-
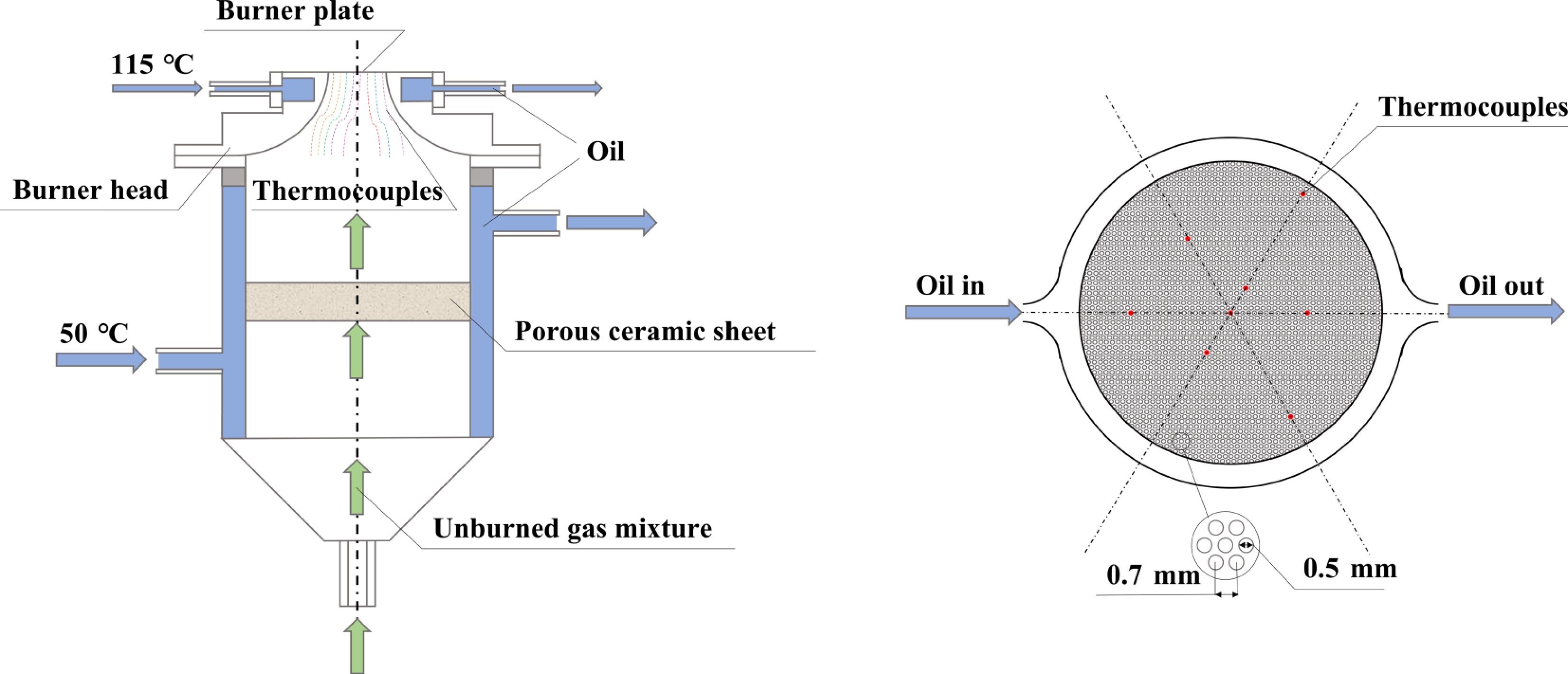
Figure 1.
Heat flux burner configuration and thermocouple arrangement on the disk.
