-

Figure 1.
The chemical composition of Euodia rutaecarpa is diverse and includes alkaloids, limonoids, flavonoids, and volatile oils. The major bioactive compounds are: indole quinazolinone alkaloids, quinolone alkaloids, and limonoids.
-
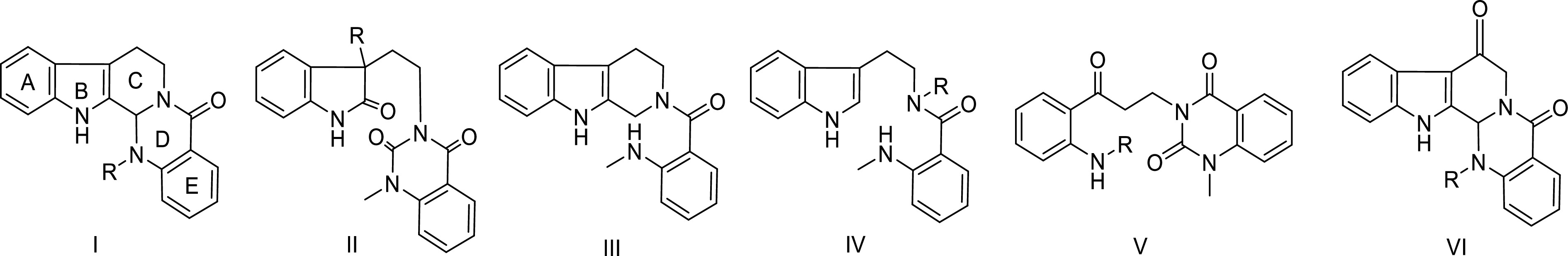
Figure 2.
Skeleton types of quinazoline alkaloids in Euodia plants.
-
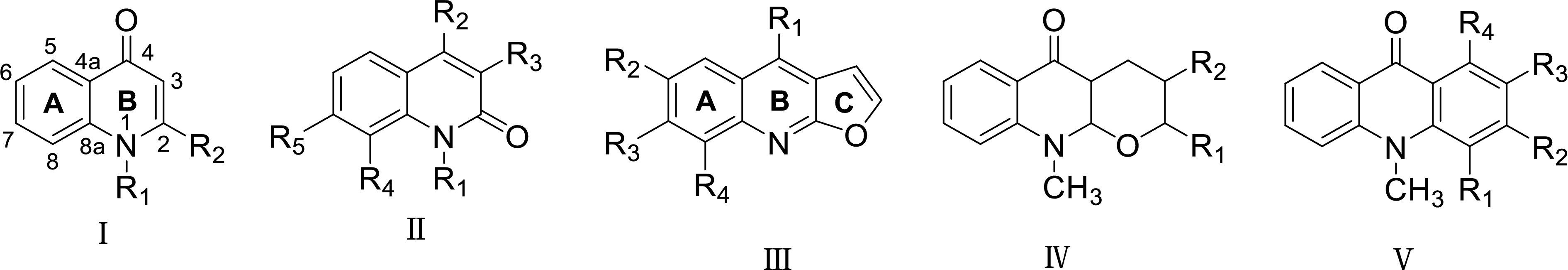
Figure 3.
The skeleton types of quinolone alkaloids in Euodia plants.
