-
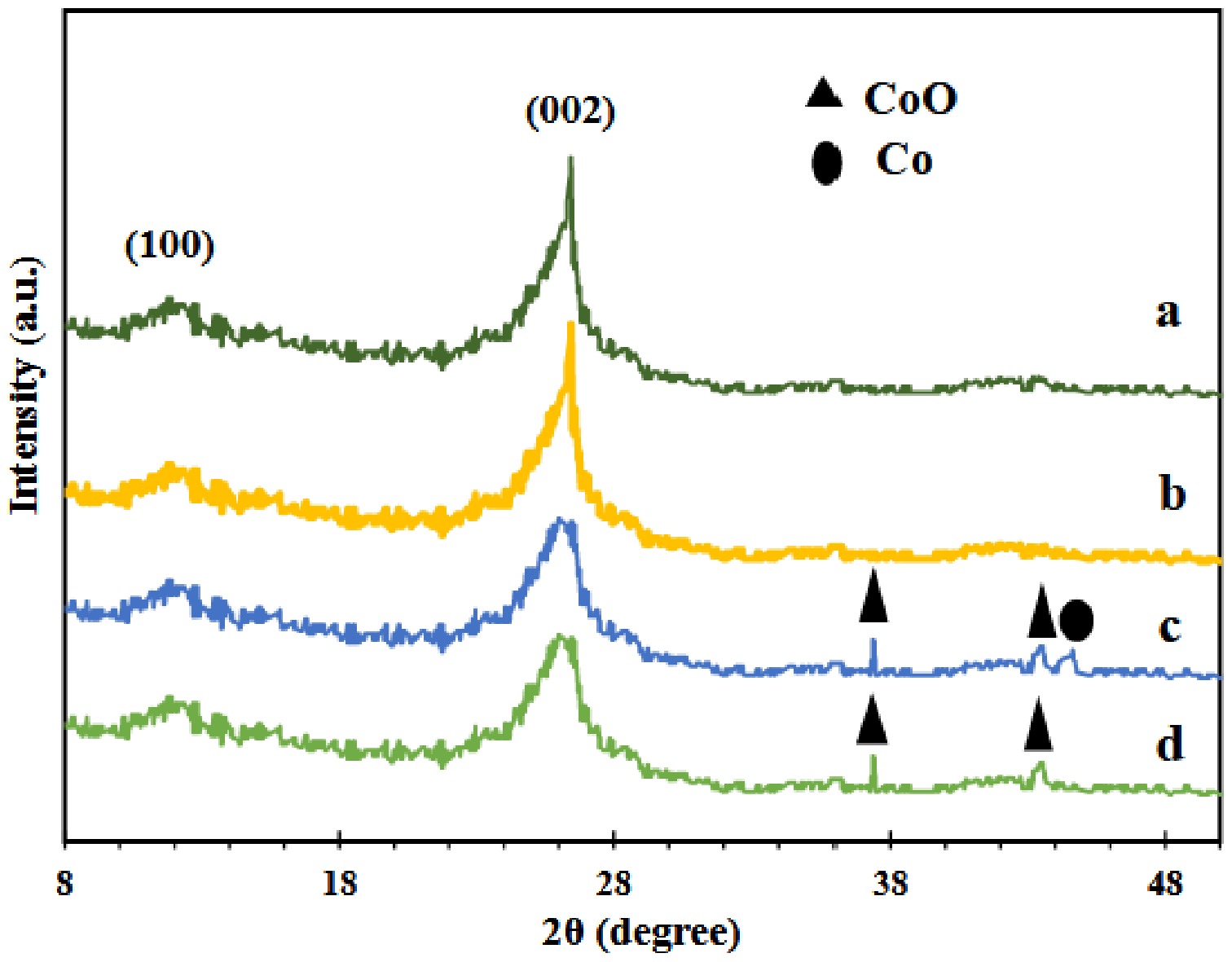
Figure 1.
XRD images of samples. (a) GNU, (b) GNM, (c) Co/GNU, (d) Co/GNM.
