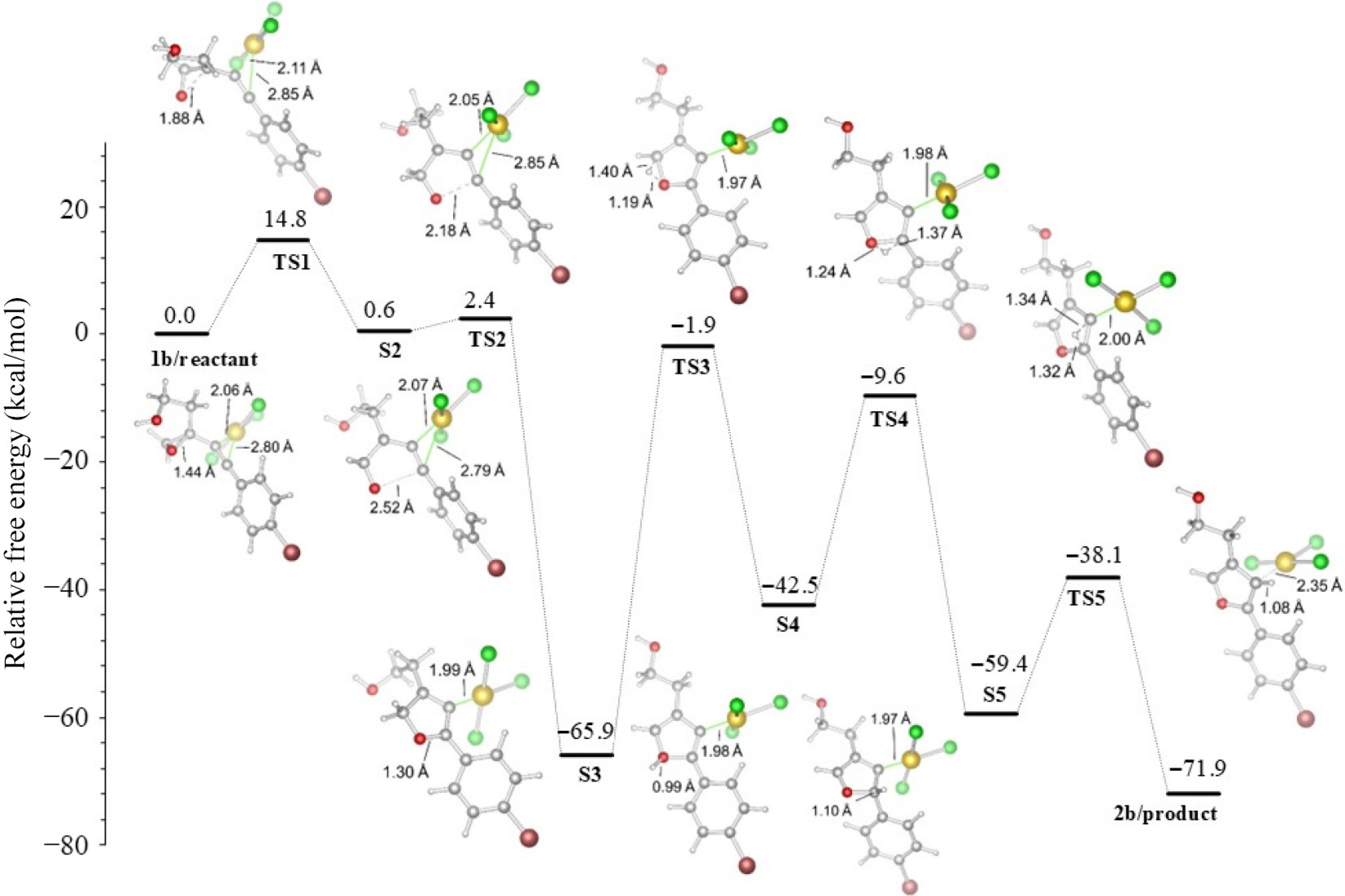
Figure 5.
R2 = p-BrPh derived furan formation in acetonitrile.