-
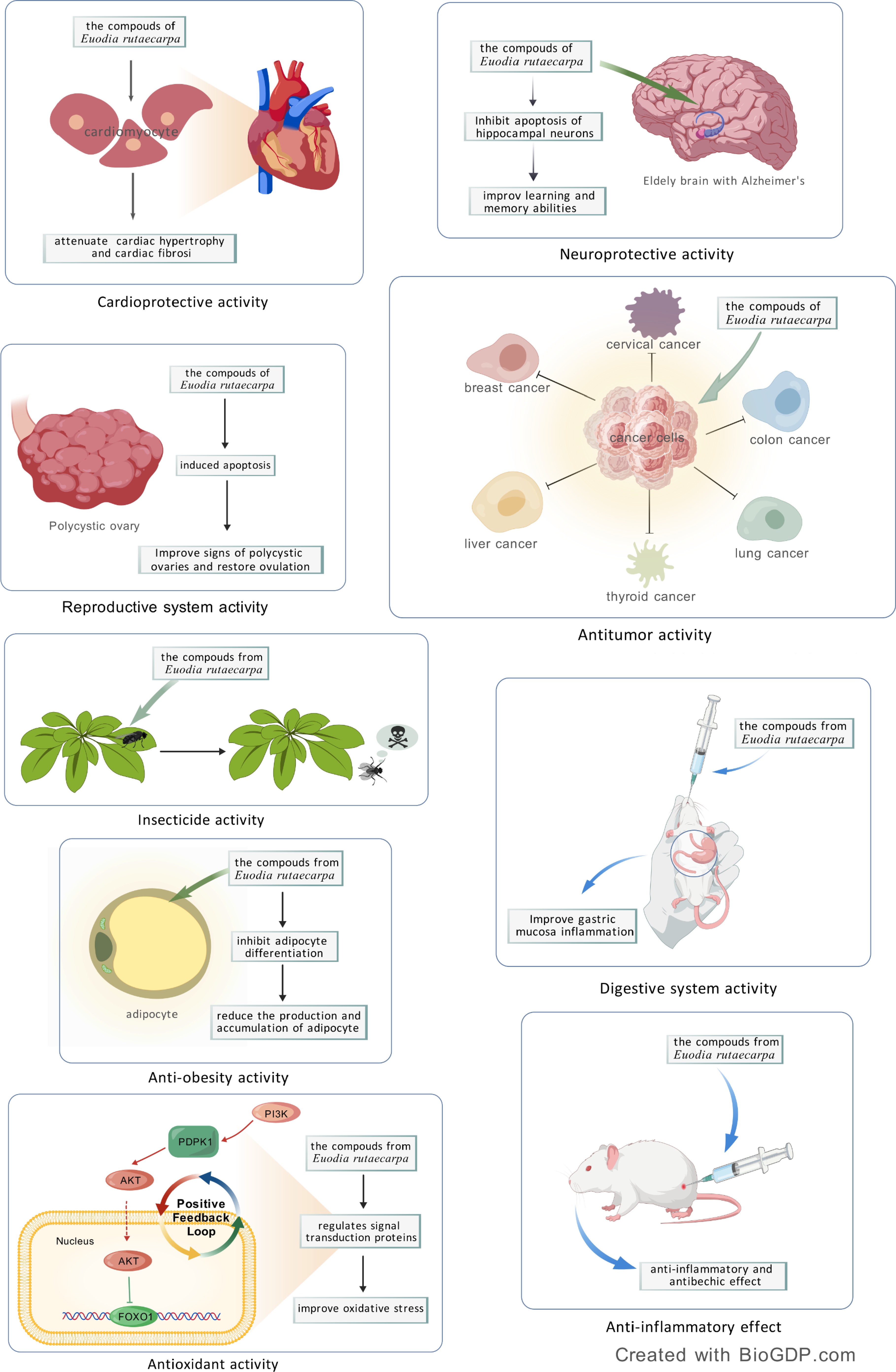
Figure 4.
Pharmacological effects of Euodia rutaecarpa: cardioprotective activity, neuroprotective activity, reproductive system activity, anti-tumor effect, insecticide activity, anti-obesity activity, antioxidant activity, digestive system activity, anti-inflammatory effect.
