-
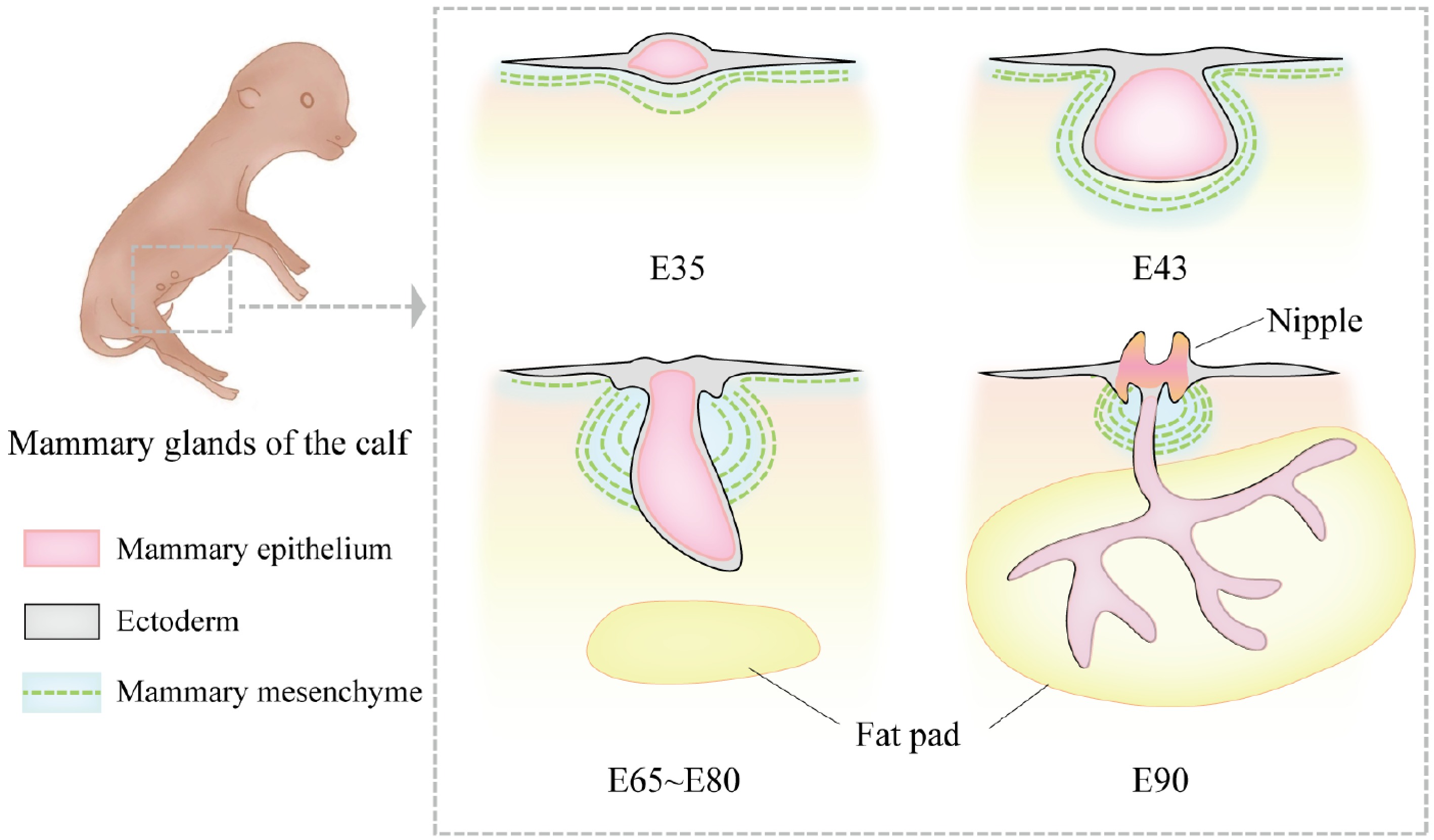
Figure 1.