-
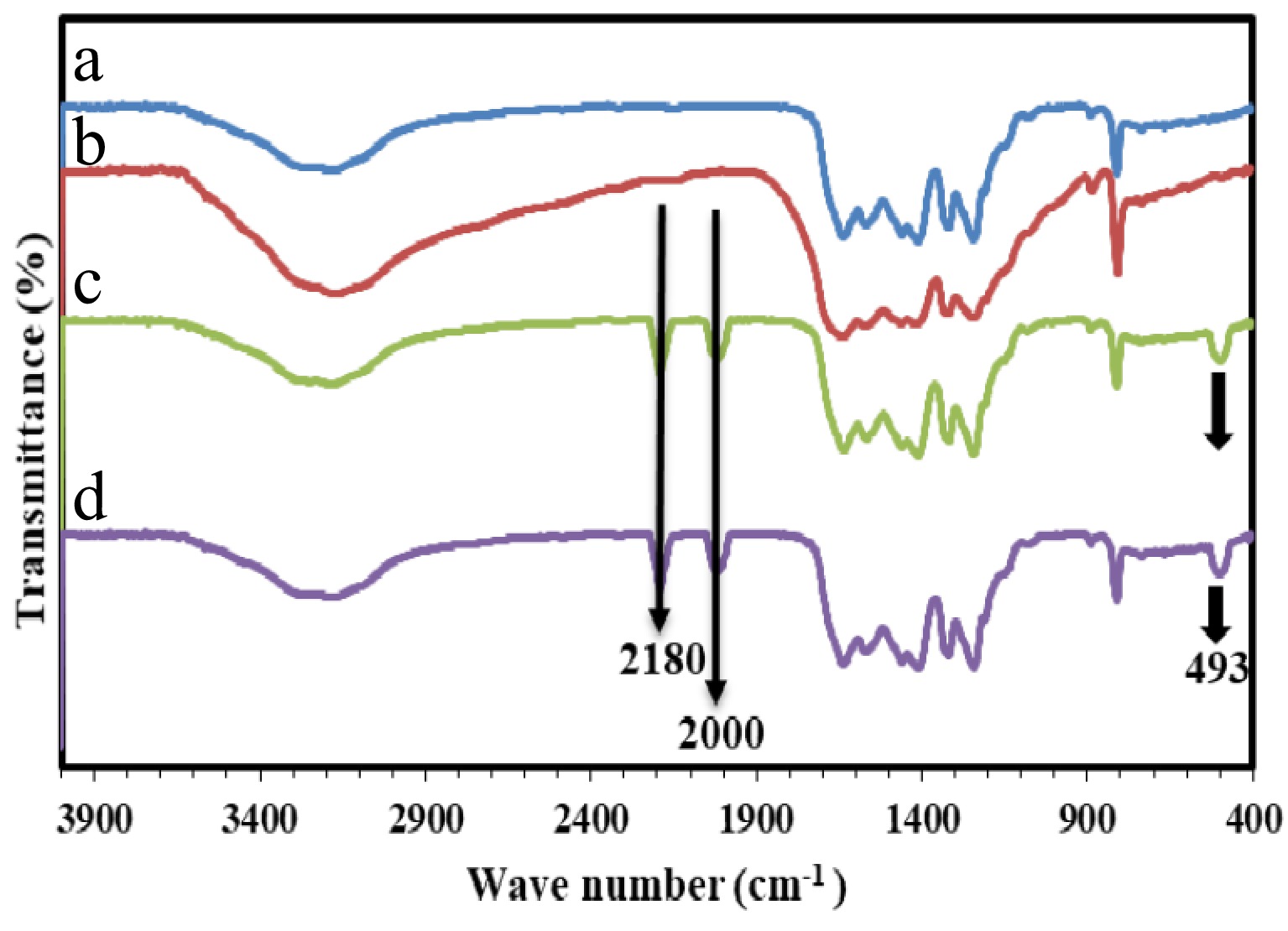
Figure 2.
FT-IR spectrum of the prepared samples. (a) GNU, (b) GNM, (c) Co/GNU, (d) Co/GNM.