-
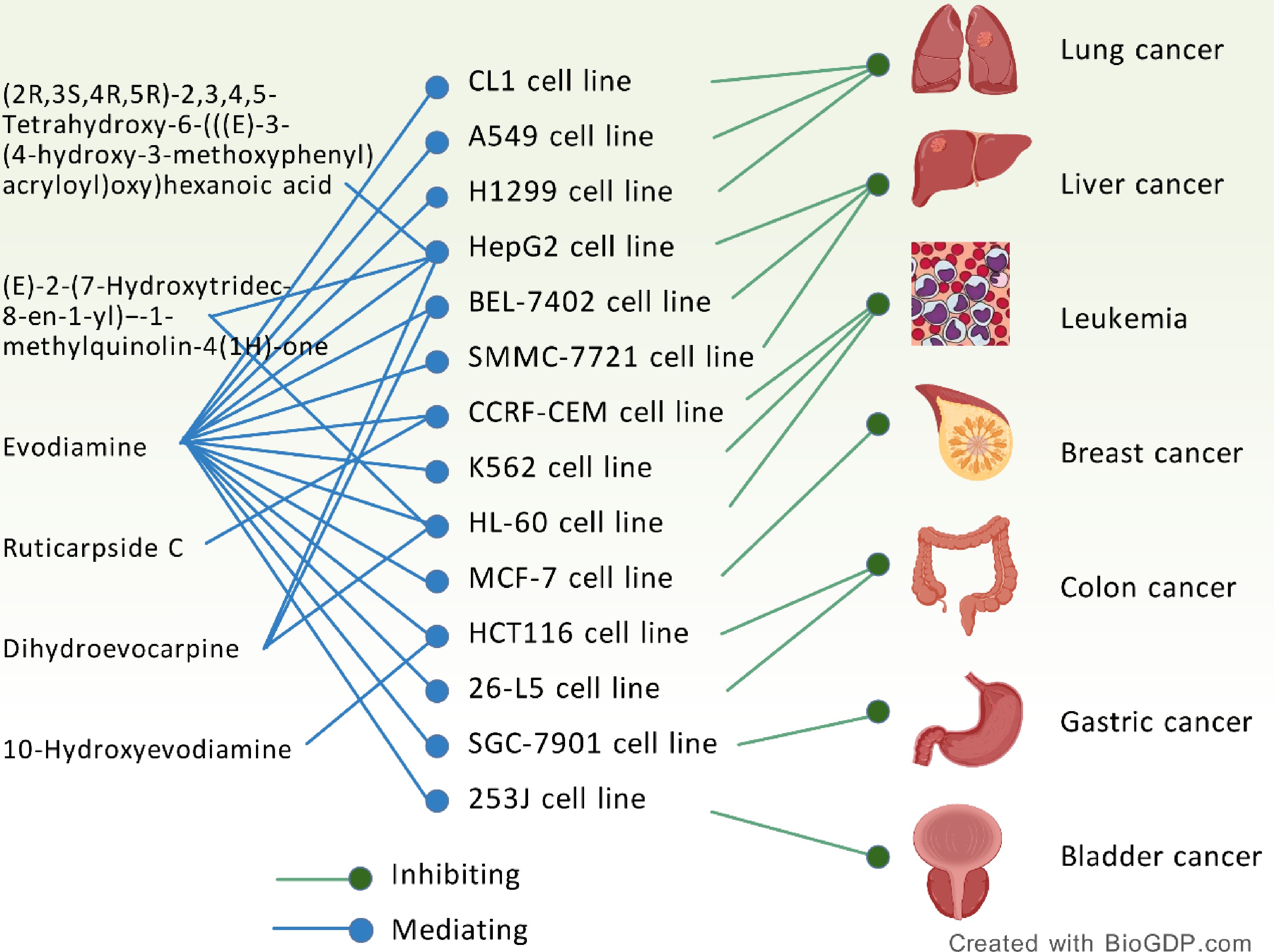
Figure 5.
Anti-tumor effect of E. rutaecarpa.