Morphological changes in bovine mammary gland development during the embryonic period. At embryonic day 35 (E35), the bovine mammary gland is in the mammary line stage. The mammary line is a narrow ridge-like structure formed by the thickening of ectodermal cells. At this stage, the mammary structure has not yet developed into a distinct protrusion but appears as a linear hyperplasia. By E43, the mammary bud forms, and the ectoderm invaginates into the mammary mesenchyme, creating a depressed structure. At this point, morphological differences between female and male bovine mammary glands begin to emerge. The female mammary gland is smaller and oval-shaped, while the male mammary gland is larger and spherical. Between approximately E65 and E80, the primary sprout begins to develop. During this stage, the rapid proliferation of mesenchymal cells pushes the bud-like structure toward the epidermis, transforming it from a round shape into a more elongated form, marking the formation of the solid bud of the primary sprout. By E90, the primary sprout extends further in the dorsoventral direction and begins to branch, gradually invading the fat pad region and forming the initial ductal system.
-
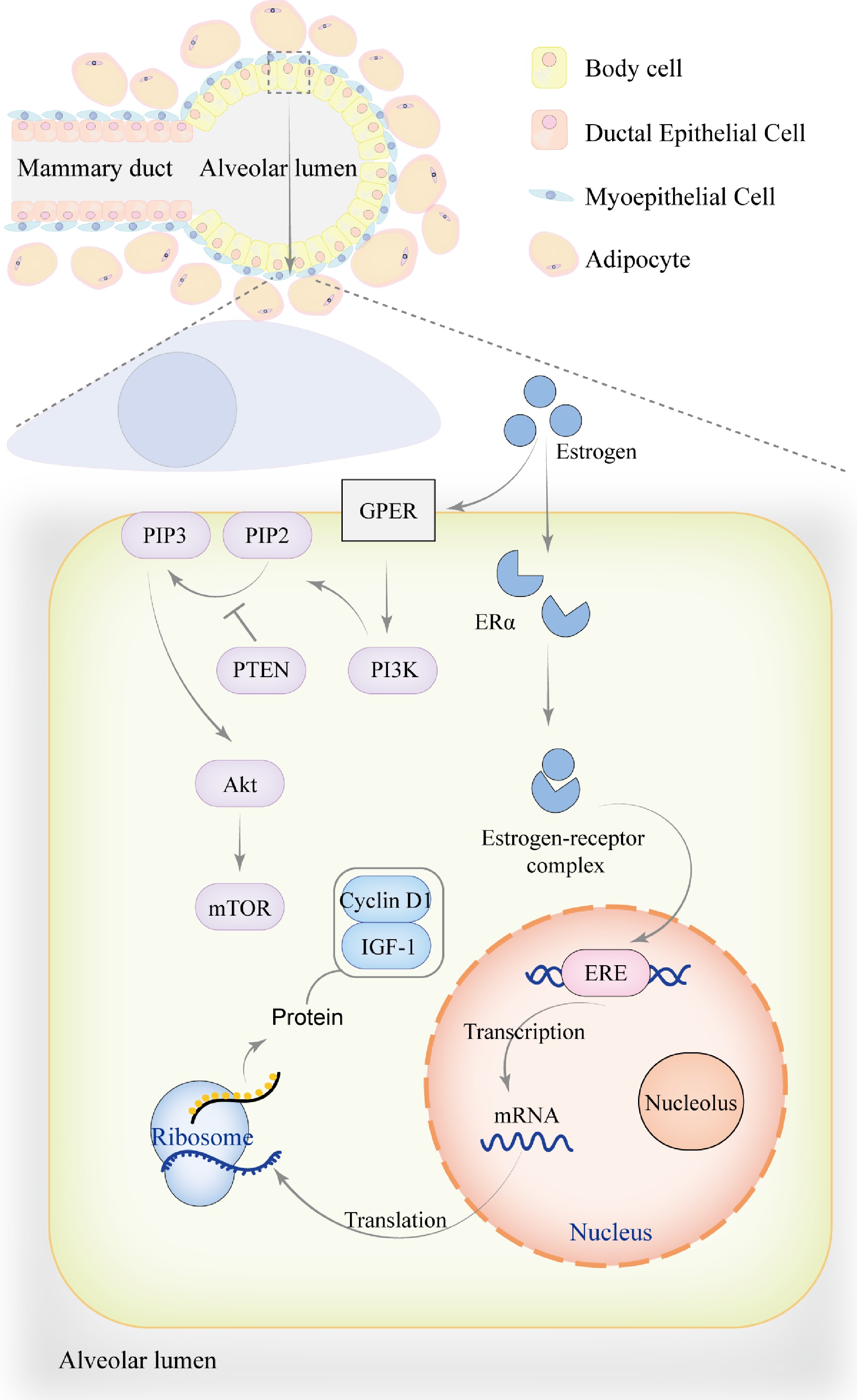
Figure 2.