-
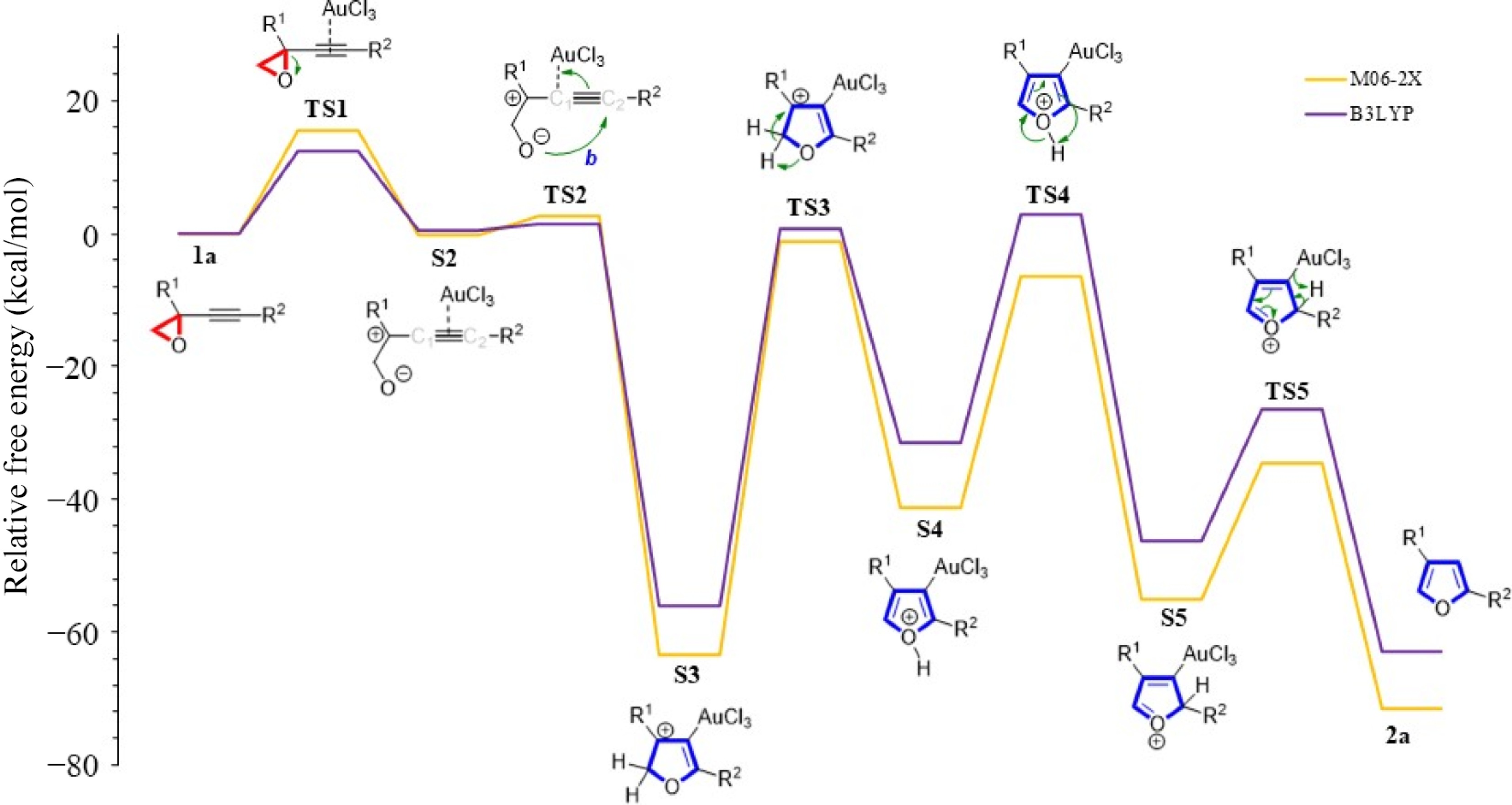
Figure 6.
R2 = Ph and R1 = CH2CH2OH derived furan formation using various functionals in acetonitrile.