-
No. Indole quinazoline alkaloids Molecular formula Relative molecular mass Structure type Ref. 1 Evodiamine (EVO) C19H17N3O 303 I [20−25] 2 Hydroxyevodiamine C19H17N3O2 319 I [26] 3 13β-acetongl hydroxyl evodiamine C22H21N3O2 359 I [27] 4 Carboxyevodiamine C20H17N3O3 347 I [28] 5 13,14-dihydrorutaecarpine C18H15N3O 289 I [29] 6 14-N-formyldihydrorutaecarpine C19H15N3O2 317 I [22,23] 7 13β-hydroxymethylevodiamine C20H19N3O2 333 I [30] 8 Rutaecarpine (RUT) C18H13N3O 287 I [21−25] 9 Hortiacine C19H15N3O2 317 I [31] 10 1-hydroxyrutaecarpine C18H13N3O2 303 I [32] 11 3-hydroxyrutaecarpine C18H13N3O2 303 I [29] 12 7β-hydroxyrutaecarpine C18H13N3O2 303 I [22,23,25,33,34] 13 10-hydroxyrutaecarpine C18H13N3O2 303 I [35] 14 (7R,8S)-7,8-hydroxyrutaecarpine C18H13N3O3 319 I [32] 15 (7R,8S)-7-hydroxy-8-methoxy-rutaecarpine C19H15N3O3 333 I [32] 16 (7R,8S)-7-hydroxy-8-ethoxy-rutaecarpine C20H17N3O3 347 I [32] 17 N13-methyl rutaecarpine C19H15N3O 301 I [36] 18 1-O-β-D-glucopyranosylrutaecarpine C24H23N3O7 465 I [37] 19 Rutaecarpine-2-O-β-D-glucopyranoside C24H23N3O7 465 I [37] 20 Rutaecarpine-10-O-β-D-glucopyranoside C24H23N3O7 465 I [38] 21 Rutaecarpine-10-O-β-D-rutinoside C30H33N3O11 611 I [38] 22 13-methyl-13H-indolo [2',3':3,4] pyrido[2,1b] quinazolin-5-one C19H13N3O 299 I [39] 23 7,8-dehydrorutaecarpine C18H11N3O 285 I [30] 24 Pseudorutaecarpine C18H13N3O 287 I [32] 25 Dehydroevodiamine C19H15N3O 301 I [22,23,40] 26 Hortiamine C20H17N3O2 331 I [41] 27 Dehydroevodiamine chloride C19H16N3OCl 337 I [31] 28 Evodianinine C19H13N3O 299 I [20] 29 Wuzhuyurutine A C17H11N3O2 289 I [36] 30 Evodiagenine C19H13N3O 299 I [42] 31 Evodiaxinine C20H15N3O 313 I [20,43] 32 (+) evodiakine C19H17N3O3 335 I [44] 33 (−) evodiakine C19H17N3O3 335 I [44] 34 Dievodiamine C38H30N6O2 602 I, II [42] 35 Wuchuyuamide I C19H17N3O4 351 II [21,22,25,31] 36 Wuchuyuamide II C19H17N3O3 335 II [45] 37 Evollionines B C20H19N3O5 381 II [46] 38 Goshuyuamide-II C19H17N3O2 319 II [25,47] 39 13-hydroxymethyl goshuyuamide-II C20H19N3O3 349 II [48] 40 10-methoxygoshuyuamide-II C20H19N3O3 349 II [29] 41 Wuzhuyurutine C C18H13N3O3 319 II [30] 42 Wuzhuyurutine D C17H11N3O3 305 II [30] 43 (s)-7-hydroxysecorutaecarpine C18H15N3O3 321 II [25,37] 44 Wuzhuyurutine B C17H11N3O3 305 II [30] 45 Bouchardatine C17H11N3O2 289 II [30] 46 Evodiamide A C20H19N3O5 381 II [40] 47 Evodiamide B C19H16N4O2 332 II [40] 48 Evodiamide C C37H32N6O6 656 II, IV [40] 49 Goshuyuamide-I C19H19N3O 305 III [23,25,47] 50 Rhetsinine C19H17N3O2 319 III [49] 51 Evollionines A C19H15N3O2 317 III [46] 52 Evodiamide C19H21N3O 307 IV [23,27] 53 Dimethyl evodiamide C18H19N3O 293 IV [47] 54 Nb-demethylevodiamide C18H19N3O 293 IV [50] 55 Wuchuyuamide III C18H17N3O3 323 V [40,51] 56 Wuchuyuamide IV C19H17N3O4 351 V [51] 57 Wuchuyuamide V C20H19N3O5 381 V [21] 58 Evodamide A C19H15N3O2 317 VI [29] Table 1.
Quinazoline alkaloids isolated from Euodia plants.
