-
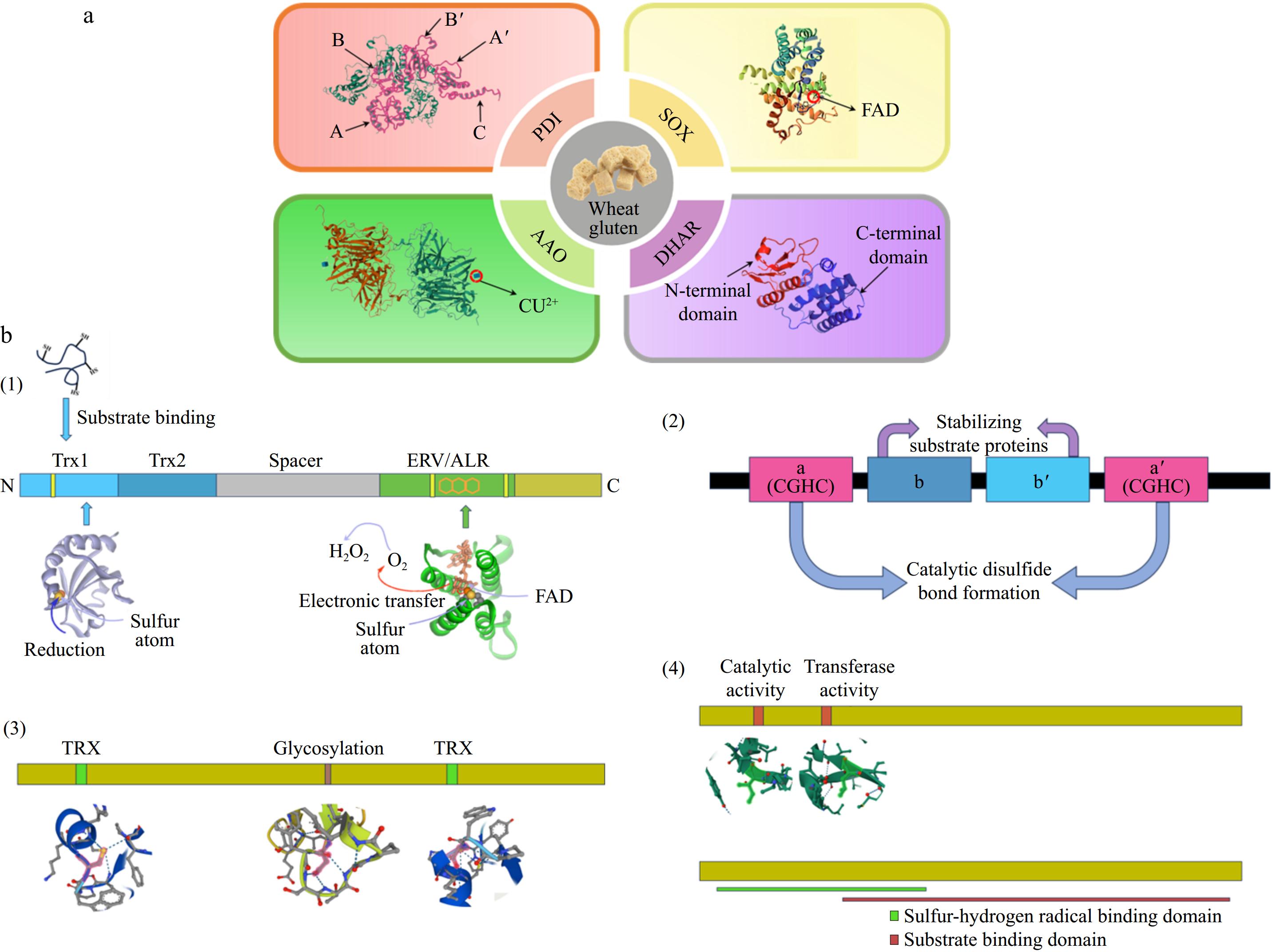
Figure 1.
