-
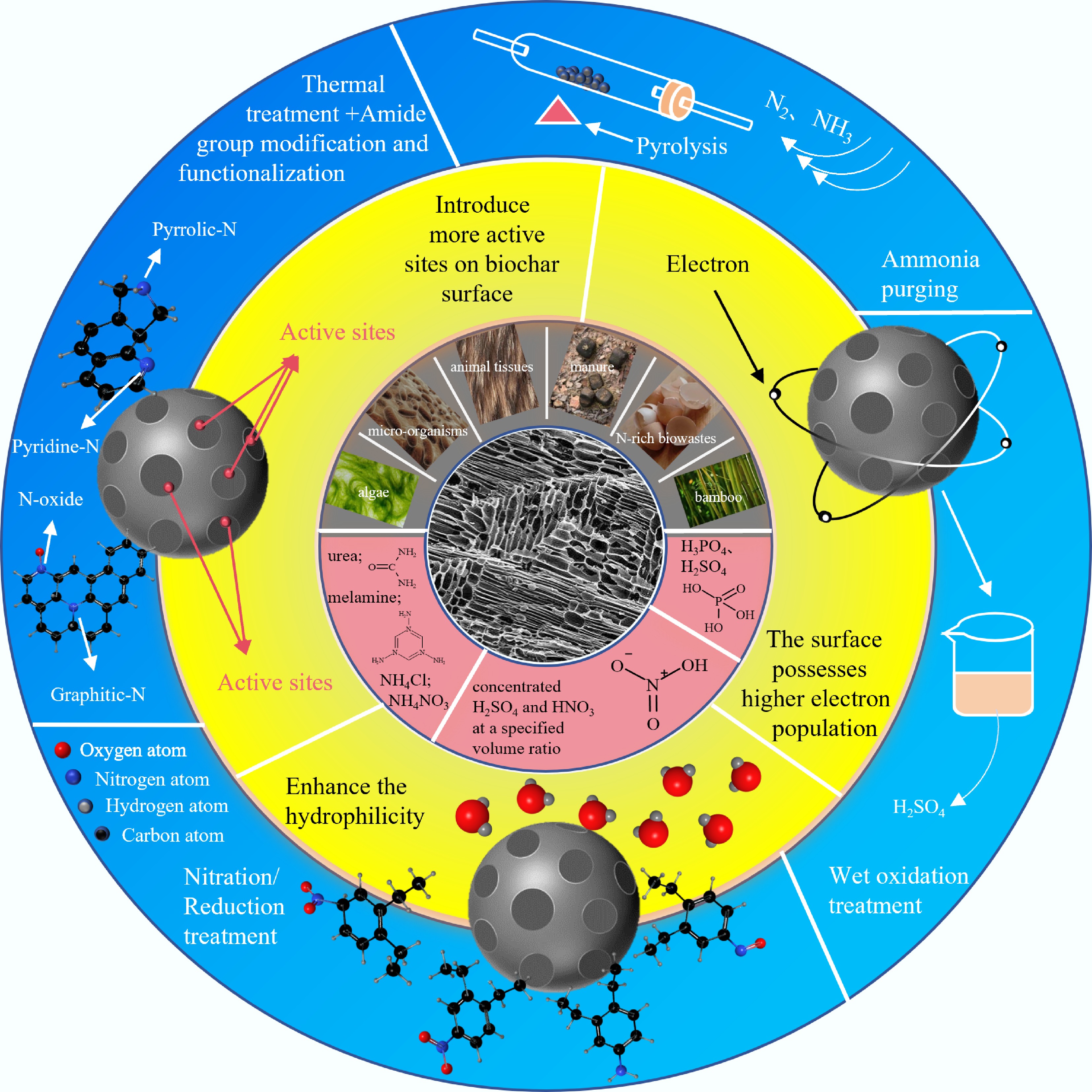
Figure 1.
Preparation of BC, synthesis technology, and performance regulation toward nitrogen-doped BC.