-
No. Quinolone alkaloids Molecular formula Relative molecular mass Structure type Ref. 1 2-ethyl-1-methyl-4(1H)-quinolone C12H13NO 187 I [52] 2 Quinolone A C14H15NO3 245 I [53] 3 1-methyl-2-pentyl-4(1H)-quinolone C15H19NO 229 I [52] 4 Methyl 5-(1,4-dihydro-1-methyl-4-oxoquinolin-2-yl) pentanoate C16H19NO3 273 I [46] 5 1-methyl-2-heptyl-4(1H)-quinolone C17H23NO 257 I [52] 6 1-methyl-2-octyl-4(1H)-quinolone C18H25NO 271 I [52] 7 1-methyl-2-nonyl-4(1H)-quinolone C19H27NO 285 I [25,52] 8 1-methyl-2-[(Z)-4-nonenyl]-4(1H)-quinolone C19H25NO 283 I [54] 9 1-methyl-2-decyl-4(1H)-quinolone C20H29NO 299 I [52] 10 1-methyl-2-undecyl-4(1H)-quinolone C21H31NO 313 I [25,52] 11 1-methyl-2-[(E)-1-undecenyl]-4(1H)-quinolone C21H29NO 311 I [54] 12 1-methyl-2-[(Z)-5-undecenyl]-4(1H)-quinolone C21H29NO 311 I [30] 13 1-methyl-2-[(Z)-6-undecenyl]-4(1H)-quinolone C21H29NO 311 I [30] 14 1-methyl-2-[6-carbonyl-(E)-4-undecenyl]-4(1H)-quinolone C21H27NO2 325 I [30] 15 1-methyl-2-[7-carbonyl-(E)-5-undecenyl]-4(1H)-quinolone C21H27NO2 325 I [54] 16 1-methyl-2-[(1E,5Z)-1,5-undecadienyl]-4(1H)-quinolone C21H27NO 309 I [54] 17 1-methyl-2-[(Z)-1-undecenyl]-4(1H)-quinolone C21H29NO 311 I [55] 18 1-methyl-2-[6-carbonyl-(E)-7-undecenyl]-4(1H)-quinolone C21H27NO2 325 I [54] 19 1-methyl-2-dodecyl-4(1H)-quinolone C22H33NO 327 I [25,52] 20 1-methyl-2-[(Z)-5’-dodecenyl]-4(1H)-quinolone C22H31NO 311 I [52] 21 1-methyl-2-tridecyl-4(1H)-quinolone or Dihydroevocarpine C23H35NO 341 I [52] 22 Evocarpine C23H33NO 339 I [56]. 23 1-methyl-2-[(Z)-4-tridecenyl]-4(1H)-quinolone C23H33NO 339 I [57] 24 1-methyl-2-[(Z)-7-tridecenyl]-4(1H)-quinolone C23H33NO 339 I [30] 25 1-methyl-2-[(Z)-8-tridecenyl]-4(1H)-quinolone C23H33NO 339 I [30] 26 1-methyl-2-[12-tridecenyl]-4(1H)-quinolone C23H33NO 339 I [58] 27 1-methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone C23H31NO 337 I [54] 28 1-methyl-2-[6-carbonyl-(E)-7-tridecenyl]-4(1H)-quinolone C23H31NO2 353 I [30] 29 1-methyl-2-[7-carbonyl-(E)-8-tridecenyl]-4(1H)-quinolone (correct)
1-methyl-2-[7-carbonyl (E)-9-tridecenyl]-4(1H)-quinolone (reference)C23H31NO2 353 I [30] 30 1-methyl-2-[8-carbonyl-(E)-9-tridecenyl]-4(1H)-quinolone C23H31NO2 353 I [30] 31 1-methyl-2-[9-carbonyl-(E)-7-tridecenyl]-4(1H)-quinolone C23H31NO2 353 I [52] 32 1-methyl-2-[7-hydroxy-(E)-8-tridecenyl]-4(1H)-quinolone (correct)