-
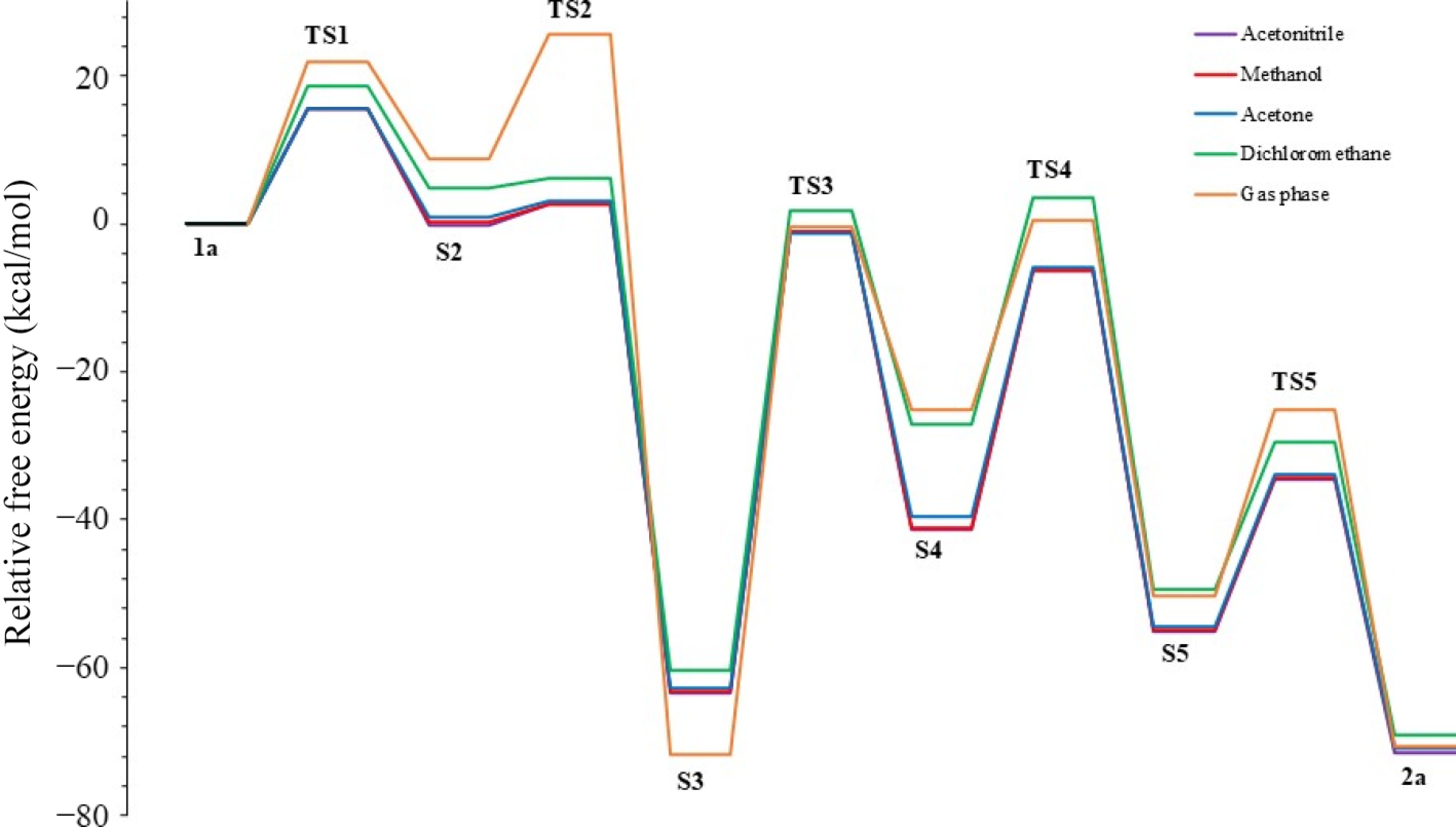
Figure 7.
R2 = Ph and R1 = CH2CH2OH substituted furan formation profile in various solvents.