-
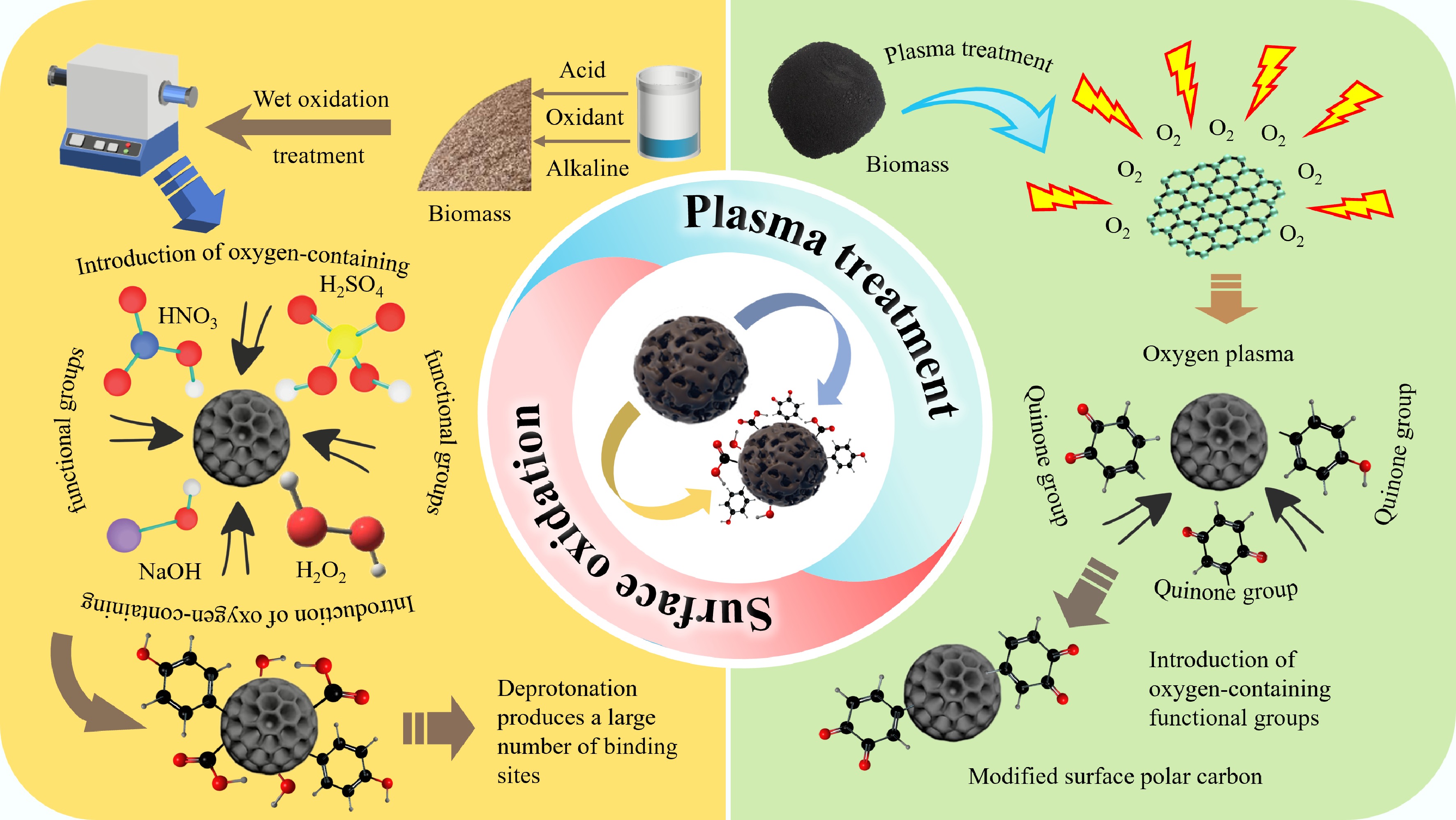
Figure 2.
The preparation methods of oxygen-doped BC and its according enhancement mechanism.