(a) Structures of the four endogenous enzymes SOX (sulfhydryl oxidase), PDI (protein disulfide bond isomerase), AAO (ascorbate oxidase), and DHAR (dehydroascorbate reductase) that function in gluten. (b) Schematic structures of the four endogenous enzymes. (1) Homology models for the Trx1 and Erv/ALR structural domains were constructed based on the structure of QSOX using the crystal structures of the yeast PDI structural domain and yeast Erv2p, respectively. (2) Schematic structure of PDI, Catalytic thioredoxin-like domains (a and a') are colored pink, and non-catalytic domains (b and b') are blue. (3) Schematic structure of AAO, including glycosylation site and disulfide bonding region, respectively. (4) Schematic structure of DHAR, including the catalytic active site and the transferase active site, respectively.
-
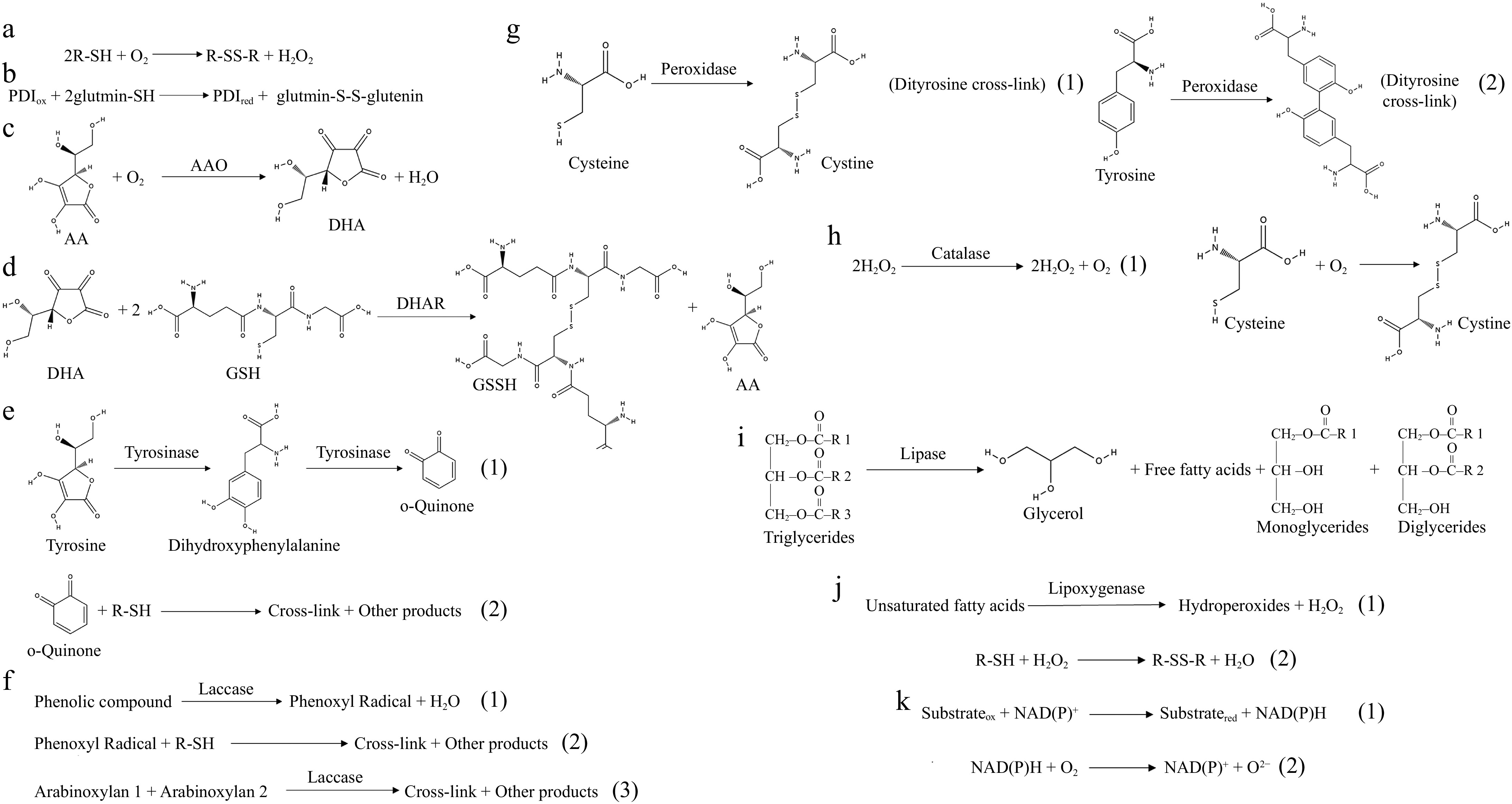
Figure 2.