-
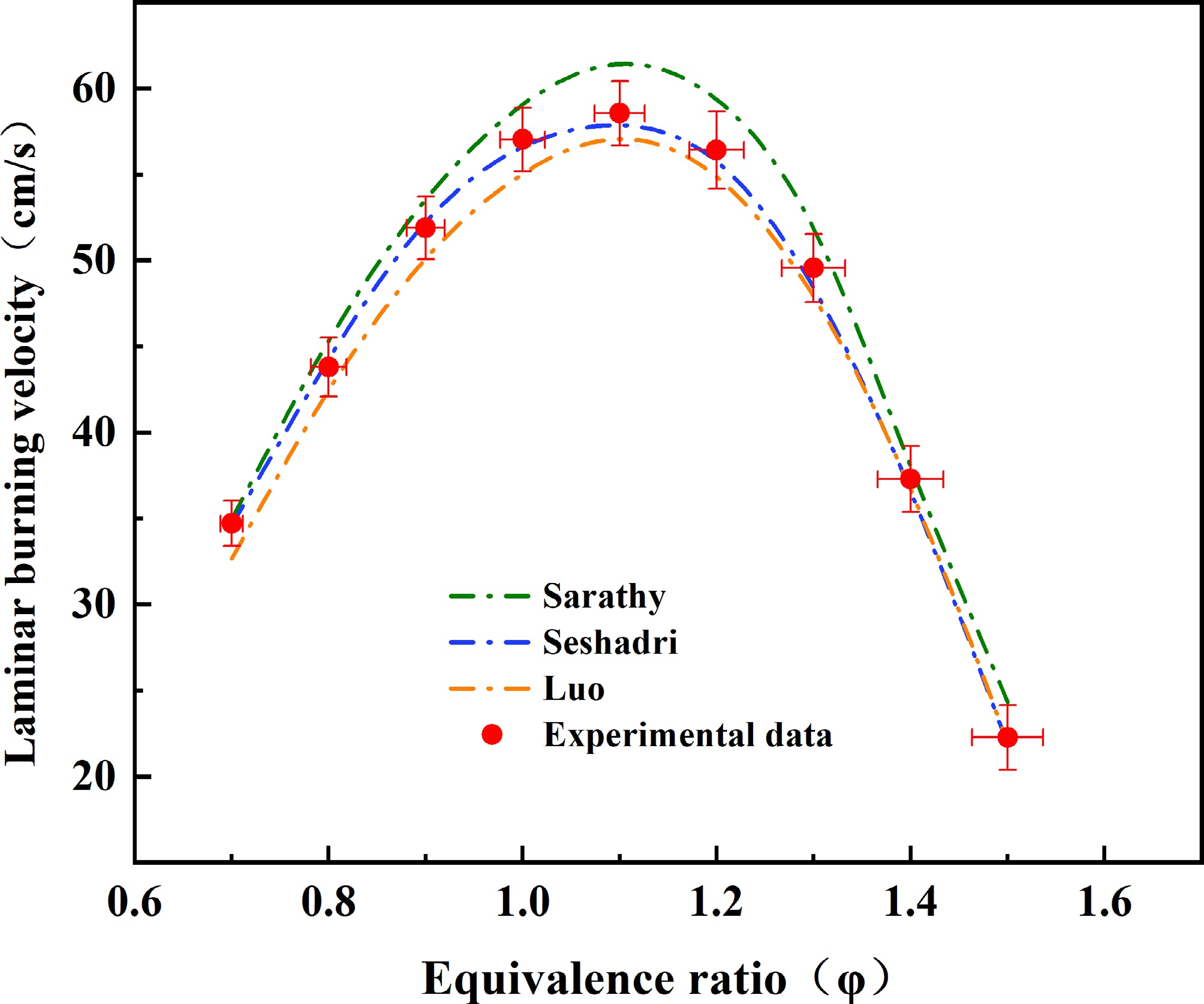
Figure 2.
Comparison of laminar burning velocity (LBV) data for methyl decanoate (MD)-air mixtures at 373 K and 1 atm. Data from Sarathy et al.[49], Seshadri et al.[50], and Luo et al.[51] were obtained using combustion kinetic mechanism models via Chemkin II simulation calculations. Error bars indicate the uncertainties in equivalence ratio and laminar burning velocity within this study.