-
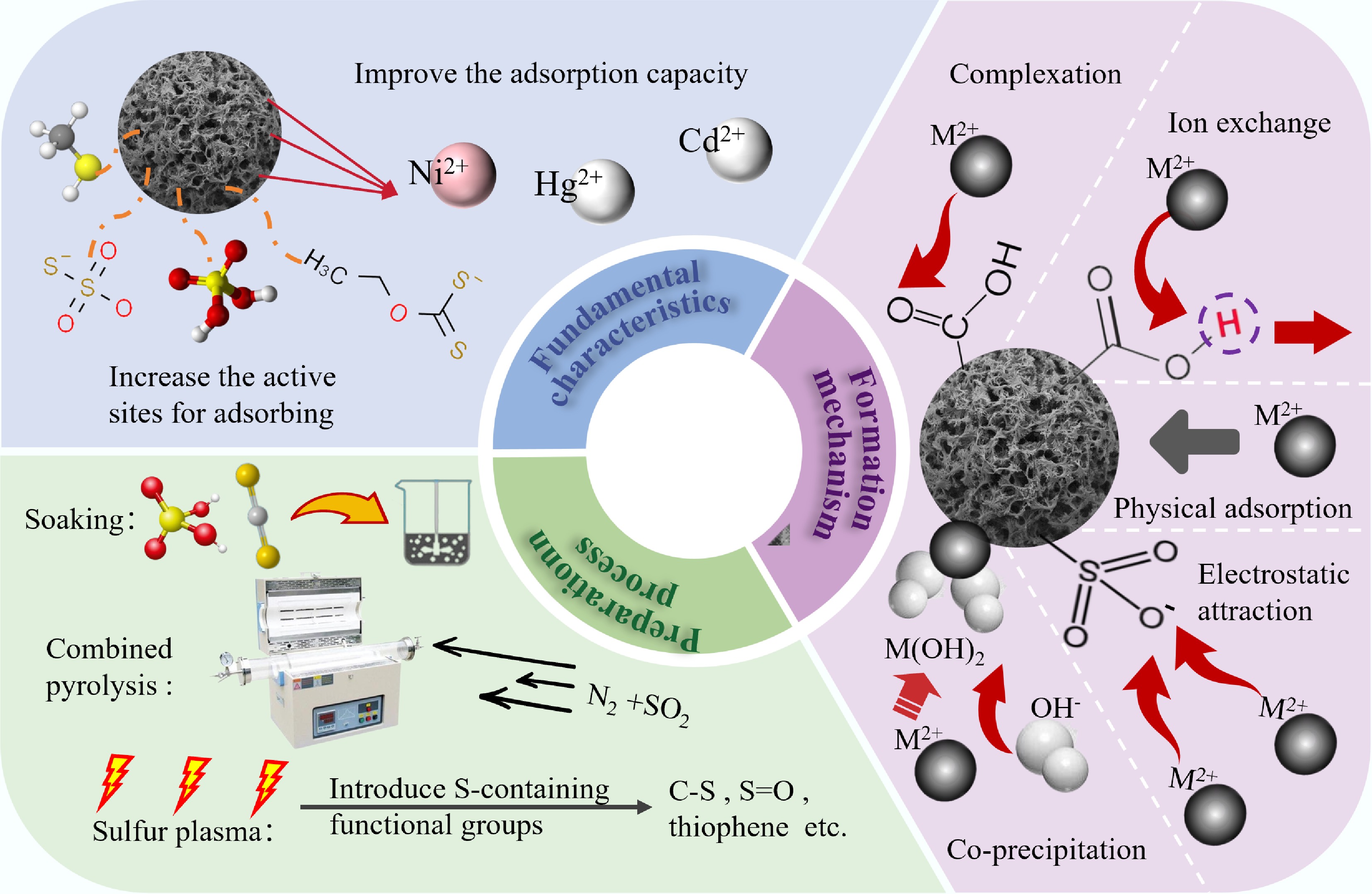
Figure 3.
The preparation methods of sulfur-doped BC and its according enhancement mechanism.
