-
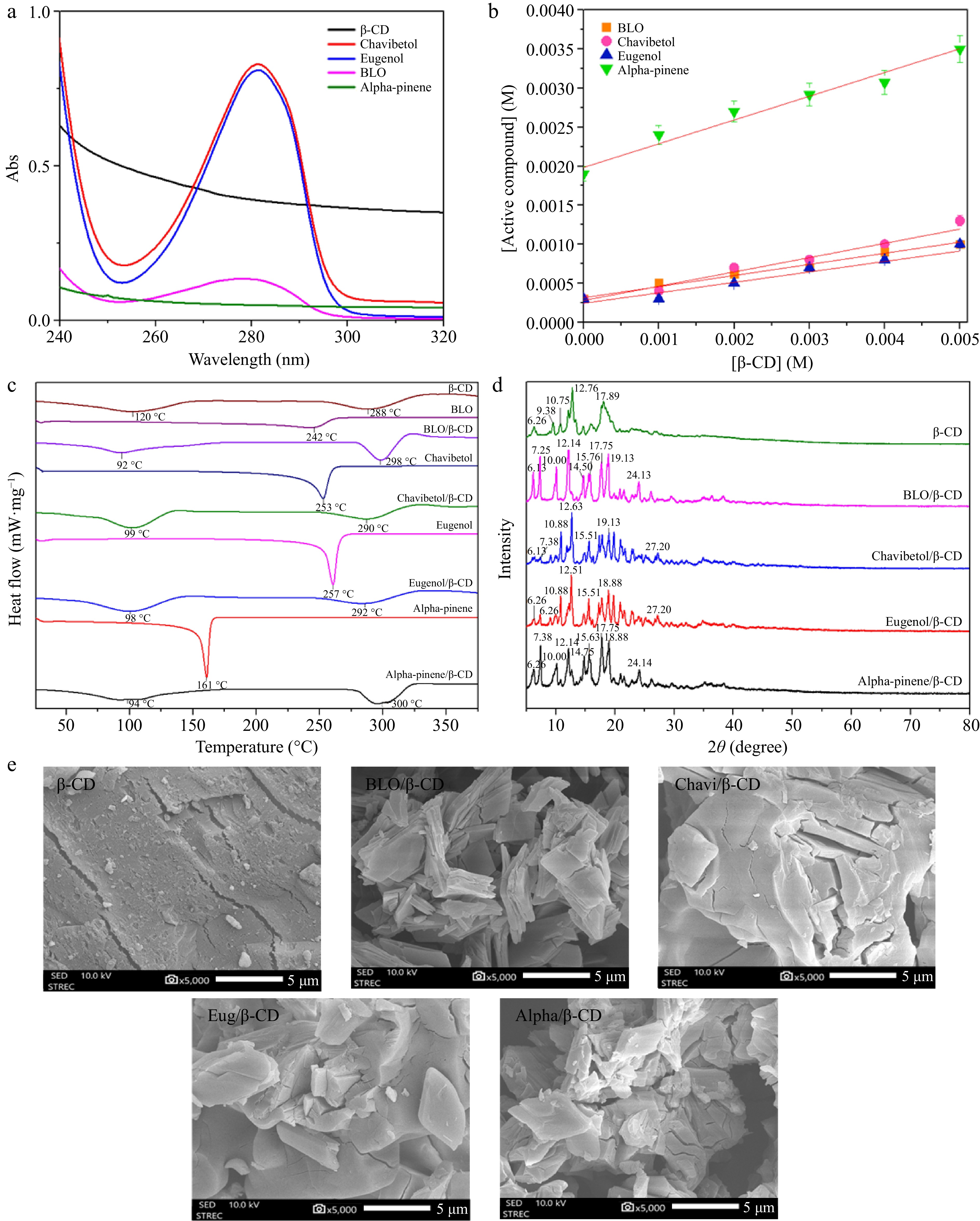
Figure 1.
(a) UV absorption spectrum of β-CD, chavibetol, eugenol, BLO, and alpha-pinene. (b) Phase solubility of β-CD with BLO, chavibetol, eugenol, and alpha-pinene. (c) DSC thermograms for β-CD, BLO, BLO/β-CD, chavibetol, chavibetol/β-CD, eugenol, eugenol/β-CD, alpha-pinene, and alpha-pinene/β-CD. (d) XRD patterns of β-CD, BLO/β-CD, chavibetol/β-CD, eugenol/β-CD, and alpha-pinene/β-CD. (e) SEM images of β-CD, BLO/β-CD, chavibetol/β-CD, eugenol/β-CD, and alpha-pinene/β-CD.