-
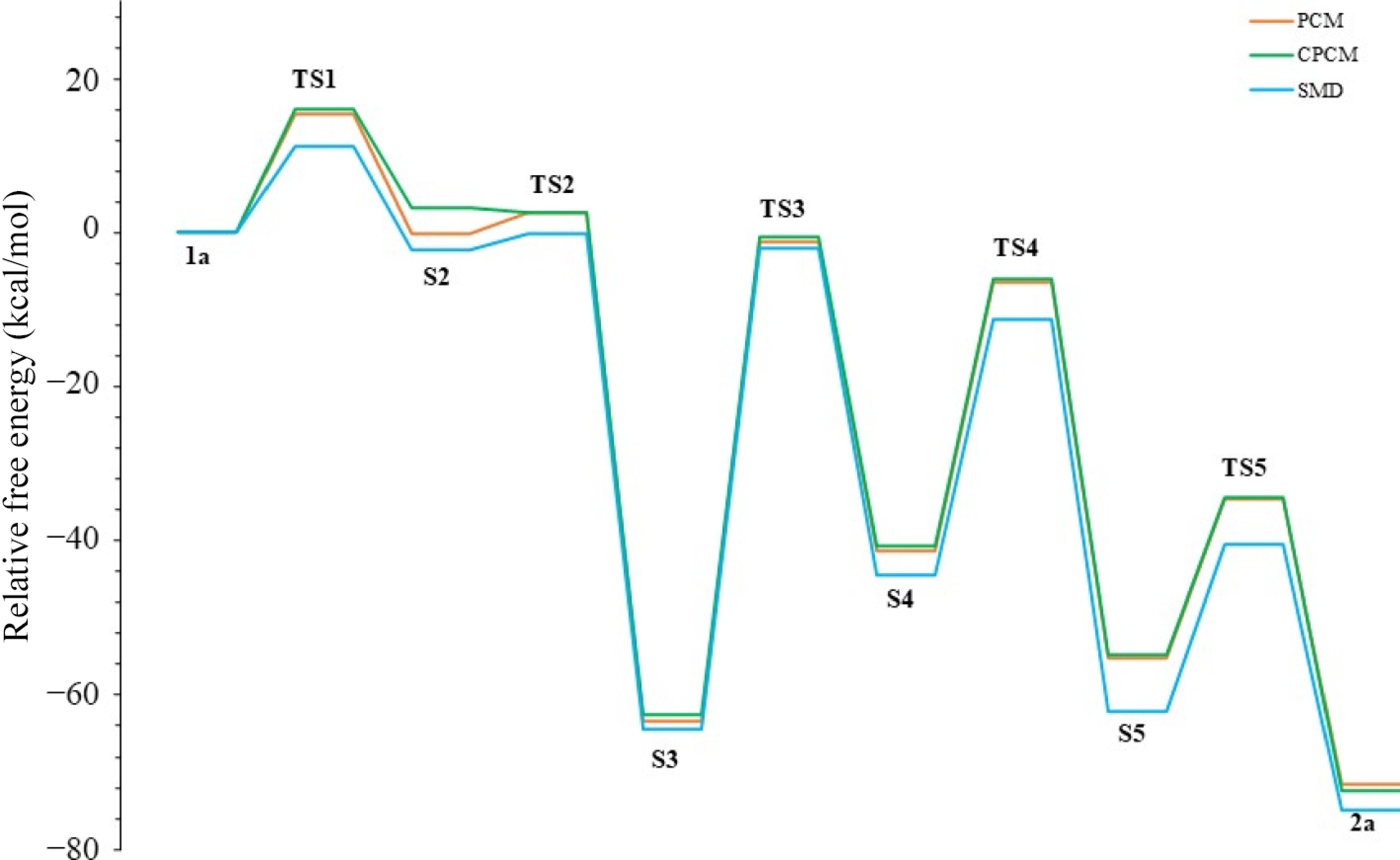
Figure 8.
Furan formation profile using various solvation models.
-
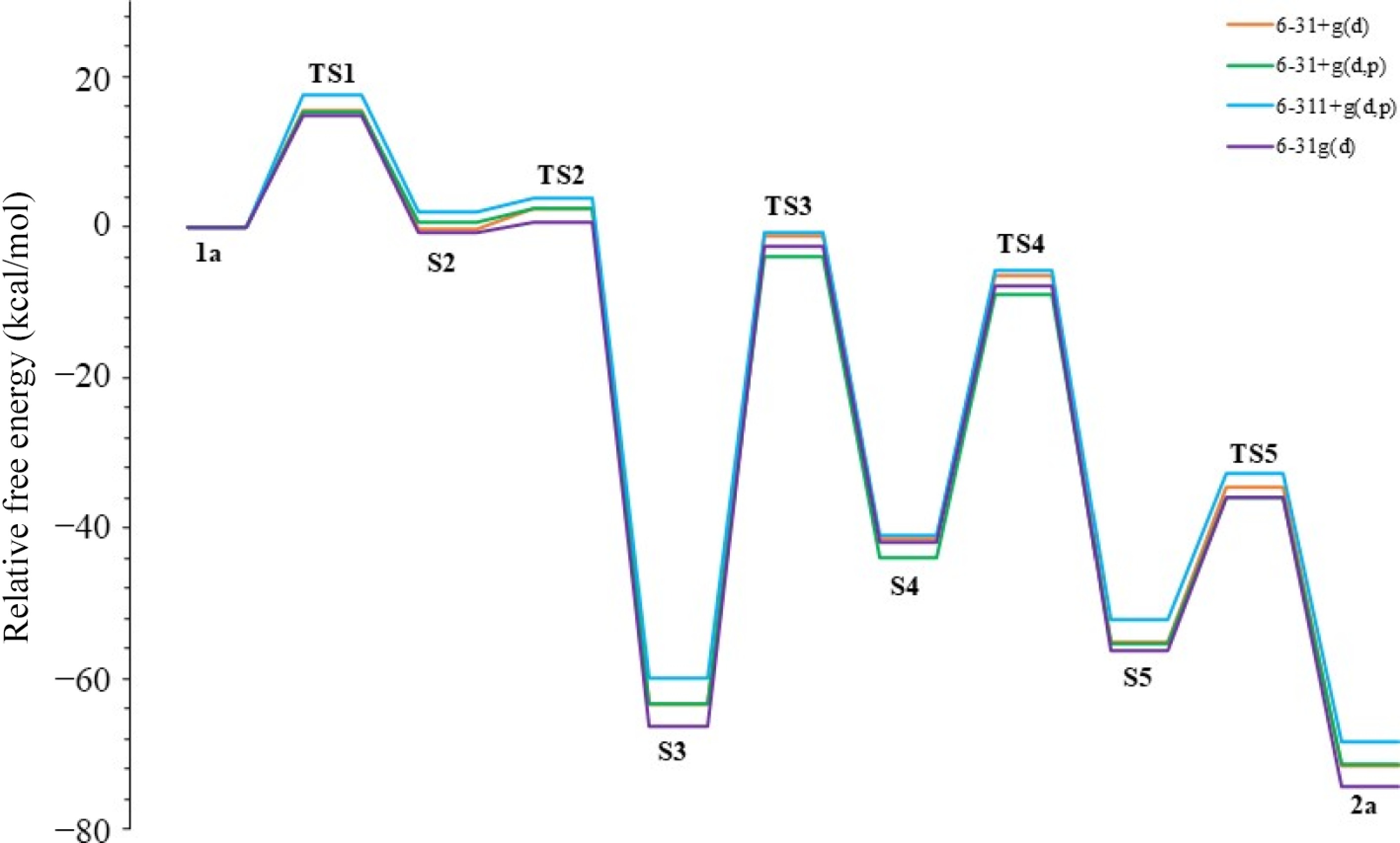