-
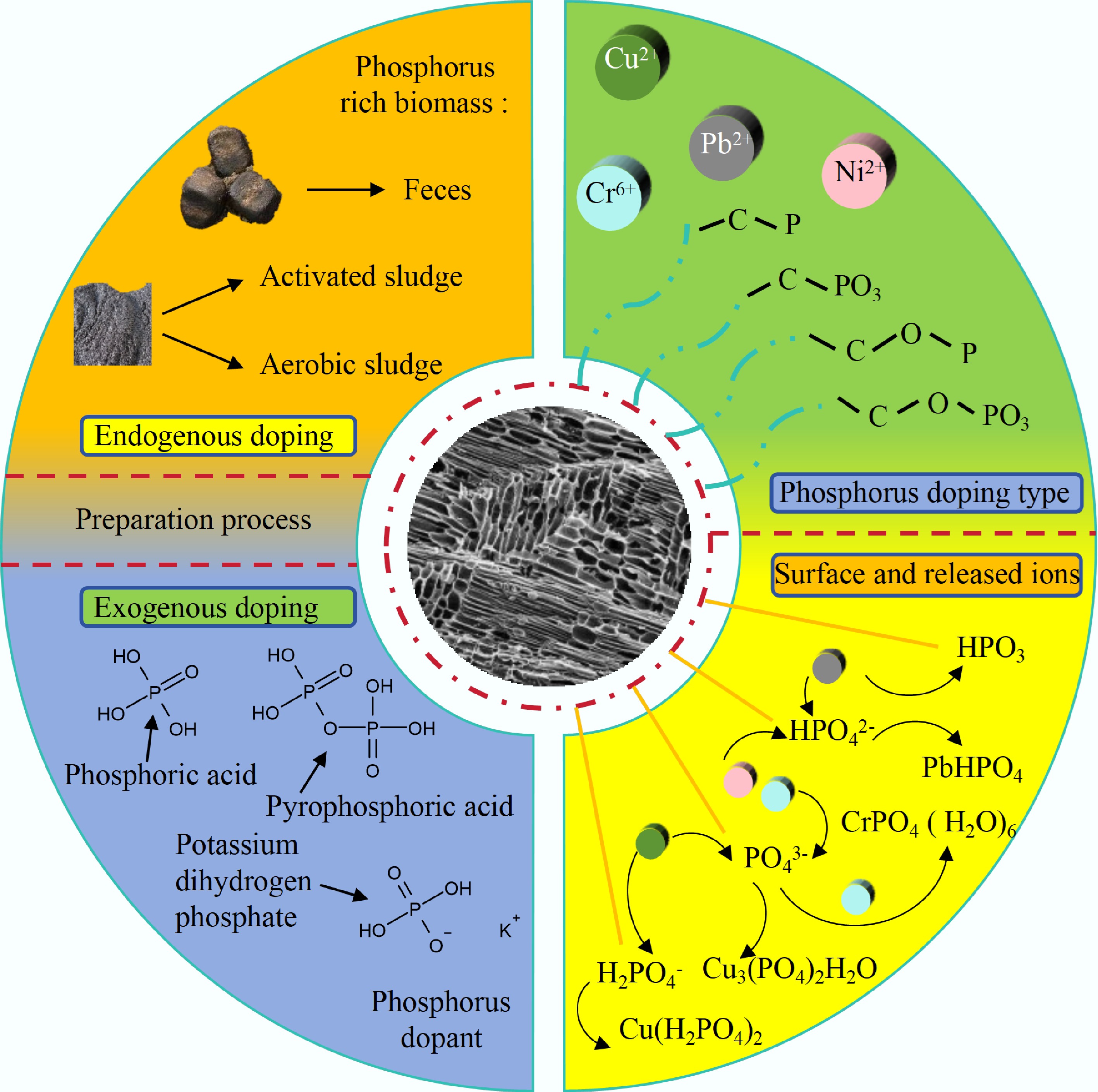
Figure 4.