-
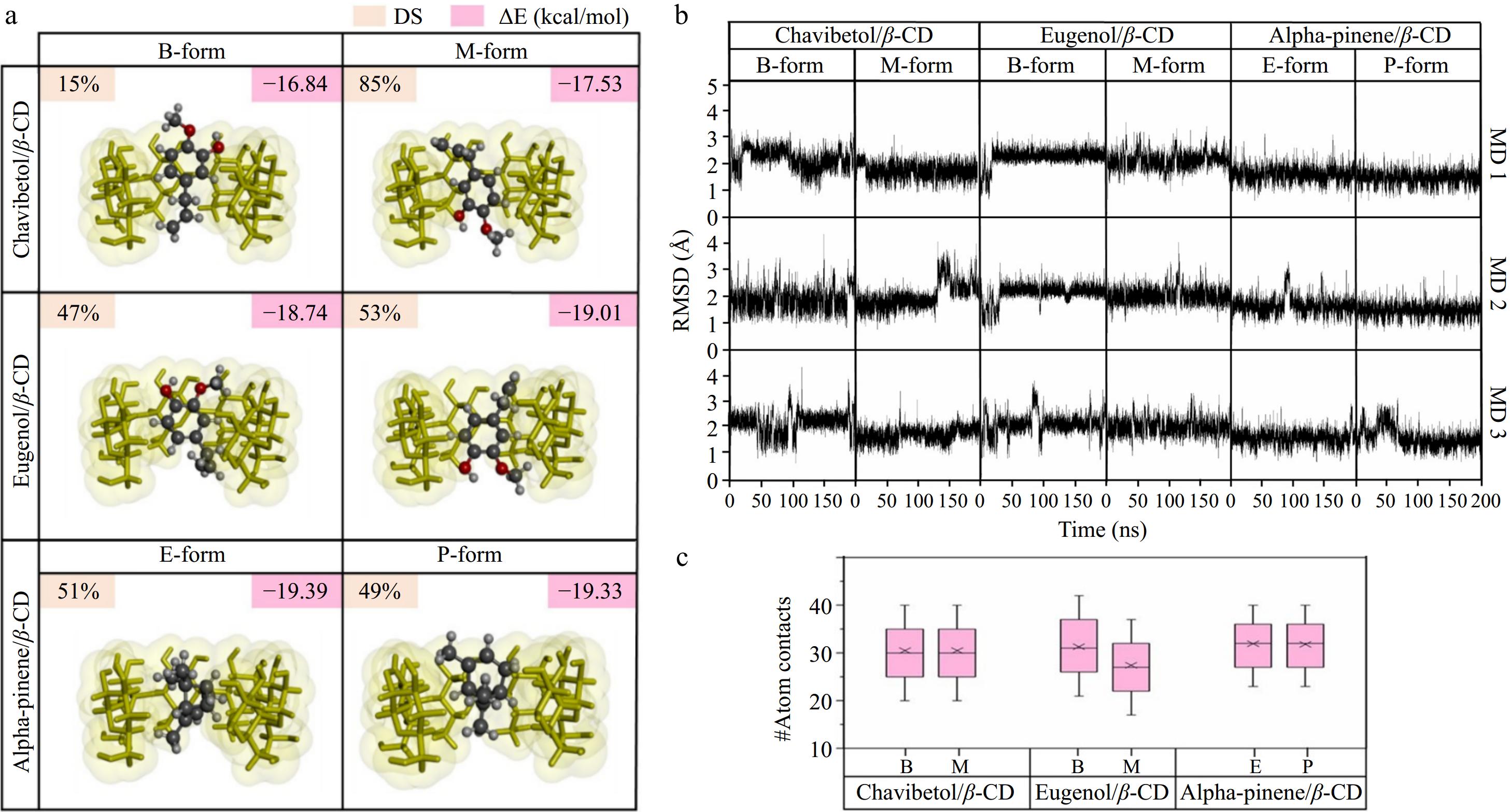
Figure 2.
(a) Percentage of docked conformations (%DCs) and CDOCKER interaction energy (ΔE) of all docked complexes. (b) RMSD profiles for all forms of the chavibetol/β-CD, eugenol/β-CD, and alpha-pinene/β-CD complexes plotted over 200 ns across three independent replicates (MD1–MD3). (c) Number of atomic interactions calculated over the last 100 ns for all ICs.