1-methyl-2-[7-hydroxy-(E)-9-tridecenyl]-4(1H)-quinolone (reference)C23H33NO2 355 I [54] 33 1-methyl-3-[(7E,9E,12Z)-7,9,12-pentadecadienyl]-4(1H)-quinolone C25H33NO 363 I [59] 34 1-methyl-3-[(7E,9E,11E)-7,9,11-Pentadecadienyl]-4(1H)-quinolone C25H33NO 363 I [59] 35 1-methyl-2-(13-hydroxy-tridecenyl)-4(1H)-quinolone C23H35NO2 357 I [30] 36 1-methyl-2-tetradecyl-4(1H)-quinolone C24H37NO 355 I [52] 37 1-methyl-2-[13-tetradecenyl]-4(1H)-quinolone C24H35NO 353 I [58] 38 1-methyl-2-pentadecyl-4(1H)-quinolone C25H39NO 369 I [52,60] 39 1-methyl-2-[(Z)-5-pentadecenyl]-4(1H)-quinolone C25H37NO 367 I [52] 40 1-methyl-2-[(Z)-6-pentadecenyl]-4(1H)-quinolone C25H37NO 367 I [54] 41 1-methyl-2-[(Z)-9-pentadecenyl]-4(1H)-quinolone C25H37NO 367 I [56] 42 1-methyl-2-[(Z)-10-pentadecenyl]-4(1H)-quinolone C25H37NO 367 I [56] 43 1-methyl-2-[(6Z,9Z)-6,9-pentadecadienyl]-4(1H)-quinolone C25H35NO 365 I [30,39] 44 1-methyl-2-[12-hydroxy-tridecyl]-4(1H)-quinolone C25H35NO2 381 I [58] 45 1-methyl-2-[(6Z,9Z,12Z)-6,9,12-pentadecatriene]-4(1H)-quinolone C25H33NO 363 I [55] 46 1-methyl-2-(15-hydroxy-pentadecenyl)-4(1H)-quinolone C25H39NO2 385 I [30] 47 1-methyl-2-hexadecyl-4(1H)-quinolone C26H41NO 383 I [58]. 48 2-nonyl-4(1H)-quinolone C18H25NO 271 I [61] 49 2-undecyl-4(1H)-quinolone C20H29NO 299 I [61] 50 2-tridecyl-4(1H)-quinolone C22H33NO 327 I [56] 51 1-methyl-2-[(6Z,9Z,12E)-pentadecatriene]-4(1H)- quinolone C25H33NO 363 I [58] 52 1-methyl-2-[(9E,13E)-9,13-heptadecadienyl]-4(1H)- quinolone C27H39NO 393 I [58] 53 2-hydroxy-4-methoxy-3-(3’-methyl-2’-butenyl)- quinolone C15H17NO2 243 I [62] 54 4-hydroxy-1-methyl-2-nonyl-quinolinium C19H27NO 285 I [63] 55 Atanine I C15H17NO2 243 II [33] 56 4-methoxy-3-(3-methylbut-2-en-1-yl)-2-quinolone-8-O-β-D-glucopyranoside C21H27NO8 421 II [53] 57 Quinolone B C21H27NO8 421 II [53] 58 3-Dimethylallyl-4-methoxy-2-quinolone C15H17NO2 244 II [64] 59 Edulinine C16H21NO4 291 II [65] 60 Dictamnine C12H9NO4 199 III [33,66,67] 61 6-methoxydictamnine C13H11NO3 229 III [33] 62 Evolitrine C13H11NO3 229 III [33,67] 63 Confusameline C12H13NO3 215 III [67] 64 Kokusaginine C14H13NO4 259 III [66] 65 Skimmianine C14H13NO4 259 III [23,25,33,67] 66 Haplophine C13H11NO3 229 III [68,69] 67 Robustine C12H9NO3 215 III [69] 68 7-isopentenyl-γ-fagarine C18H19NO4 313 III [65] 69 Melineurine C18H19NO4 313 III [70] 70 Leptanoine A C17H15NO3 281 III [71] 71 Leptanoine B C18H17NO4 311 III [71] 72 Leptanoine C C18H19NO4 313 III [71] 73 Evodine C18H19NO5 329 III [72] 74 Roxiamine A C18H19NO5 329 III [73] 75 Roxiamine B C18H17NO5 327 III [73] 76 Roxiamine C C16H27NO4 287 III [73] 77 Evoxoidine C18H19NO5 329 III [74] 78 Ribalinine C15H17NO3 259 IV [65] 79 1-hyddroxy-2,3-dimetoxy-10-methylacridone C16H15NO4 285 V [65] 80 2,3-Dimethoxy-4-hydroxy-10-methylacridone C16H15NO4 285 V [75] 81 2,3,4-trimethoxy-10-methylacridone C17H17NO4 299 V [76] 82 Xanthoxoline C15H13NO4 271 V [77] 83 Evoxanthidine C15H11NO4 269 V [77] 84 Xanthevodine C16H13NO5 299 V [77] 85 Melicopidin C17H15NO5 313 V [68] Table 2.
Quinolone alkaloids isolated from Euodia plants.