The preparation methods of phosphorus-doped BC and its according enhancement mechanism.
-
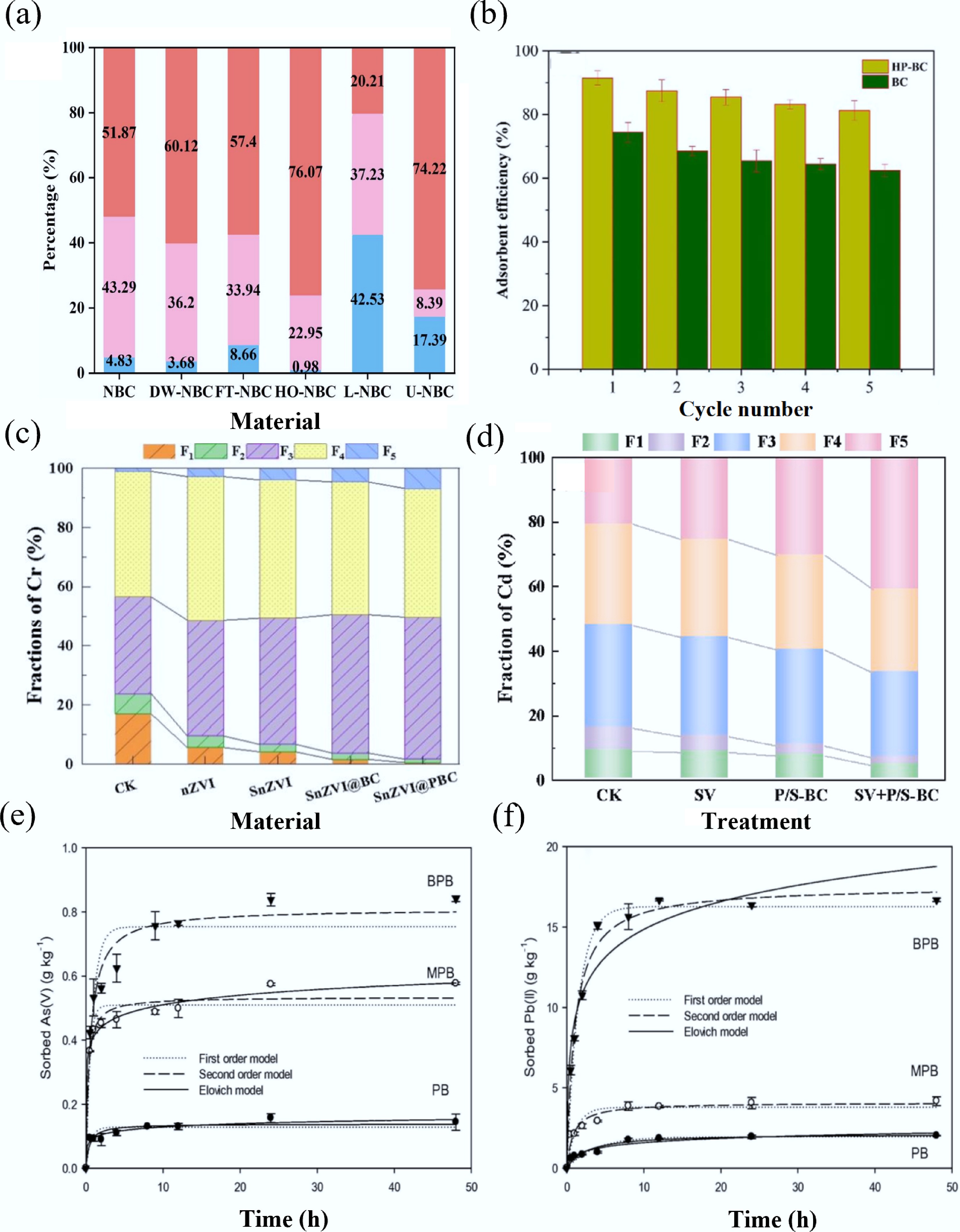
Figure 5.