-
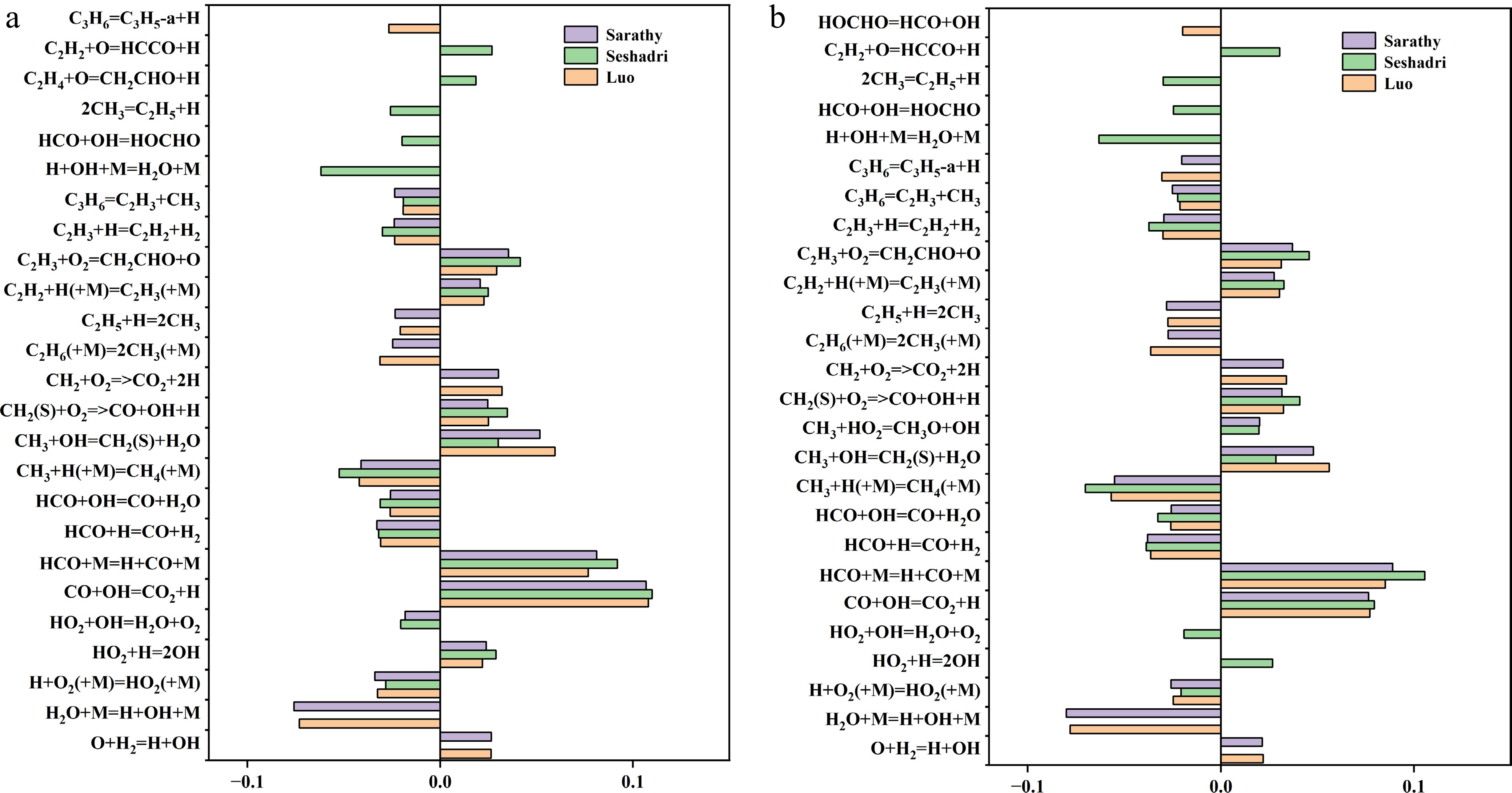
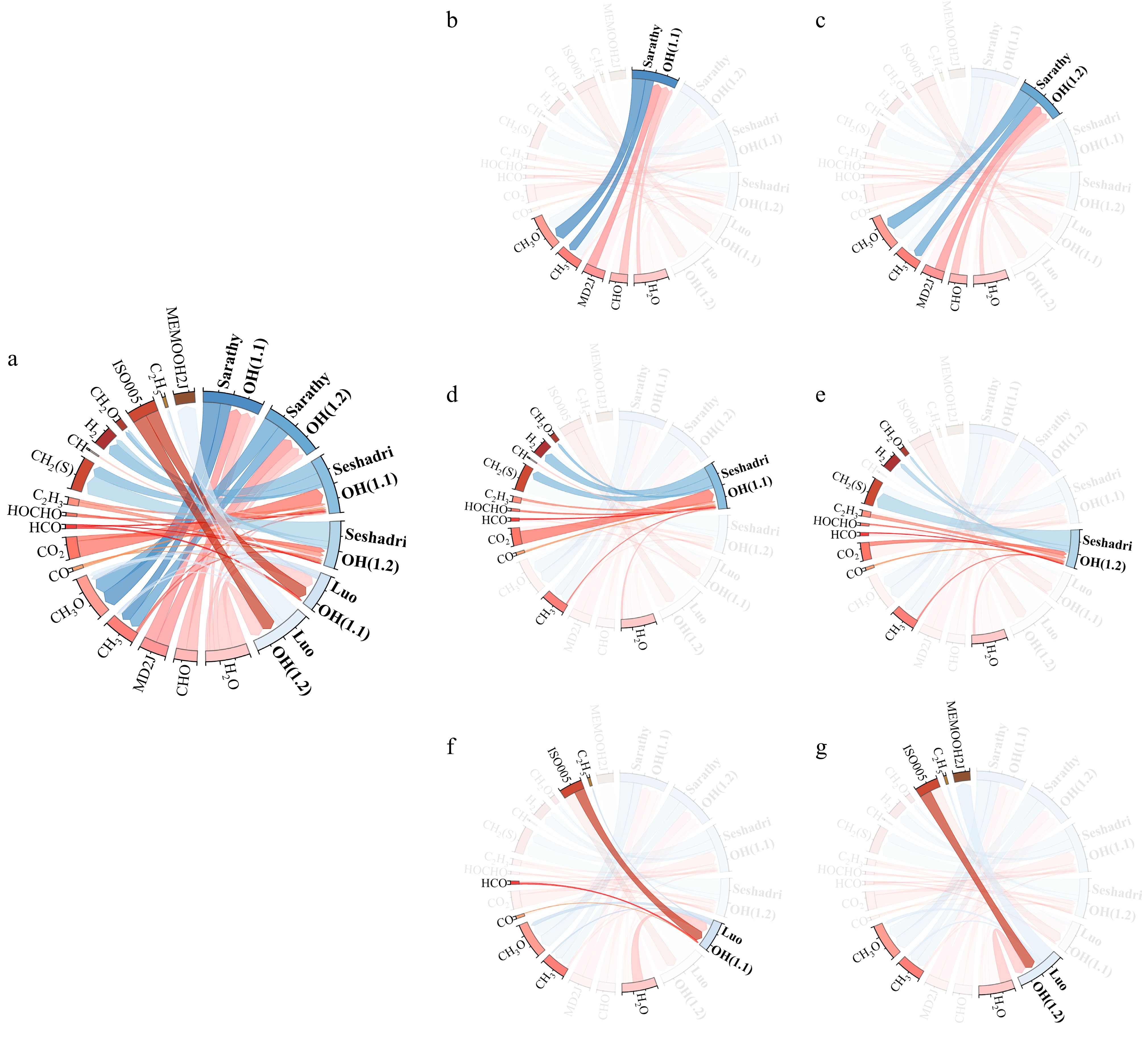
Figure 3.
Sensitivity analysis of laminar burning velocity (LBV) for MD-air premixed combustion at 373 K and equivalence ratio (a) Φ = 1.1, and (b) Φ = 1.2. Top 