Figure 9.
Energy profiles of the mechanism in various basis sets.
-
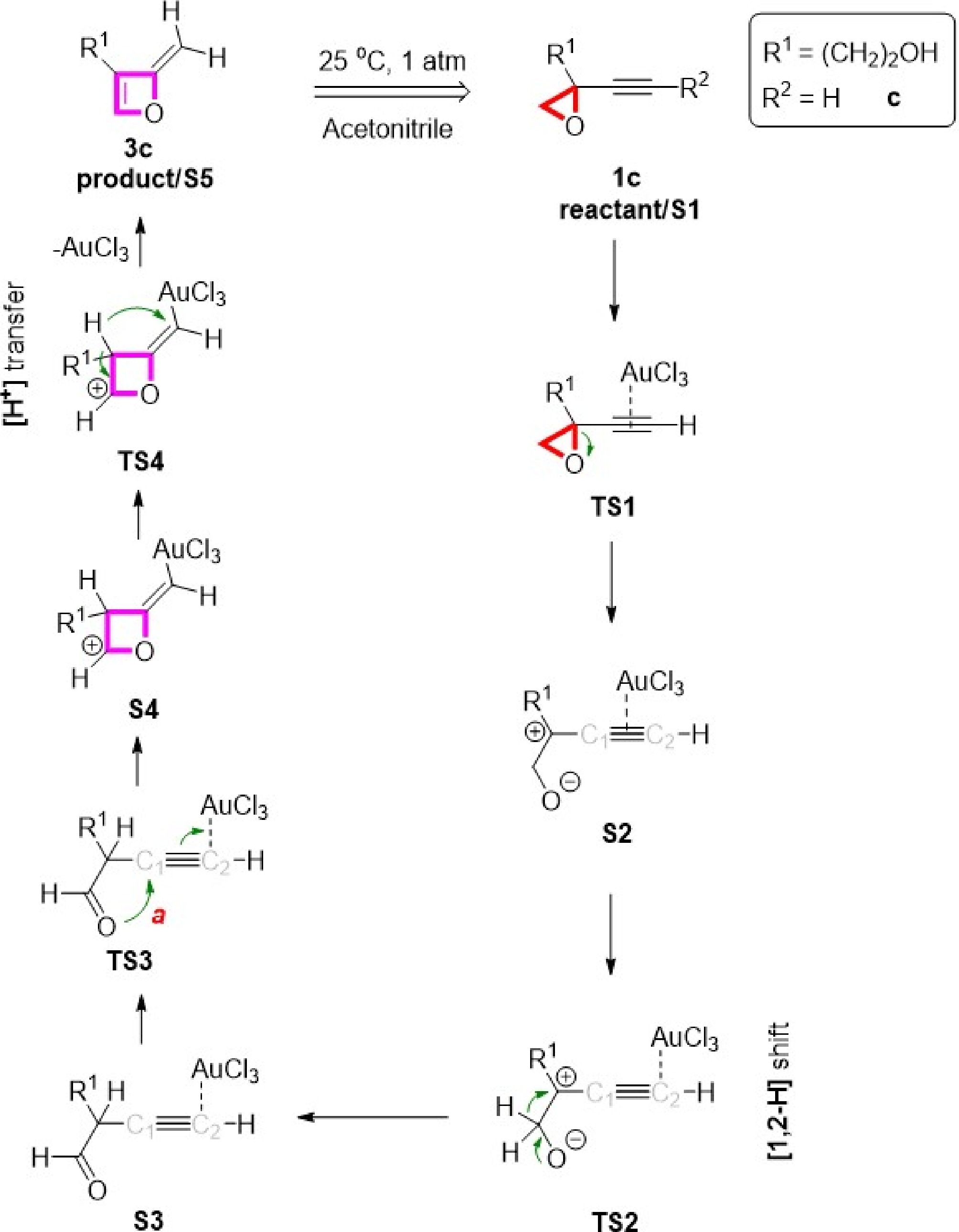
Figure 2.
Proposed gold-catalyzed