(a) Percentage distribution of Cd speciation on NBC and aged NBC[125]. (b) Regeneration and reusability of biochars[129]. (c) Influence of various materials on percentage variation of Cr fractions in the contaminated soil[112]. (d) Effects of different passivators on the morphology distribution of Cd[134]. (e) As(V), (f) Pb(II) adsorption kinetics data[143].
-
BC/material Pyrolysis parameters Heavy
metalsMaximum adsorption
capacity (mg/g)Kinetic model Isotherm Removal mechanism Ref. Hickory wood 600 °C Pb(II) 153.1 Richie Langmuir Surface adsorption [19] Cu(II) 34.2 Elovich Langmuir Cd(II) 28.1 Richie Langmuir Coconut shells 800 W microwave Pb(II) 4.77 Pseudo-second-order (PSO) Langmuir Surface adsorption and Van der Waals forces [20] Cd(II) 4.96 PSO Langmuir Oleifera shells 550 °C Pb(II) 52.9 PSO Langmuir Chemisorption [21] Waste-art-paper 600 °C Pb(II) 1,555 PSO Langmuir Precipitation [22] Corn straw 500 + 800 °C Cr(VI) 116.97 Avrami fractional-order (AFO) Langmuir Electrostatic attraction, complexation, ion exchange and reduction action [23] Douglas fir 900–1,000 °C Pb(II) 140 PSO Langmuir Chelation, electrostatic-attraction, and ion exchange [24] Cr(VI) 127.2 PSO Langmuir Cd(II) 29 PSO Langmuir Rice husks 450 °C Cr(VI) 195.24 PSO Langmuir Ion exchange [25] Tea waste 450 °C Cr(VI) 197.5 PSO Langmuir Nitrogenous bone 600 °C Pb(II) 558.88 PSO Langmuir Surface complexation, cation exchange, chemical precipitation, electrostatic interaction and cation-π bonding [26] Cu(II) 287.58 PSO Langmuir Cd(II) 165.77 PSO Langmuir Water hyacinths 700 °C Cd(II) 35.1 Pseudo-first-order (PFO) Langmuir Precipitation [27] nZVI-BC 600 °C Cu(II) 31.53 PSO Langmuir Precipitation [28] Swine manure, sawdust 400 °C Pb(II) 268 PSO Langmuir Electrostatic attraction and ion exchange [29] Table 1.
Summary of removal mechanisms for BC-based adsorbents and various feedstocks
-
BC Preparation technology Contaminant Maximum adsorption capacity (mg/g)—Langmuir Adsorption mechanism Ref. Oleifera shells Ammonium polyphosphate pretreatment, high temperature carbonization Pb(II) 723.6 Chemisorption [21] Coffee shells (NH4)3PO4 pretreatment, in-situ doping Cr(VI) 235.5 Adsorption/reduction [72] Rice straw Nitrification and nitro reduction method Cd(II) 84.266 Complexation [68] Lignocellulosic biomass Nitrification and nitro reduction method Cu(II) 17.12 Complexation, onexchange, chelation, and microprecipitation [69] Wood dust HNO3 pretreatment, autoclave heating Cr(VI) 107.2939 Ion exchange, electrostatic effect [73] Sb(V) 167.2042 N-enriching hydrophyte biomass Biomass of N-rich aquatic plants loaded with MgCl2 by rapid pyrolysis, endogenous doping Pb(II) 893 Ion-exchange, surface adsorption [74] Shrimp shells Microwave-assisted self-modification of nitrogen doped oxygen graphene hydrogels Cr(VI) 360.05 Electrostatic adsorption (ion-pair), pore filling, reduction and coordination. [75] Table 2.