20 most sensitive reactions (with the reaction: H + O2 = O + OH is omitted) identified in the Sarathy et al.[49], Seshadri et al.[50], and Luo et al.[51] combustion mechanisms.
-
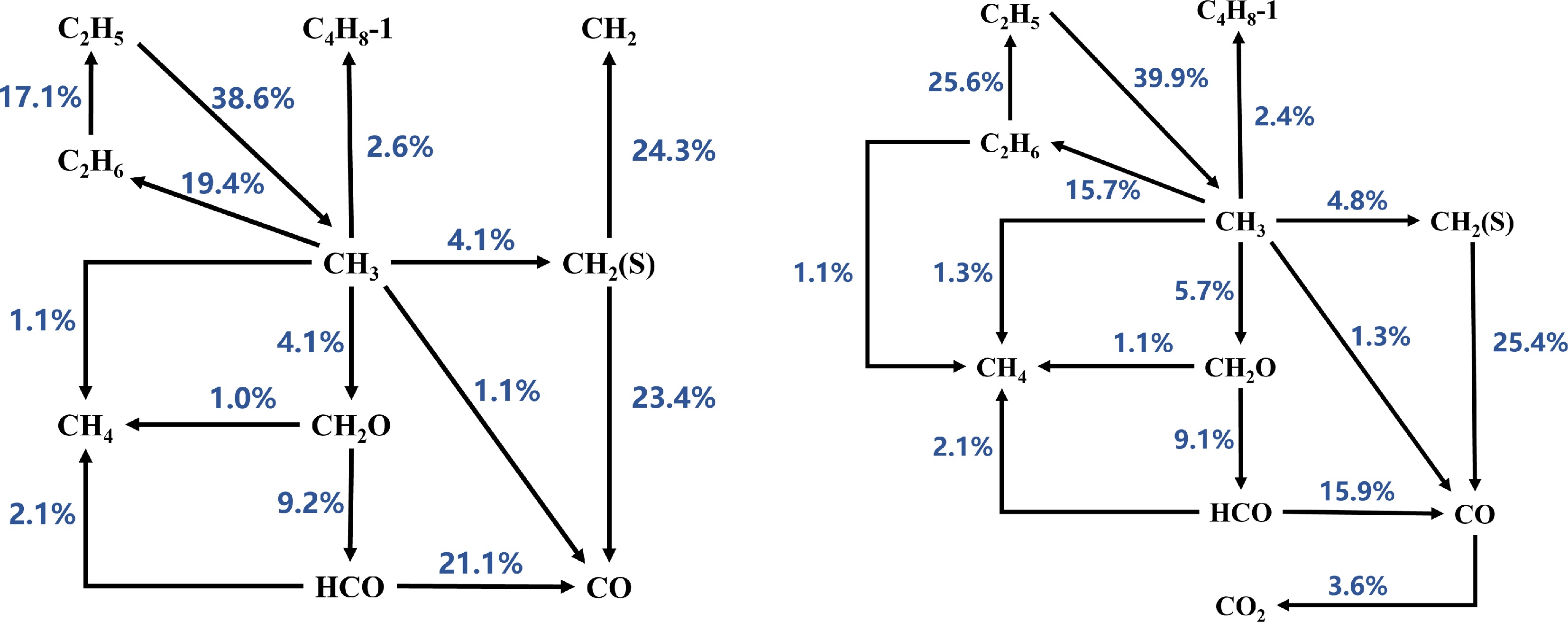
Figure 4.