-
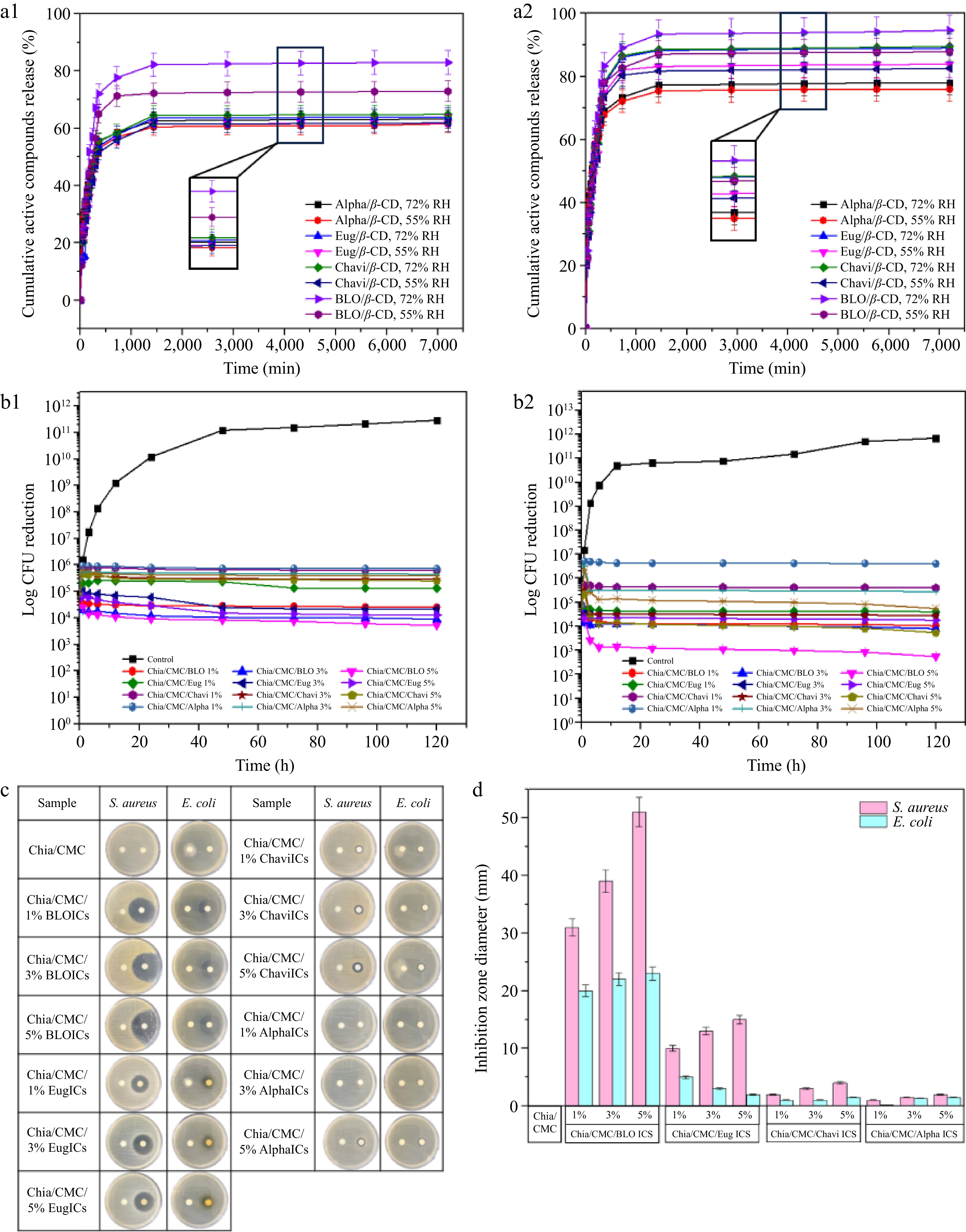
Figure 3.
(a) Cumulative release of active compounds (%) from ICs for 3 d at various temperatures and pH conditions. (a1) 25 °C (55% RH and 72% RH), and (a2) 40 °C (55% RH and 72% RH). The results are expressed as the mean ± SD, n = 3. (b) Bacterial reduction of (b1) S. aureus, and (b2) E. coli. The results are expressed as the mean ± SD, n = 3. (c) Clear zone after 24 h. (d) Inhibition zone diameters of S. aureus and E. coli.