Enzymatic catalysts in dough chemistry. (a) Mode of action of sulfhydryl oxidase. (b) Mode of action of disulfide bond isomerase. (c) Mode of action of ascorbate oxidase. (d) Mode of action of dehydroascorbate. (e) Mode of action of tyrosinase. (1) Tyrosinase catalyzes the production of dihydroxyphenylalanine from tyrosine to continue the production of o-quinone. (2) O-quinone can interact nonenzymatically with thiol groups and amino groups in proteins, resulting in covalent cross-linking. (f) Mode of action of laccase. (1) Phenolic compounds are catalyzed by laccase to generate phenoxy radicals. (2) Phenoxy radicals can interact with thiol groups to form SH radicals for SH/SS intercalation. (3) Laccase can cross-link arabinoxylan in whole flour to form distearic acid, resulting in a powerful network. (g) Mode of action of peroxidase. (1) Peroxidase catalyzes the formation of disulfide bonds from cysteine. (2) Peroxidase catalyzes the formation of a double tyrosine cross-link from tyrosine. (h) Mode of action of catalase. (1) Catalase catalyzes the breakdown of hydrogen peroxide into water and oxygen. (2) Molecular oxygen promotes the formation of disulfide bonds between cysteine residues. (i) Mode of action of lipase. Lipase catalyzes the hydrolysis of triglycerides into free fatty acids and glycerol, thereby modifying the lipid architecture of dough. (j) Mode of action of lipoxygenase. (1) Lipoxygenase catalyzes the oxidation of unsaturated fatty acids to produce hydrogen peroxide and Hydroperoxides. (2) The H2O2 produced promotes the formation of disulfide bonds between thiol groups in gluten. (k) Mode of action of NAD(P)-dependent dehydrogenases. (1) Electrons are transferred from the substrate to NAD(P)+ to produce NAD(P)H. (2) NAD(P)H can react with molecular oxygen to produce superoxide radicals.
-
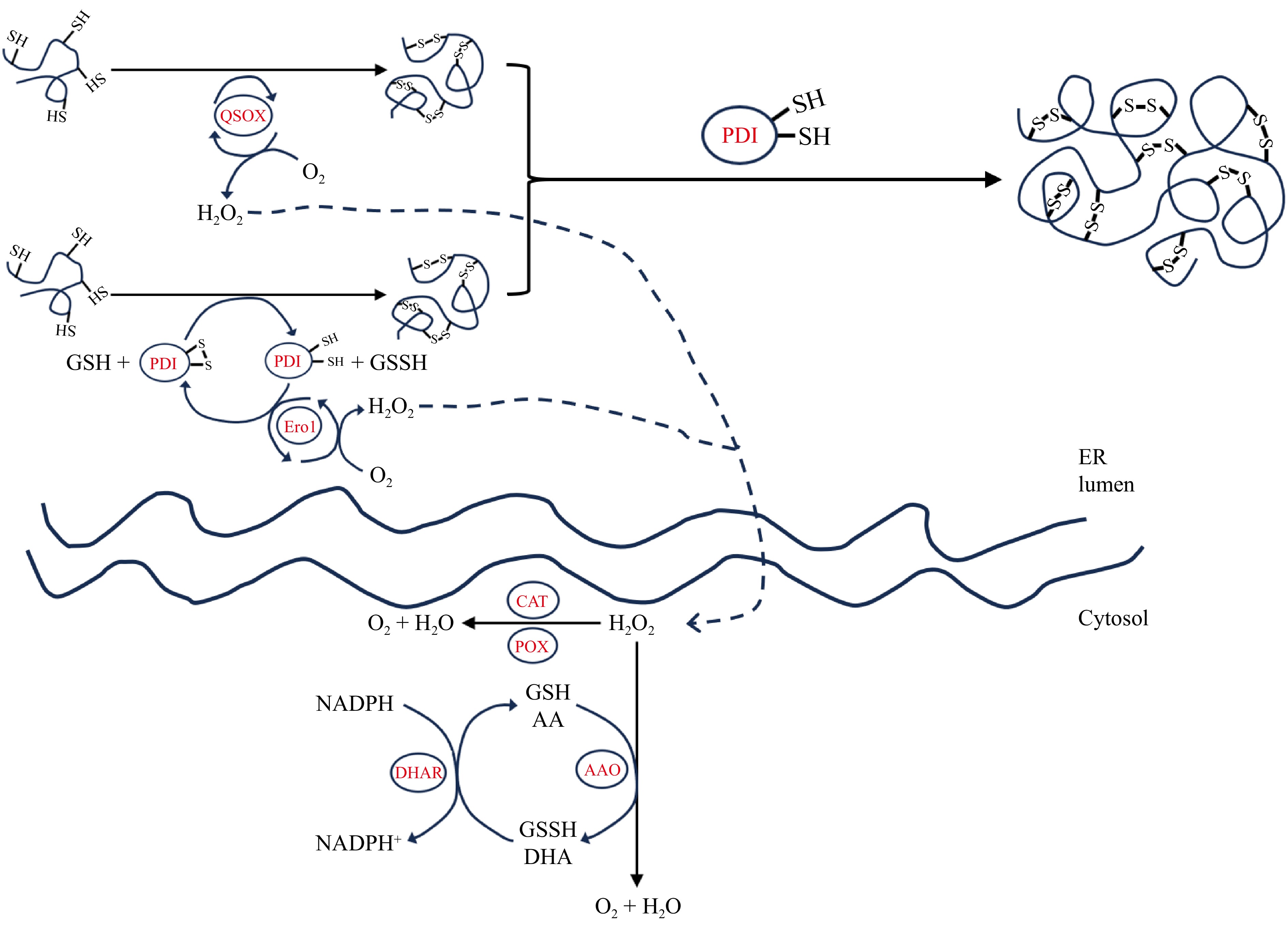
Figure 3.
Schematic representation of the reaction mechanisms of different endogenous enzymes in the endoplasmic reticulum and cytoplasm.
-
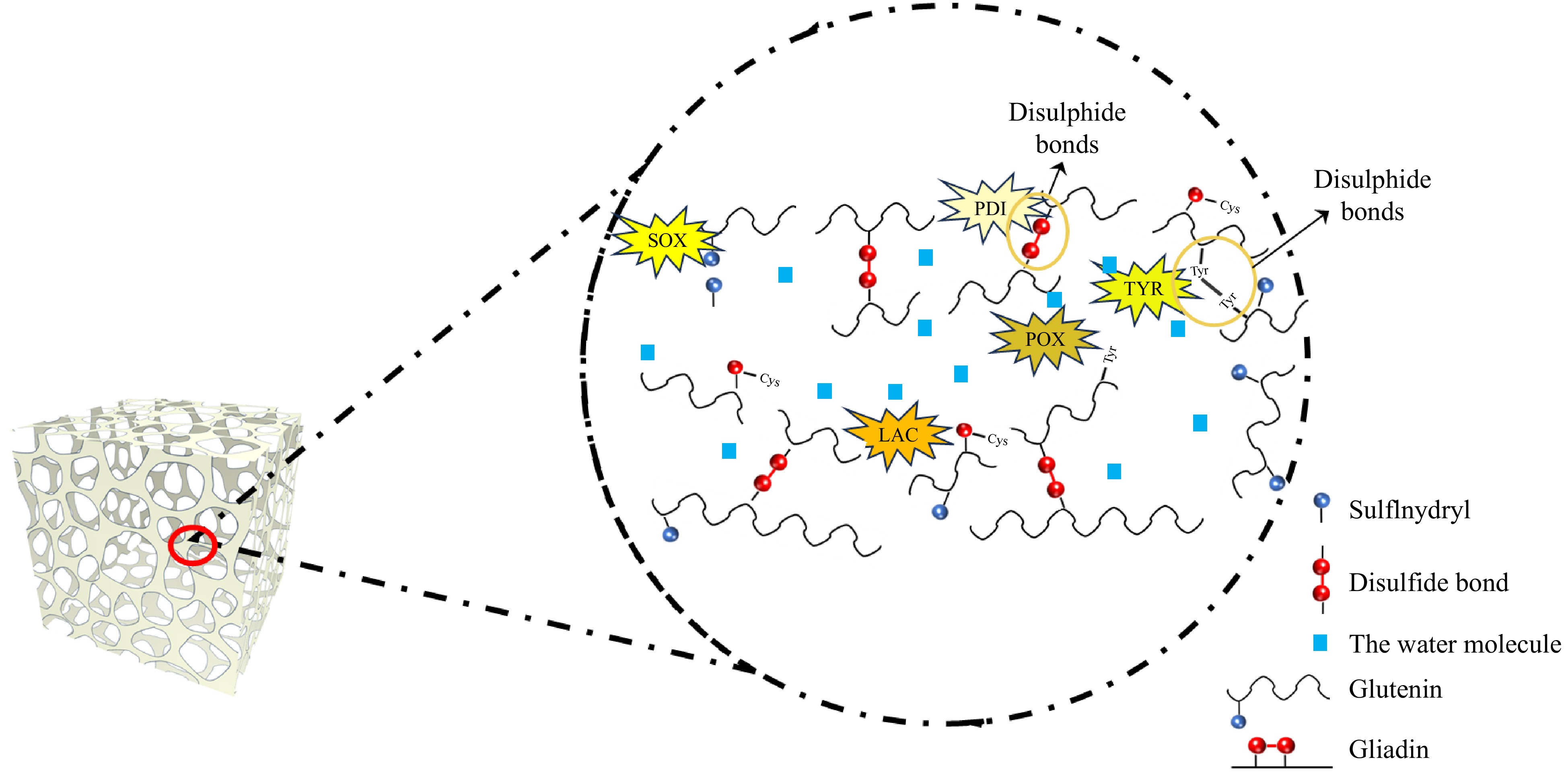
Figure 4.