Reaction path analysis based on laminar burning velocity (LBV) for MD-air combustion at 373 K using the Sarathy et al.[49] combustion mechanism, comparing equivalence ratios (left) Φ = 1.1 and (right) Φ = 1.2.
-
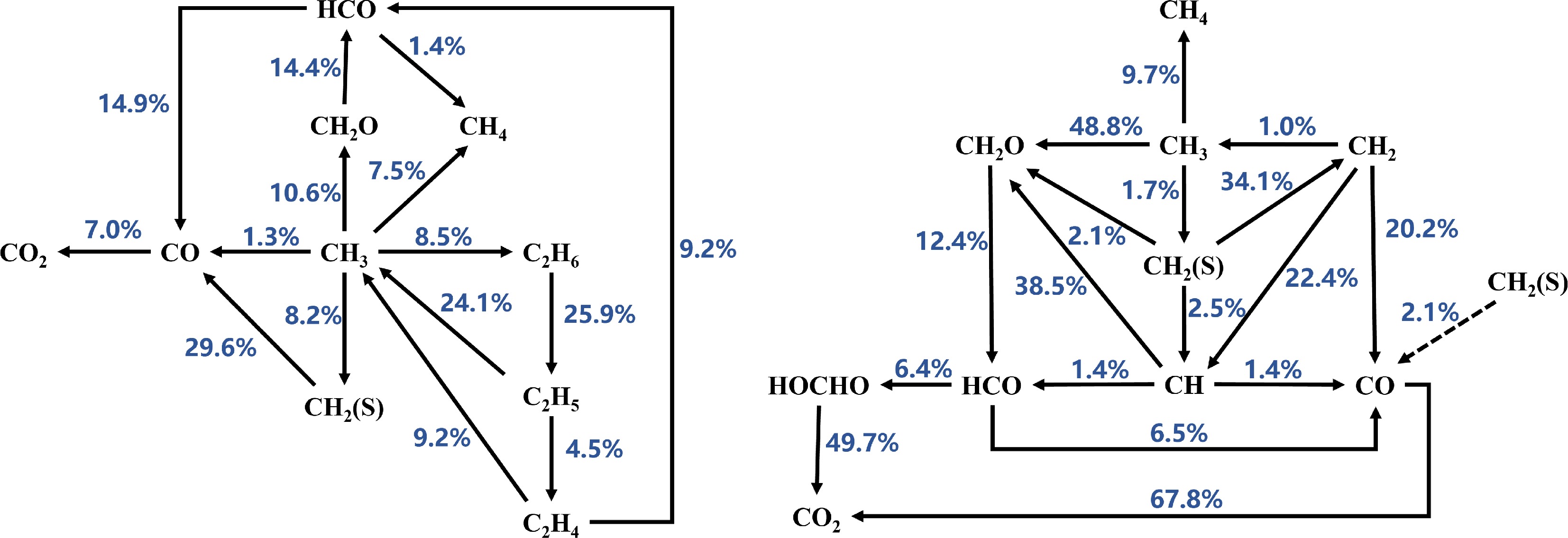
Figure 5.