-
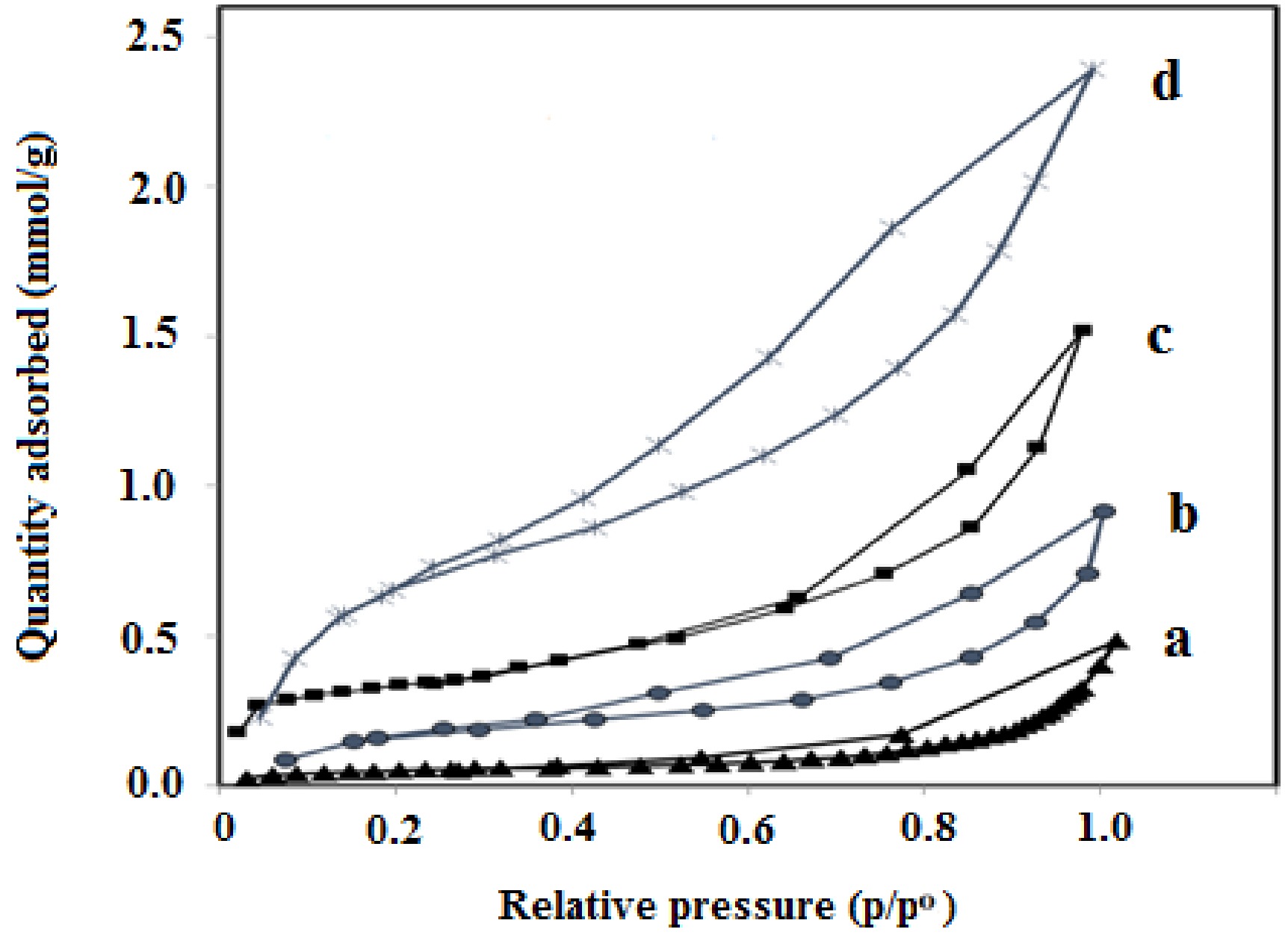
Figure 3.
Nitrogen adsorption-desorption isotherms of the prepared samples obtained at 77 K. (a) GNM, (b) GNU, (c) Co/GNM, (d) Co/GNU.