-
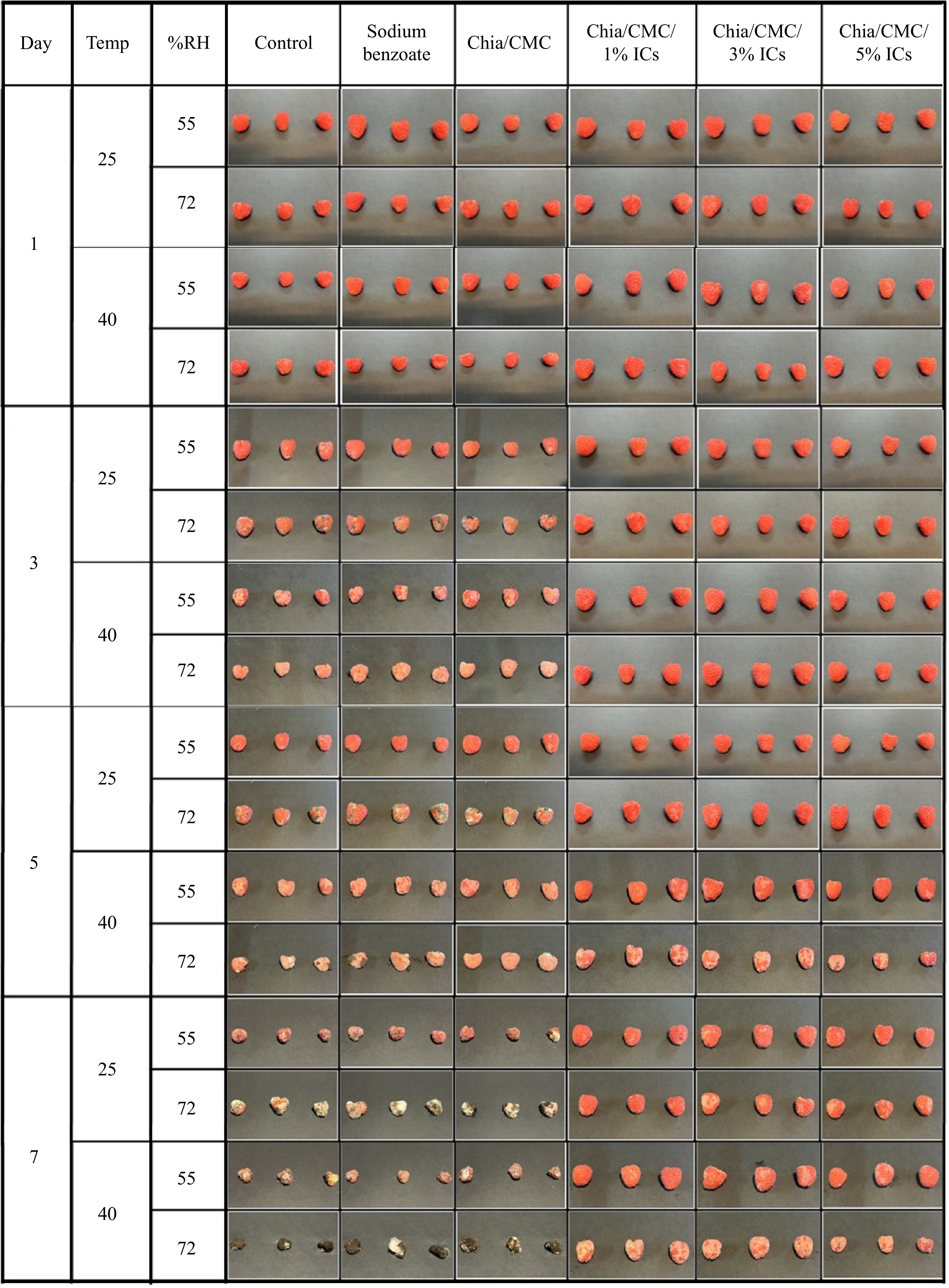
Figure 4.
The time-dependent appearance of raspberry, as observed over a 7-day period.