The role of estrogen in mammary gland development. During the pre-pregnancy period, mammary ducts gradually branch and extend into the surrounding adipose tissue to support mammary gland development. The mammary alveoli undergo partial development but are not yet fully mature. Estrogen exerts its effects through genomic mechanisms, primarily by binding to ERα to form an estrogen-receptor complex, which translocates into the nucleus. Within the nucleus, the complex binds to estrogen response element (ERE) to regulate the transcription of genes associated with mammary gland development, including Cyclin D1 and IGF-1, thereby promoting mammary cell proliferation, metabolism, and growth. In addition to genomic effects, estrogen can also bind to the G protein-coupled estrogen receptor (GPER) on the cell membrane, activating PI3K to convert PIP2 into PIP3, which recruits and activates Akt. PTEN acts as a negative regulator of this process by inhibiting the conversion of PIP2 to PIP3. This non-genomic signaling pathway activates downstream targets such as mTOR through phosphorylation, regulating cell proliferation, survival, and metabolism. The genomic effects of estrogen may also directly or indirectly influence non-genomic signaling pathways by modulating signaling factors.
-
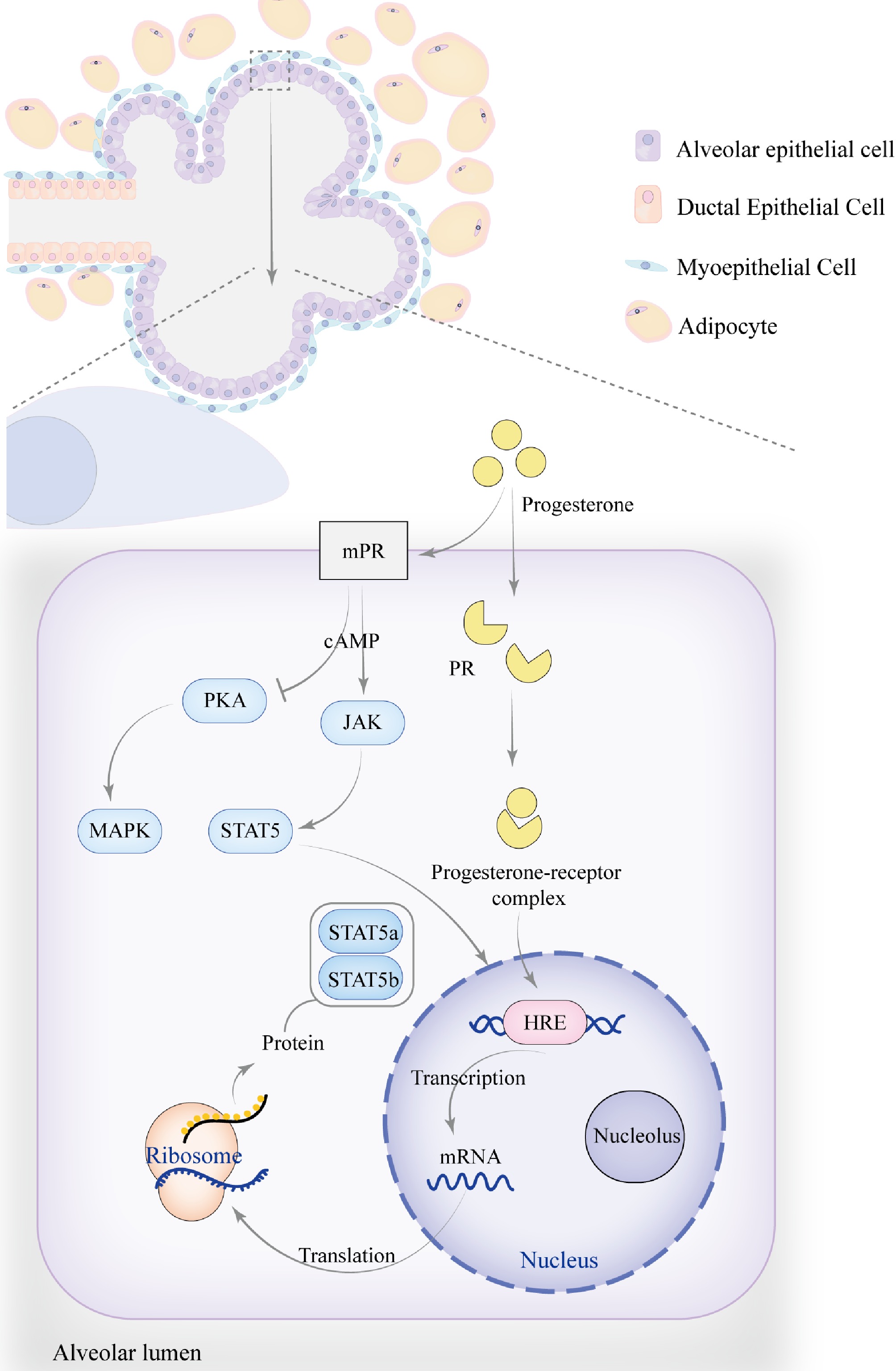
Figure 3.