-
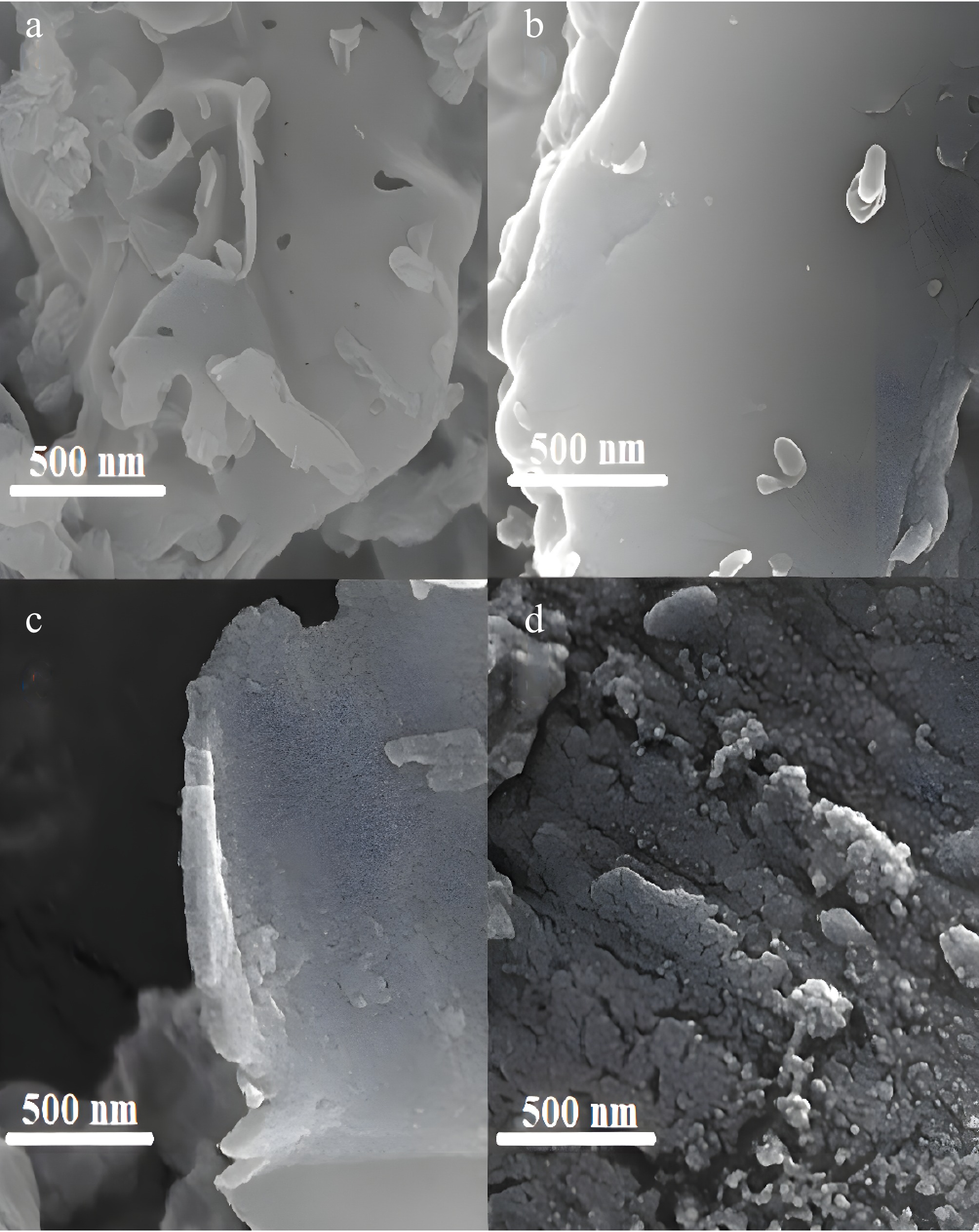
Figure 4.
SEM images of the prepared samples. (a) GNU, (b) GNM, (c) Co/GNU, (d) Co/GNM.