-
No. Limonoids Molecular formula Relative molecular mass Ref. 1 Limonin C26H30O8 470 [69,78−82] 2 12α-hydroxylimonin C26H30O9 486 [83−85] 3 6α-acetoxy-5-epilimonin (glaucin B) C28H32O10 528 [82,86] 4 6β-acetoxy-5-epilimonin C28H32O10 528 [87] 5 Jangomolide C26H28O10 500 [88] 6 Limonin diosphenol C26H28O9 484 [82−84] 7 Evodol (graucin C) C26H28O11 516 [78,80] 8 12α-hydroxyevodol C26H28O10 500 [80,89] 9 12α-hydroxyrutaevin (glaucinA) C26H30O10 502 [82] 10 Rutaevin C26H30O9 486 [80,82,90] 11 7β-acetoxy-5-epilimonin (ruteavine acetate) C27H32O10 516 [91] 12 Isolimonexic acid C26H30O10 502 [81] 13 Shihulimonin A1 C26H30O10 502 [78] 14 Evorubodinin C27H32O10 516 [59] 15 Evolimorutanin C28H36O11 548 [79] 16 Evodirutaenin A C26H29O11 517 [92] 17 Euodirutaecins A C26H28O11 516 [92] 18 Euodirutaecins B C26H28O11 516 [92] 19 Graucin A C26H30O10 502 [83] Table 3.
Ring-integrating limonoids isolated from Euodia.
-
No. Limonoids Molecular formula Relative molecular mass Ref. 20 Obacunone C26H30O7 454 [91] 21 7-deacetylproceranone C25H29O5 409 [87] 22 Nomilin C29H38O9 530 [87] 23 Clauemargine L C26H34O7 458 [91] 24 19-hydroxy methyl isoobacunoate diosphenol C28H36O10 532 [91] 25 Obacunonsaeure C26H32O8 472 [91] Table 4.
Rearranged limonoids isolated from Euodia.
-
Table 5.
Degradable limonoids isolated from Euodia.
-
Table 6.
Anti-inflammatory activity.
-
Activities Mechanism of action Signaling pathway Ref. Protective effect of gastric mucosa injury Stimulation of CGRP release TRPV1 [163] Reducing the inflammatory response [164] Effects on gastrointestinal transit Inhibiting gastrointestinal transit and gastric emptying CKK, CKK1 receptor [165] Increasing normal jejunal contractility [13] Table 7.
Effects on the digestive system.
-
Activities Mechanism of action Signaling pathway Ref. The positive inotropic and chronotropic effects Vanilloid receptors, the calcitonin gene related peptide antagonist [166,168] Anti-hypertensive Attenuation of VSMC migration PPAR [170,171] Regulating endothelial to mesenchymal transition The transforming growth factor-beta1/Smd [173] Anti-platelet and anti-thrombotic activities Inhibiting HUVECs tube formation and invasion VEGF, p44/p42, MAPK, ERK [174] Inhibiting thromboxane formation and phosphoinositide breakdown [11,166] The vasodiatory effect Endothelium, receptor-mediated Ca2+ channels [176] Anti-arrhythmic Decreasing the Na+ and Ca2+ currents [177] Vascular disease Attenuate the phosphorylation of mitogen-activated protein kinase promote the reverse transport of cholesterol VSMCs
ABCA1[179]
[180]Protective effect against valvular heart disease Decrease the expression of phosphate cotransporter PiT-1 [181] Table 8.
Effects on the cardiovascular system.
-
Activities Mechanism of action Ref. Anti-Alzheimer's disease Beta-secretase inhibitor [182,183] Selectivity over AChE, strong neuroprotection [184] Inhibited tau phosphorylation on Ser202/Thr205, Ser262, and Ser396 and downregulated tau aggregation and neuronal cell apoptosis. [185] Anti-anoxic and
anti-cerebral ischemia activitiesEquivalent to vinpocetine [153] Upregulation of pGSK3b, pAkt, and claudin-5, and downregulation of NF-kB expression [187] Analgesic effect The activation and subsequent desensitization of TRPV1 [16] Antidepressant effect Monoamine emitters and hippocampal BDNF-TrkB signaling [189] Table 9.
Effect on the central nervous system.
Figures
(5)
Tables
(9)