Mechanisms of endogenous wheat enzymes in gluten cross-linking: reaction sites and bond types.
-

Figure 5.
Effect of endogenous oxidative enzymes on dough properties and bread quality.
-
Proteases (EC:) Mode of action/cross-linking mechanism Cofactor/metal dependence Substrate Reactive sites in gluten Application of enzymatic
cross-linkingRef. Sulfhydryl oxidase
EC:1.8.3.3Catalyzes the oxidation of a sulfhydryl group (-SH) to a disulfide bond (-S-S-) with the simultaneous generation of hydrogen peroxide. Usually contains FAD or metals such as iron or copper. Small molecule thiols such as glutathione, cysteine, or cysteine residues in proteins. Cysteine residue Promotes protein cross-linking. [12] Protein disulfide
isomerase EC:5.3.4.1Catalyzes the exchange reaction of sulfhydryl groups and disulfide bonds in proteins. − Disulfide bonds in proteins. Intermolecular disulfide bonds Affects dough quality. [13] Ascorbate oxidase
EC:1.10.3.3Oxidizes ascorbic acid, forming new disulfide bonds. Copper ion Ascorbic acid. − Improve dough properties. [14] Dehydroascorbic acid reductase
EC:1.8.5.1Reduction of dehydroascorbic acid. Formation of new disulfide bonds NAD(P)H Dehydroascorbic acid. − Reduction of oxidative stress. [15] Tyrosinase
EC:1.14.18.1Catalyzes hydroxylation and oxidation reactions of phenolic compounds. Two copper atoms. Tyrosine and L-dopa. Tyrosine residues. Catalyze protein cross-linking reactions. [16,17] Laccase
EC:1.10.3.2Oxidizes phenolic compounds to produce reactive phenol radicals. Four copper atoms. Phenolic compounds and ferulic acid etc. Tyrosine and cysteine residues Catalyze protein cross-linking reactions. [17] Lipoxygenase
EC:1.13.11.12Oxidized fats and oils produce hydroperoxides. − Fats and oils with a pentadiene 1,4 double bond. − Enhances gluten strength. [18,19] Lipase
EC:3.1.3.3Hydrolysis of fatty acid ester bonds. − Lipids. − Catalyze protein cross-linking reactions. [20,21] Peroxidase
EC:1.11.1.7Catalyzes the decomposition of hydrogen peroxide and enhances gluten strength. Iron-containing hemoglobin protein. Hydrogen peroxide. Tyrosine Catalyze protein cross-linking reactions. [22] Catalase
EC:1.11.1.6Decompose hydrogen peroxide. − Hydrogen peroxide. − Baking, dough strengthening. [23] NAD(P)H-dependent dehydrogenase Oxidized substrates that initiate free radical chain reactions between gluten. Dependent on NAD(P)(H). Includes alcohols, aldehydes, alpha-hydroxy, and beta-hydroxy carboxylic acids. − Participation in redox reactions. [5,24] Table 1.
Summary of various endogenous oxidative enzymes acting in gluten.
Figures
(5)
Tables
(1)