-
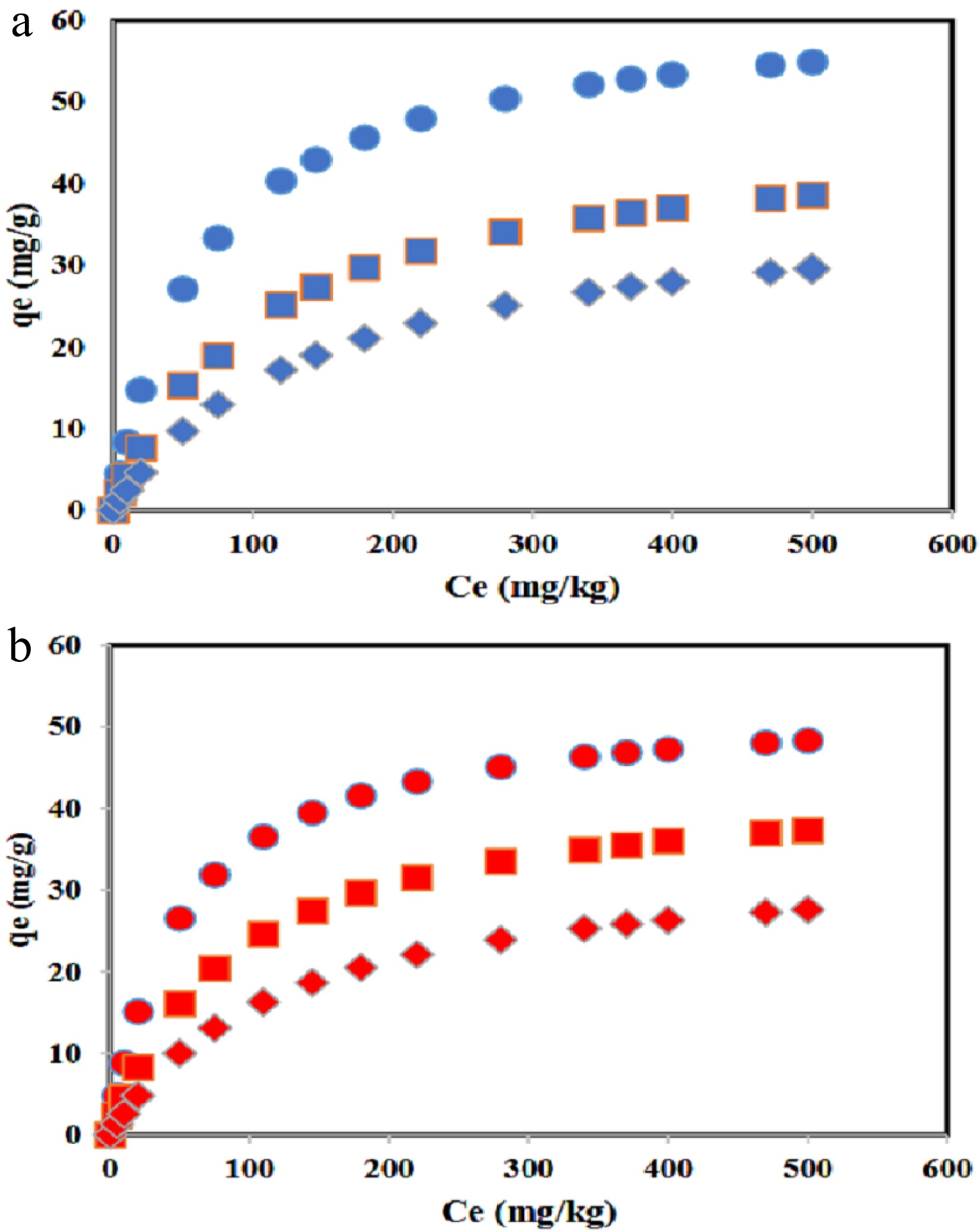
Figure 5.
The DBT adsorption capacity for (a) Co/GNU, and (b) Co/GNM adsorbents at 278 (solid cicle), 308 (solid square), and 328 K (solid diamond).
-
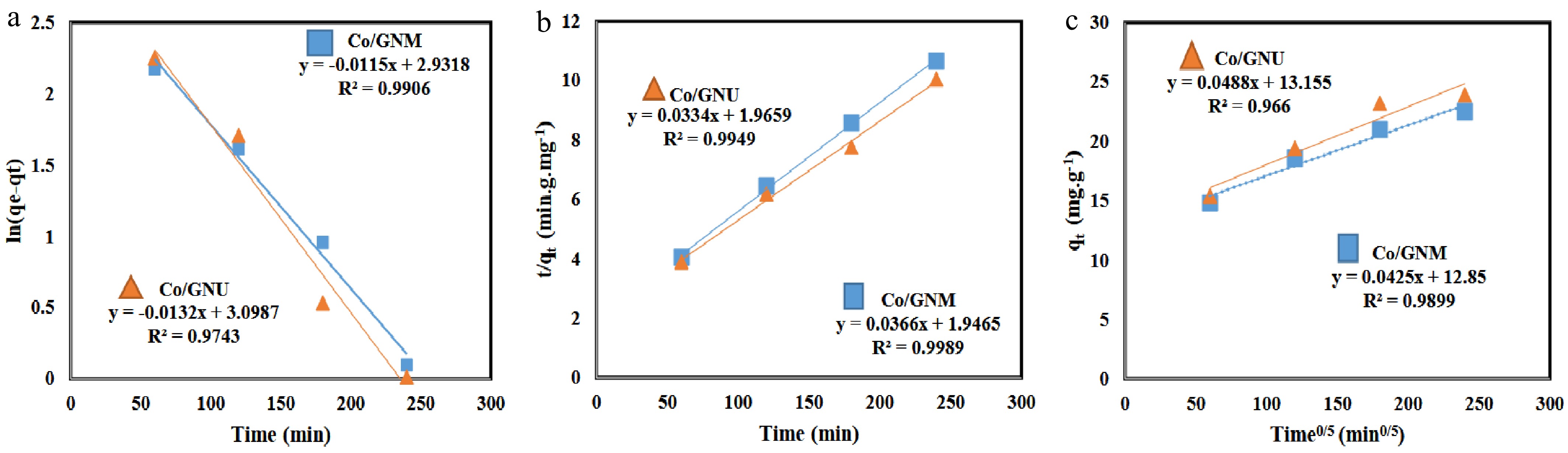
Figure 6.