Reaction path analysis based on laminar burning velocity (LBV) for MD-air combustion at 373 K using the Seshadri et al.[50] combustion mechanism, comparing equivalence ratios (left) Φ = 1.1 and (right) Φ = 1.2.
-
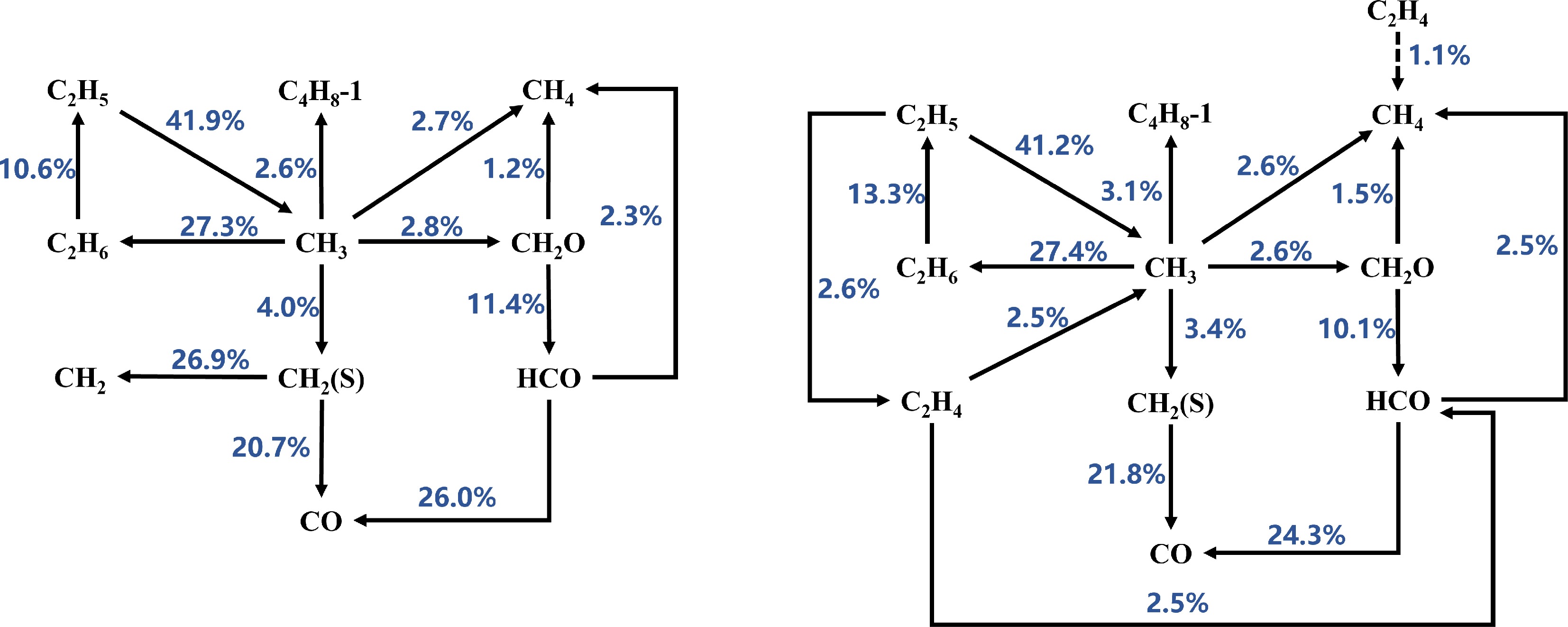
Figure 6.
Reaction path analysis based on laminar burning velocity (LBV) for MD-air combustion at 373 K using the Luo et al.[51] combustion mechanism, comparing equivalence ratios (left) Φ = 1.1 and (right) Φ = 1.2.
-
Figures
(7)
Tables
(0)