The role of progesterone in mammary gland development. As pregnancy progresses, the number and size of alveoli increase, becoming more densely packed, while the ducts continue to proliferate, leading to an overall enlargement of the mammary gland. Progesterone regulates mammary gland development and function by modulating the JAK-STAT signaling pathway, particularly through membrane progesterone receptors (mPR) and progesterone receptors (PR), which influence STAT5a and STAT5b. The signaling pathway activated by mPR transduces signals via G-protein-coupled receptors (GPCRs), which may activate the cAMP secondary messenger. This indirectly activates JAK kinases, resulting in the phosphorylation of STAT5, causing its dimerization and translocation to the nucleus, where it binds to specific DNA sequences to regulate the transcription of target genes. Progesterone can also inhibit PKA activity through mPR, reducing cAMP levels while activating the MAPK signaling pathway. Additionally, progesterone regulates the JAK-STAT signaling pathway through PR, similar to estrogen. Upon binding with PR, progesterone activates the receptor and initiates gene transcription. By interacting with the hormone response element (HRE), progesterone regulates the expression of target genes such as STAT5a and STAT5b.
-
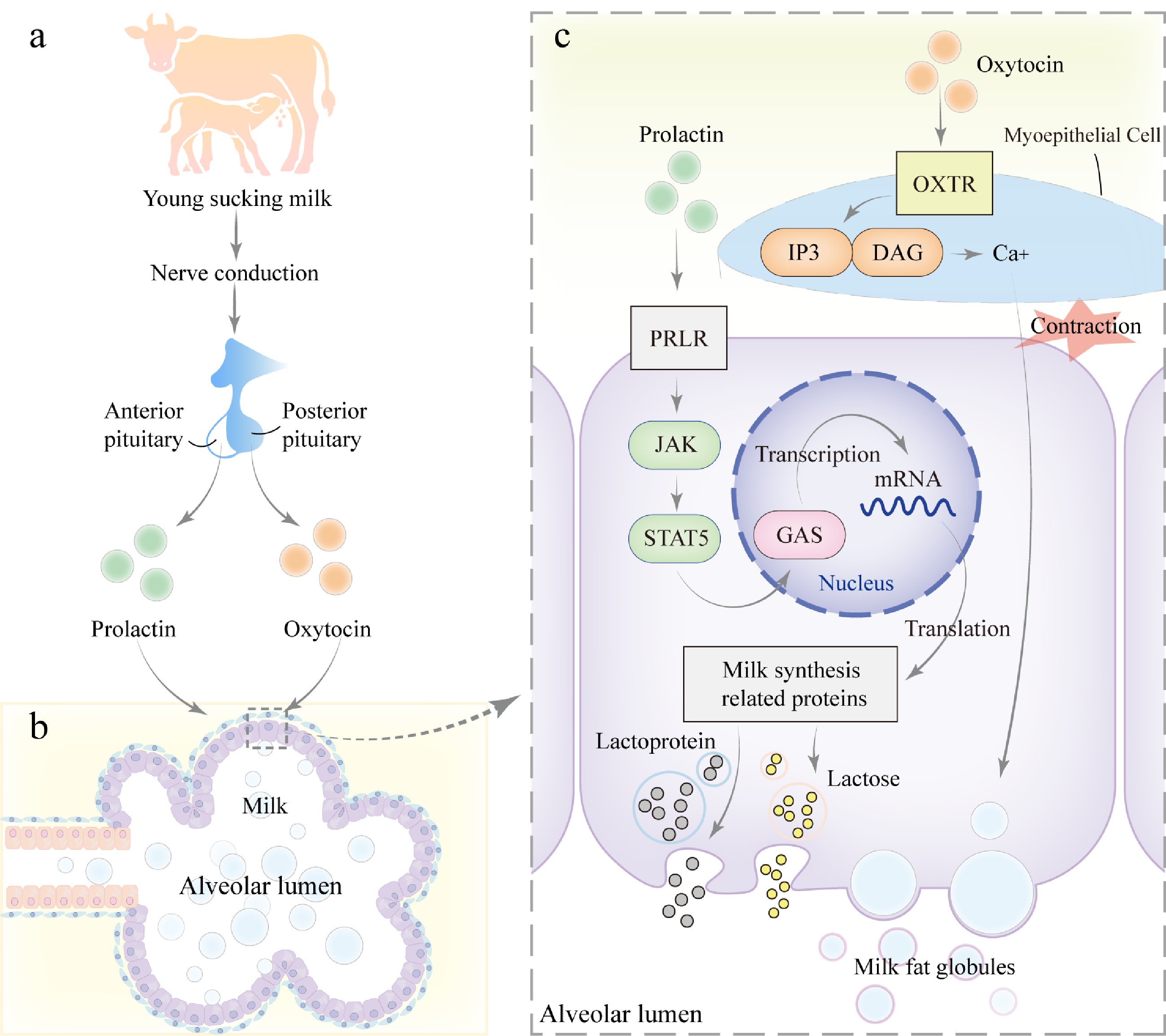
Figure 4.