mechanism to obtain oxetene from alkynyl epoxides.
-
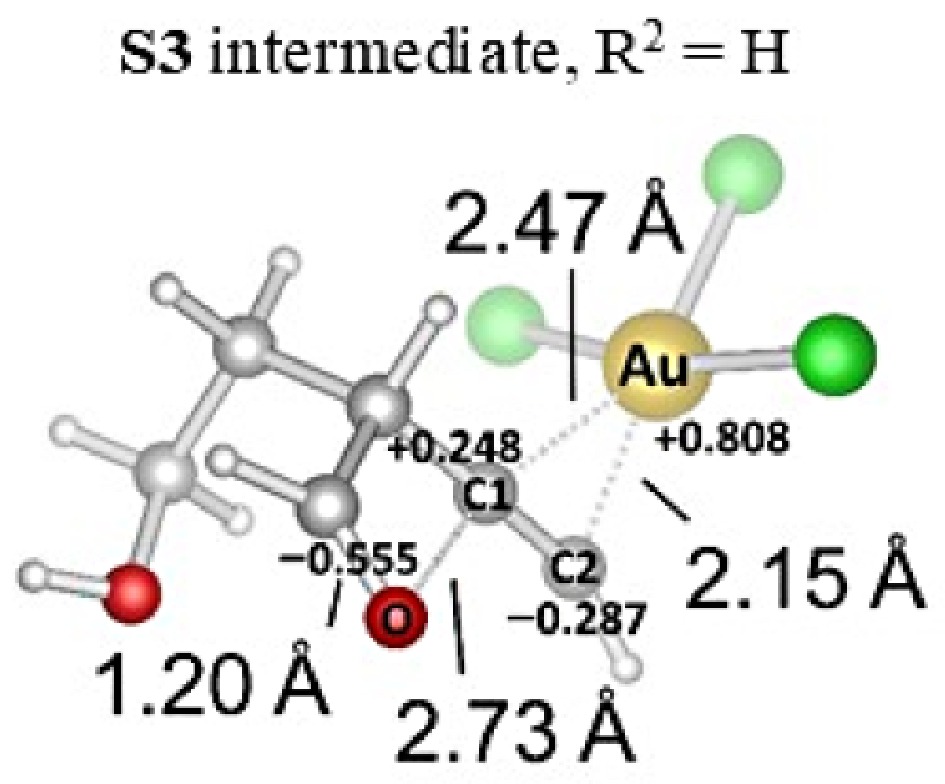
Figure 10.
Optimization of S3/AuCl3 complex in acetonitrile.