-
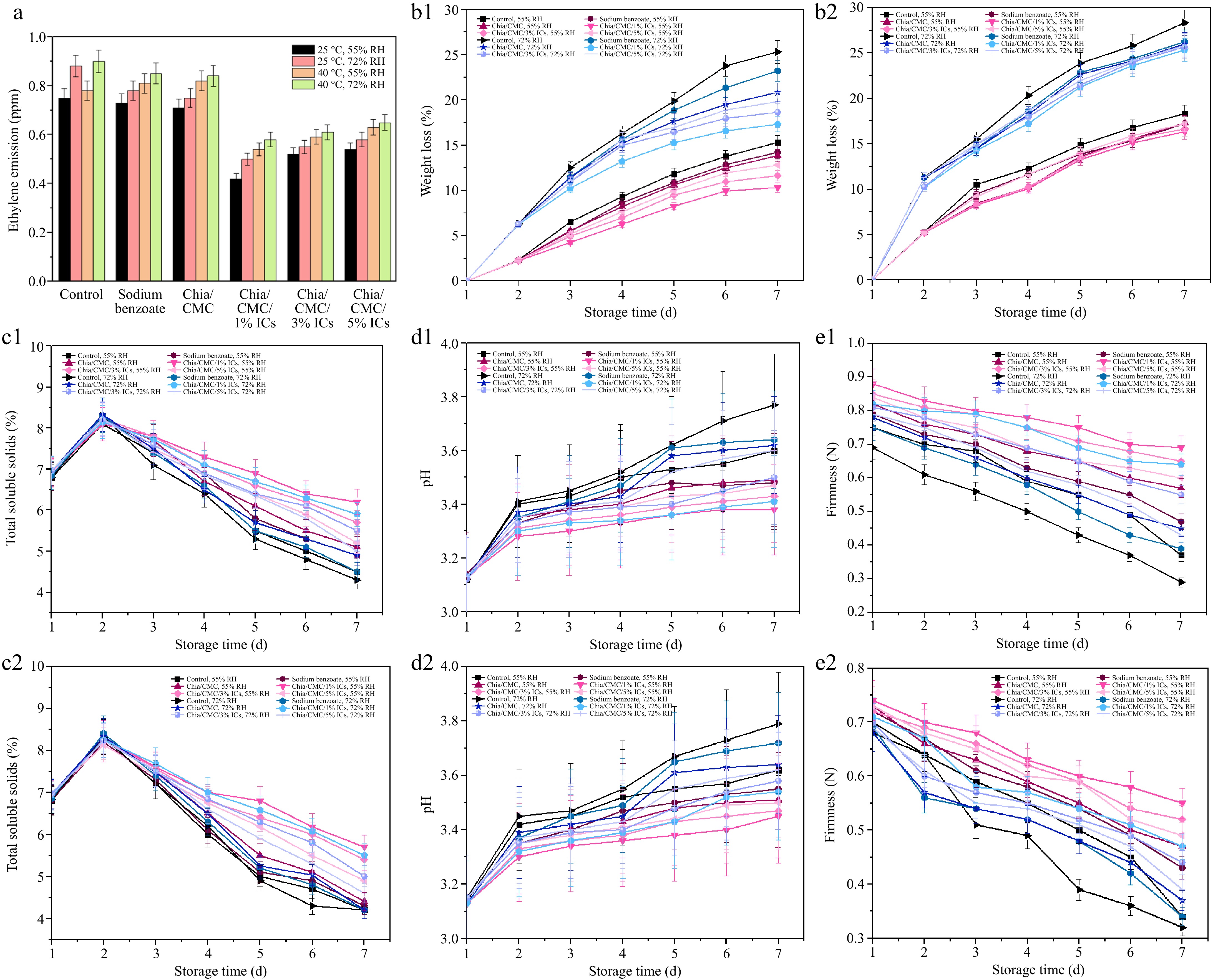
Figure 5.
Effect of different coating materials on raspberry. (a) Ethylene emitted after 7 d. (b1) Weight loss at 25 °C and (b2) Weight loss at 40 °C. (c1) TSS at 25 °C and (c2) TSS at 40 °C. (d1) pH at 25 °C and (d2) pH at 40 °C. (e1) Firmness at 25 °C and (e2) Firmness at 40 °C.