Mechanism of milk secretion during lactation. (a) During lactation, when the young suckles the teat, mechanical stimulation is transmitted via neural signals to the hypothalamus of the mother, triggering the release of prolactin from the anterior pituitary gland and oxytocin from the posterior pituitary gland. (b) Prolactin and oxytocin act on the alveolar epithelial cells and myoepithelial cells of the mammary gland, respectively. (c) PRLR on mammary epithelial cells, activating the JAK-STAT signaling pathway. JAK kinases phosphorylate the receptor and downstream STAT5, leading to STAT5 dimerization and translocation into the nucleus, where it binds to GAS elements and regulates the transcription of genes related to milk synthesis, including those involved in lactose, milk proteins, and lipids, thereby promoting milk production. Additionally, oxytocin binds to its receptor (OXTR) on myoepithelial cells, activating the GPCR signaling pathway. This process activates Inositol Triphosphate (IP3) and diacylglycerol (DAG), leading to an increase in intracellular calcium concentration, which further promotes smooth muscle contraction and facilitates milk ejection.
-
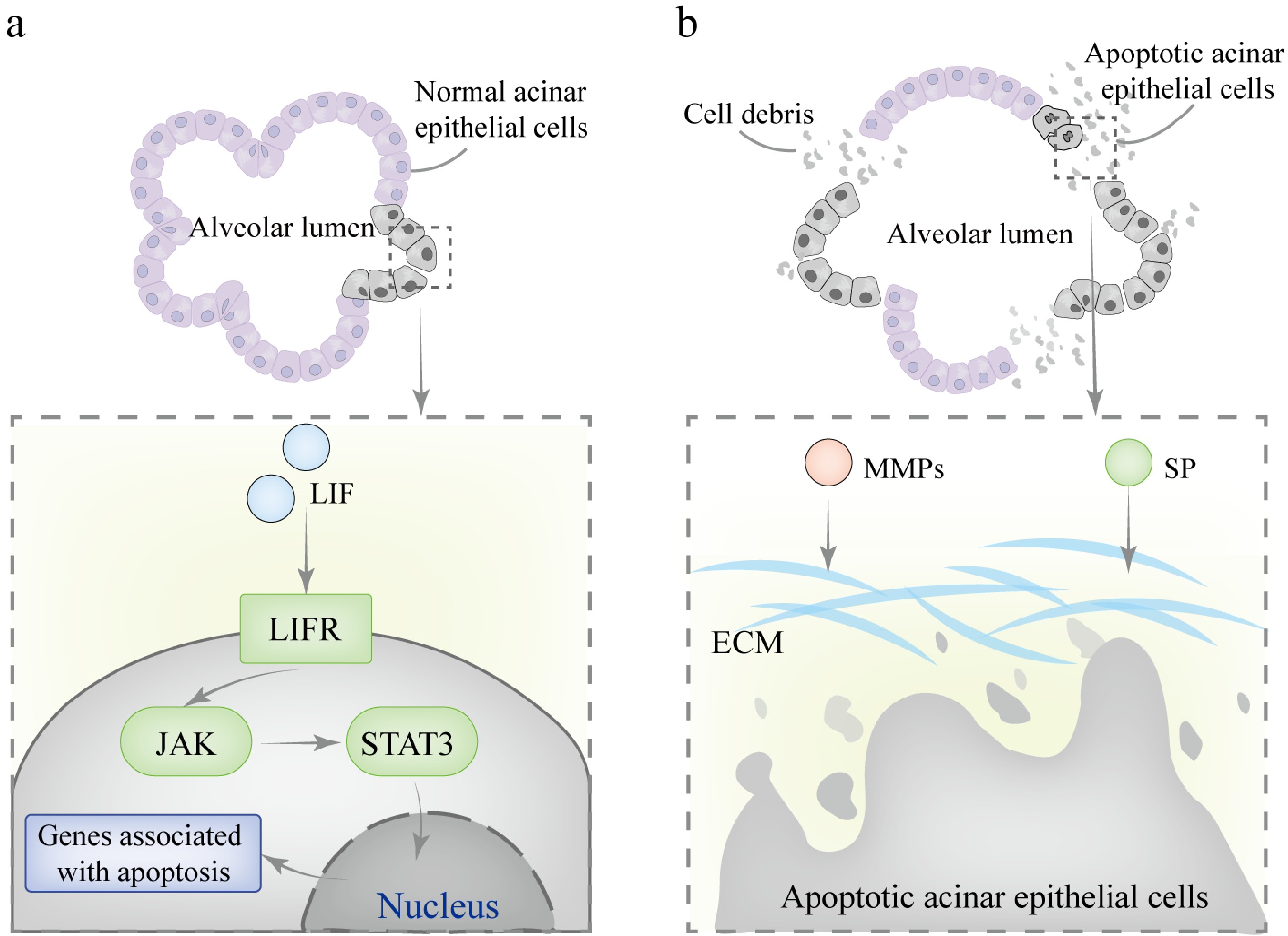
Figure 5.
Mechanisms involved in mammary gland involution during the dry period. (a) In the first phase, LIF binds to the LIFR on the cell membrane, activating JAK kinase, which mediates the phosphorylation of STAT3. The phosphorylated STAT3 forms homodimers and translocates to the nucleus, where it binds to specific DNA sequences in the promoter regions of target genes, initiating the transcription of pro-apoptotic genes. (b) In the second phase, SP and MMPs collaborate to degrade the basement membrane and ECM components. This process results in the irreversible collapse of alveolar structures.