BC adsorbents doped with N for heavy metal removal
-
BC Preparation technology Contaminant Maximum adsorption capacity (mg/g)—Langmuir Adsorption mechanism Ref. Dried hickory chips Heating with NaOH solution, alkali modification Pb(II) 19.1 Chemisorption [84] Cu(II) 17.9 Cd(II) 0.98 Corn straw Soaking in water and HNO3 activation, wet chemical oxidation Cr(VI) 36.365 Adsorption/reduction [87] Rice straw Modification of biochar with equal volume mixture of H2O2 and HNO3, wet chemical oxidation Cd(II) 117.975 Electrostatic attraction, chemisorption [88] Rice straw KMnO4 reacts with pyrolytic biochar to form MnO coating, wet chemical oxidation Pb(II) 305.25 Chemical adsorption, complexation, precipitation, ion interaction [89] Corn straw In-situ doping, acid ammonium persulfate oxidation method Cu(II) 151.67 Surface complexation, chemical reduction [90] Camellia seed husks Soaking in HCl, wet chemical oxidation, endogenous doping Pb(II) 141.86 Electrostatic attraction, sedimentation, coordination [91] Cd(II) 54.3 Pecan nut shells (Caryaillinoinensis) Irradiation heating in H2O steam atmosphere Pb(II) 129.86 Complexation, strong interaction, ion interaction [92] Cu(II) 57.83 Cd(II) 21.69 Table 3.
BC adsorbents doped with O for heavy metal removal
-
BC Preparation technology Contaminant Maximum adsorption capacity (mg/g)—Langmuir Adsorption mechanism Ref. Corn straw Acid ammonium persulfate oxidation process, hydrophilic treatment Pb(II) 184.5 Surface complexation, precipitation and reduction reactions [90] Corn straw Mixing and stirring modification of biochar with Na2S solution Hg(II) 5.71 Precipitation, chemisorption, electrostatic interaction, chemical interaction mechanism, ion exchange [98] Corn straw Adding ammonium tetrathiomolybdate solution to calcined biochar, heating in autoclave Cd(II) 139 Surface complexation, electrostatic attraction, ion exchange, chelation [100] Wood chips S element impregnation pyrolysis, sulfurization Hg(II) 107.5 Surface adsorption, functional groups and chemical bonds [102] Sugarcane bagasse Single step reaction with isothiocyanates during pyrolysis Cd(II) 87.5 Surface complexation, coprecipitation, ion exchange, electrostatic attraction [103] Cr(III) 25.5 Ni(II) 41.0 Pb(II) 41.2 Pomelo peel H2SO4 treated biochar and chloroacetyl chloride mixed with N, N- two methylformamide and reflux 24 h at 100 °C Pb(II) 420 Coordination, ion exchange, chelation [104] Table 4.
BC adsorbents doped with S for heavy metal removal
-
BC Preparation technology Contaminant Maximum adsorption capacity (mg/g)—Langmuir Adsorption mechanism Ref. Oleifera shells Ammonium polyphosphate pretreatment, high temperature carbonization Pb(II) 723.6 Chemisorption [21] Coffee shells (NH4)3PO4 pretreatment, in situ pyrolysis Cr(VI) 235.5 Adsorption, reduction [72] Apricot stones H3PO4 thermochemical activation Pb(II) 179.48 Surface adsorption, chemisorption, ion exchange, ion capture [112] Cd(II) 105.84 Ni(II) 78.8 Undaria pinnatifida roots Ca (NO3)2·4H2O and (NH4) 2·HPO4 mixed, one pot hydrothermal Cu(II) 99.01 Surface complexation and cation exchange [113] Table 5.
BC adsorbents doped with P for heavy metal removal
-
BC Preparation technology Contaminant Maximum adsorption capacity (mg/g)—Langmuir Adsorption mechanism Ref. Phragmites australis Synthesis of Ce doped magnetic battery by chemical co-precipitation and dissolution thermal method Sb(V) 25 Hydrogen bonding, electrostatic attraction and ligand exchange, intrasphere complexation [116] Corn straw Biomass and boric acid were mixed, pyrolyzed, in-situ doping Fe(II) 132.78 Chemical complexation, ions exchange, and co-precipitation [119] Rice straw Iron and (3- amino propyl) triethoxy silane (APTES) complex, doped with Fe and NH2 radicals. Cr(VI) 100.59 Precipitation, surface complexation [120] Zn(II) 83.92 Electrostatic attraction, reduction, complexation Rice husks Pyrolysis, endogenous doping Cd(II) 154.22 Complexation, electrostatic attraction, sedimentation, cation exchange [121] Table 6.