-
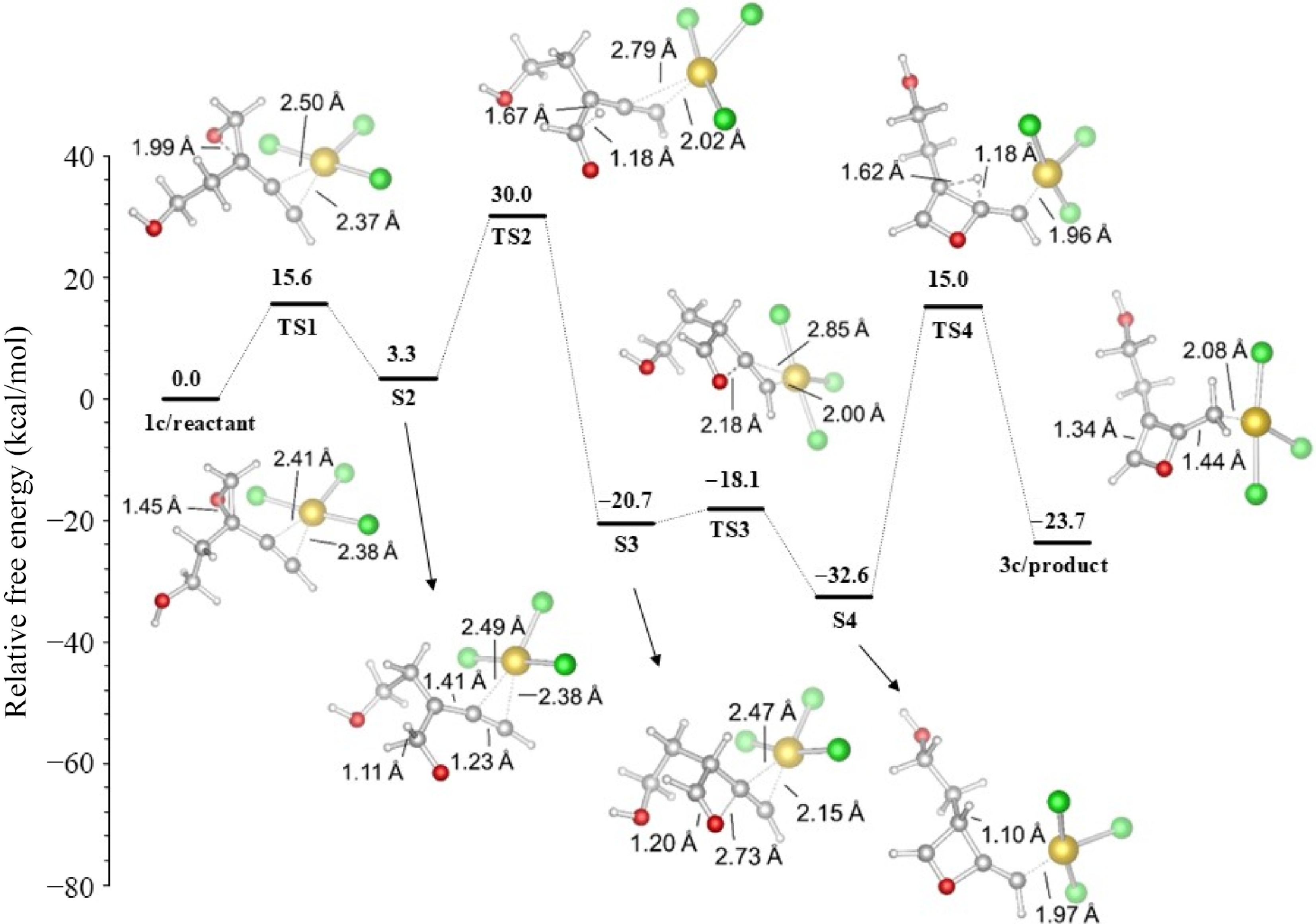
Figure 11.
The formation of oxetene (R2 = H) in acetonitrile.
-
R2 TS1 S2 TS2 S3 TS3 S4 TS4 S5 TS5 2a Ph 15.5 −0.1 2.6 −63.3 −1.1 −41.3 −6.4 −55.1 −34.5 −71.6 p−BrPh 14.8 0.6 2.4 −65.9 −1.9 −42.5 −9.6 −59.4 −38.1 −71.9 Table 1.
Energies of reaction (in kcal/mol), (R1 = (CH2)2OH), with the M06-2X functional in acetonitrile.
-
Method TS1 S2 TS2 S3 TS3 S4 TS4 S5 TS5 2a B3LYP 12.5 0.6 1.6 −56.0 0.7 −31.5 2.8 −46.2 −26.5 −63.1 Table 2.