-
Signaling pathway Gene/transcription factor Periods of possible impact Ref. Wnt Wnt3, Wnt6, Wnt10b, Wnt4, LEF1, LEF, β-catenin Embryonic, pubertal, and pregnancy stages [4,5] BMP BMP2, BMP4 Embryonic, pubertal, and pregnancy stages [6,7] PTHLH PTHrP, PTH1R Embryonic, pubertal, and pregnancy stages [8] Hedgehog SHH, PTCH1, GLI3 Embryonic, pubertal, and pregnancy stages [9,10] FGF FGF10, FGFR2b Embryonic, pubertal, and pregnancy stages [11,12] Notch NOTCH2, NOTCH4 Embryonic, pubertal, and pregnancy stages [13] JAK-STAT STAT5a, STAT5b, STAT6, STAT3, LIF Pregnancy, lactation, and involution stages [14,15] RANKL-RANK RANKL Pregnancy, lactation, and involution stages [16] PI3K-Akt PI3K, Akt1, mTOR, PIP2, PIP3, PTEN, Cyclin D1 Embryonic, pubertal, pregnancy, lactation, and involution stages [17] NF-κB NF-κB, IKK, RelA, p65, p50 Pubertal, pregnancy, lactation, and involution stages [18,19] TGF-β TGF-β1, TGF-βR, SMAD2, SMAD3, SMAD4 Embryonic, pubertal, pregnancy, and involution stages [20] MAPK RAS, RAF, MEK, ERK Pubertal, pregnancy, and lactation stages [21,22] Autophagy ATG5, Beclin1, LC3 Pubertal, pregnancy, lactation, and involution stages [23] Table 1.
Signaling pathways associated with mammary gland development/remodeling.
Figures
(5)
Tables
(1)