BC adsorbents doped with other elementals for heavy metal removal
-
Material Heavy metal Qmax (mg/g) Kinetic model Isotherm Functional groups (FGs) Ref. Coffee shells Cr(VI) 95% Pseudo-second-order (PSO) Slips -N–Q [72] Agarpowder 1,5-Diphenylcarbazide, FeCl3 Cr(VI) 144.86 PSO Langmuir -NH2, -NH3+ [64] Rice straw Cd(II) 69.3 PSO Langmuir -NH2 [68] Rice husks, chlorella pyrenoidosa Cu(VI) 29.11 PSO Langmuir -NH2 [126] Plant waste and aquatic animal waste Ni(II) 44.78 PSO Freundlich -NH2, -NH3+ [127] Table 7.
Effects of nitrogen-functional carbon adsorbents on heavy metal removal
-
Material Heavy metal Qmax (mg/g) Kinetic model Isotherm Functional groups (FG) Ref. Pines Pb(II) 55 Pseudo second-order (PSO) Freundlich -OH, -COOH [83] Rice straw Cd(II) 117 PSO Freundlich -C=O, -OH, -C-O [88] Sawdust Cr(VI) 45.88 PSO Freundlich -OH, -C=O, -C-O [130] Grape pomace Pb(II) 137 PSO Sips -OH [131] Table 8.
Effects of oxygen-containing carbon adsorbents on heavy metal removal
-
Material Heavy metal Qmax (mg/g) Kinetic model Isotherm Functional groups (FG) Ref. Corn straw Cd(II) 139 Pseudo-second-order (PSO) Langmuir Mo-S [100] Wood Hg(II) 107.5 PSO Langmuir C-SOx- [102] Rice husks Cd(II) 137.16 PSO Langmuir C=S [135] Luffa sponge Cr(VI) 312.50 PSO Langmuir C-SH [136] Sugarcane bagasse Pb(II) 95.7 PSO Langmuir -N=C=S [103] Cr(III) 90.7 Cd(II) 87.5 Pine-tree needle Hg(II) 48.2 PSO Freundlich C-S [137] Wheat straw Hg(II) 95.5% PSO Langmuir C-S [138] Table 9.
Effects of sulfur-containing carbon adsorbents on heavy metal removal
-
Material Heavy metal Qmax (mg/g) Kinetic model Isotherm Functional groups (FGs) Ref. Camellia oleifera shells Pb(II) 723.6 Pseudo-second-order (PSO) Langmuir O-P-O, P=O, P-N-C, P-O-C [21] Corn stalk Ce(III) 177.26 PSO Langmuir P-O [139] Lotus leaves Pb(II) 321.52 PSO Freundlich P=O, P-O-C, HO-P=O [140] Fish scales Cu(II) 505.8 PSO Langmuir P-O, P=O [141] Cd(II) 327.2 Pb(II) 661.2 Olive oil mill press waste Pb(II) 57.78 PSO − PO43−, ester sulphate [142] Table 10.
Effects of phosphorus-containing carbon adsorbents on heavy metal removal
-
Material Heavy metal Qmax (mg/g) Kinetic model Isotherm Functional groups (FG) Ref. Rice husks Cd(II) 154.22 Pseudo-second-order (PSO) Freundlich -OH, -COOH, C=N, -NH2 [121] Chicken manure Cu(II) 258.22 PSO Langmuir Fe-O, Fe-OH, C-OH [146] Pb(II) 390.60 As(V) 5.78 Rice husks Cd(II) 104.68 PSO Langmuir Mg-O, -OH [147] Cu(II) 173.22 Zn(II) 104.38 Cr(VI) 47.02 Table 11.
Effects of heteroatom-containing carbon adsorbents on heavy metal removal
Figures
(5)
Tables
(11)