-
Peak no. Retention
time (min)Compositions Relative contents (%) BLO BLO/β-CD ICs 1 7.01 3-Thujene 1.294 0.664 2 7.19 Alpha-pinene 17.977 38.018 3 7.61 Camphene 2.554 0.001 4 8.37 Sabinen 1.959 0.001 5 8.45 Beta-pinene 0.109 0.001 6 8.94 Beta-myrcene 0.785 0.000 7 9.48 3-Carene 1.006 0.007 8 9.68 Alpha-terpinen 1.426 0.001 9 9.92 beta-cymene 1.508 0.004 10 10.05 Beta-phellandrene 1.685 0.001 11 10.11 Eucalyptol 2.317 0.003 12 10.68 Trans-beta-ocimene 2.467 0.004 13 10.98 Gamma-terpinene 1.785 0.001 14 11.88 Alpha-terpinolene 1.227 0.001 15 12.24 Linalool 0.674 0.002 16 14.51 Terpinen-4-ol 2.274 0.001 17 17.65 Safrole 0.748 0.037 18 19.02 Delta-elemene 0.870 0.021 19 19.34 Alpha-cubebene 1.118 0.064 20 19.55 Eugenol 6.559 10.849 21 19.91 Chavibetol 31.528 49.793 22 20.04 Copaene 5.700 0.049 23 20.45 (−)-Cis-beta-elemene 0.783 0.008 24 21.07 Cis-alpha-bergamotene 1.676 0.419 25 22.01 Humulene 3.685 0.000 26 23.15 Alpha-muurolene 1.118 0.001 27 23.34 Beta-bisabolene 0.913 0.001 28 23.58 (−)-Alpha-panasinsen 0.855 0.018 29 23.90 (E)-Gamma-bisabolene 1.924 0.018 30 26.73 Tau-muurolol 1.476 0.012 The values in boldface indicate the main chemical composition of BLO and BLO/β-CD ICs. Table 1.
Chemical composition of BLO and BLO/β-CD ICs by GC-MS analysis.
-
Molar ratio
host/guest% DL % EE % Recovery BLO/β-CD Chavi/β-CD Eug/β-CD Alpha/β-CD BLO/β-CD Chavi/β-CD Eug/β-CD Alpha/β-CD BLO/β-CD Chavi/β-CD Eug/β-CD Alpha/β-CD 1:1 19.66 20.12 18.77 20.93 98.56 98.21 97.34 98.66 96.67 95.72 95.32 97.87 9:1 4.37 3.33 3.57 4.02 27.81 25.33 24.98 25.73 78.97 78.88 78.65 78.98 1:9 13.02 12.07 11.03 14.11 13.56 13.33 12.09 13.42 74.90 74.88 74.65 74.89 8:2 4.57 3.62 3.65 3.21 72.58 73.23 72.58 73.49 78.14 78.20 78.12 78.25 2:8 14.40 12.48 12.32 12.67 17.32 17.54 17.23 17.22 88.60 88.65 88.44 88.63 7:3 8.46 8.33 8.21 8.44 72.08 72.12 71.34 72.32 85.95 85.76 85.53 85.84 3:7 13.02 13.11 12.59 12.66 28.23 27.32 27.21 27.43 72.62 72.65 72.34 72.60 6:4 13.34 13.21 12.76 13.22 78.35 78.43 78.22 78.32 71.67 72.63 71.83 72.13 4:6 12.40 12.43 12.33 12.21 37.15 37.20 37.12 37.32 90.37 90.24 90.21 90.56 Table 2.
Properties of the ICs.
-
ICs Proton δ (ppm) Δδ (ppm) β−CD pure ICs BLO/β−CD H-1 4.813 4.813 0.000 H-2 3.298 3.298 0.000 H-3 3.656 3.653 −0.003 H-4 3.338 3.338 0.000 H-5 3.537 3.535 −0.002 H-6 3.620 3.620 0.000 Chavibetol/β−CD H-1 4.813 4.813 0.000 H-2 3.298 3.298 0.000 H-3 3.656 3.651 −0.005 H-4 3.338 3.338 0.000 H-5 3.537 3.533 −0.004 H-6 3.620 3.620 0.000 Eugenol/β−CD H-1 4.813 4.813 0.000 H-2 3.298 3.299 +0.001 H-3 3.656 3.654 −0.002 H-4 3.338 3.338 0.000 H-5 3.537 3.536 −0.001 H-6 3.620 3.620 0.000 Alpha-pinene/β−CD H-1 4.813 4.813 0.000 H-2 3.298 3.299 +0.001 H-3 3.656 3.646 −0.010 H-4 3.338 3.339 +0.001 H-5 3.537 3.533 −0.004 H-6 3.620 3.620 0.000 Table 3.
Chemical shifts of protons (in ppm) corresponding to pure β-CD and ICs.
Figures
(5)
Tables
(3)