Energies (in kcal/mol) with R2 = Ph and R1 = CH2CH2OH using B3LYP functional.
-
Solvent ε TS1 S2 TS2 S3 TS3 S4 TS4 S5 TS5 2a Acetonitrile 37.5 15.5 −0.1 2.6 −63.3 −1.1 −41.3 −6.4 −55.1 −34.5 −71.6 Methanol 32.7 15.6 0.3 2.7 −63.3 −1.1 −41.2 −6.3 −55.0 −34.0 −71.0 Acetone 20.7 15.5 1.0 3.0 −62.8 −1.3 −39.6 −5.8 −54.5 −33.9 −70.9 Dichloromethane 8.9 18.6 4.8 6.2 −60.3 1.7 −27.0 3.5 −49.4 −29.5 −69.1 Gas phase 0 22.0 8.7 25.6 −71.7 −0.4 −25.2 0.4 −50.3 −25.1 −70.7 Table 3.
Energies of reaction steps (in kcal/mol) with R2 = Ph and R1 = CH2CH2OH substituents in various solvents.
-
Solvation model TS1 S2 TS2 S3 TS3 S4 TS4 S5 TS5 2a PCM 15.5 −0.1 2.6 −63.3 −1.1 −41.3 −6.4 −55.1 −34.5 −71.6 C-PCM 16.0 3.3 2.7 −62.6 −0.6 −40.6 −6.1 −54.7 −34.4 −72.5 SMD 11.3 −2.2 −0.1 −64.4 −2.0 −44.5 −11.2 −62.1 −40.5 −75.0 Table 4.
Energies of reaction steps (in kcal/mol) (R2 = Ph and R1 = CH2CH2OH) in acetonitrile and various solvation models.
-
Basis set TS1 S2 TS2 S3 TS3 S4 TS4 S5 TS5 2a 6-31+g(d) 15.5 −0.1 2.6 −63.3 −1.1 −41.3 −6.4 −55.1 −34.5 −71.6 6-31+g(d,p) 15.2 0.7 2.4 −63.2 −3.9 −43.9 −8.9 −55.4 −35.8 −71.3 6-311+g(d,p) 17.5 2.0 4.0 −59.8 −0.7 −40.9 −5.8 −52.2 −32.7 −68.3 6-31g(d) 14.9 −0.6 0.6 −66.3 −2.4 −41.8 −7.7 −56.3 −35.9 −74.2 Table 5.
Energies of reaction steps (in kcal/mol) using various basis sets in acetonitrile.
-
Basis set Job CPU time TS1 S2 TS2 S3 TS3 S4 TS4 S5 TS5 2a 6-31+g(d) 0 d 23 h
38 min 40.8 s1 d 3 h
55 min 22.9 s1 d 3 h
28 min 33.5 s1 d 4 h
0 min 53.6 s1 d 6 h
12 min 33.0 s1 d 8 h
7 min 2.3 s0 d 14 h
36 min 50.0 s0 d 16 h
58 min 7.2 s0 d 16 h
34 min 4.6 s0 d 15 h
54 min 57.4 s6-31+g(d,p) 1 d 6 h
6 min 9.9 s1 d 12 h
5 min 10.6 s1 d 13 h
50 min 18.4 s1 d 10 h
23 min 31.2 s1 d 14 h
7 min 27.6 s1 d 13 h
45 min 27.4 s1 d 1 h
46 min 46.4 s0 d 17 h
17 min 24.7 s0 d 18 h
51 min 57.7 s0 d 18 h
33 min 27.2 s6-311+g(d,p) 1 d 0 h
48 min 59.0 s1 d 2 h
3 min 56.9 s1 d 2 h
37 min 44.5 s1 d 5 h
23 min 41.2 s1 d 2 h
46 min 16.9 s1 d 5 h
12 min 7.6 s1 d 6 h
10 min 27.9 s1 d 5 h
27 min 23.0 s1 d 8 h
10 min 44.4 s1 d 2 h
55 min 8.0 s6-31g(d) 0 d 3 h
12 min 54.9 s0 d 3 h
12 min 50.8 s0 d 3 h
17 min 53.9 s0 d 3 h
22 min 20.4 s0 d 3 h
18 min 26.8 s0 d 3 h
13 min 54.5 s0 d 3 h
18 min 24.1 s0 d 3 h
23 min 56.9 s0 d 3 h
26 min 13.0 s0 d 3 h
22 min 26.0 sTable 6.
The computational cost (CPU time) for each basis set.
Figures
(13)
Tables
(6)