Kinetics modeling of adsorbent. (a) Pseudo-first-order, (b) pseudo-second-order, and (c) intraparticle diffusion model.
-
Sample BET surface area
(m2·g-1)Pore volume
(cm3·g-1)Mean pore diameter
(nm)GNU 29 0.083 8.3 GNM 15 0.045 4.8 Co/GNU 66 0.117 4.7 Co/GNM 41 0.068 3.3 Table 1.
Textural properties of the prepared samples.
-
Adsorbent H2 uptake (μm·g−1) Co particle size (nm) Dispersion (%) Co/GNM 66.2 18.4 5.2 Co/GNU 142 8.3 11.2 Table 2.
H2 uptake, Co particle size, and the dispersion of Co.
-
Adsorbent T (K) Langmuir isotherm Temkin isotherm Freundlich isotherm KL (kg·mg−1) qm (mg·g−1) R2 KT (kg·mg−1) B (J·mol−1) R2 KF (mg1-(1/n)·g−1·kg1/n) n R2 Co/GNM 278 0.019 53.200 1 0.282 10.170 0.990 0.813 1.335 0.927 308 0.011 43.735 1 0.181 8.343 0.987 0.429 1.247 0.951 328 0.008 34.372 1 0.145 6.304 0.975 0.248 1.198 0.964 Co/GNU 278 0.015 61.980 1 0.225 11.940 0.991 0.772 1.292 0.940 308 0.009 46.479 1 0.161 8.738 0.982 0.393 1.222 0.958 328 0.006 38.245 1 0.132 6.779 0.966 0.233 1.175 0.971 Table 3.
Isotherm parameters for DBT adsorption of DBT.
-
Adsorbent Sulfur compound C0 (ppm) qm (mg S g ad−1) Ref. g-C3N4 DBT 1,000 39.10 [12] CN-xa DBT 500 37.02 [43] ACb DBT 500 10.20 [44] GOPc DBT 377 10.60 [45] MWCNTd DBT 250 23.42 [46] Co/GNM DBT 500 53.20 This work Co/GNU DBT 500 61.98 This work a Nitrogen-doped carbon materials. b Activated carbon. c Graphite oxide synthesized with phosphoric acid. d Multiwall carbon nanotubes. Table 4.
Comparison of the adsorption capacity of various adsorbents.
-
Adsorbent T (K) qe, exp (mg·g-1) Pseudo-first-order Pseudo-second-order Intra-particle diffusion K1 (min−1) qe cal (mg·g−1) R2 K2 (g·mg·min−1) qe cal (mg·g−1) R2 Kid (mg·g·min0.5−1) C (mg·g−1) R2 Co/GNM 278 25.2 0.007 16.2 0.991 0.00068 27.4 0.995 0.989 7.5 0.987 308 24.1 0.010 17.4 0.993 0.00069 27.4 0.999 1.001 7.6 0.981 328 23.6 0.011 18.7 0.990 0.00071 27.3 0.998 1.002 7.2 0.989 Co/GNU 278 26.5 0.007 18.2 0.969 0.00054 29.2 0.993 1.141 5.9 0.972 308 25.1 0.010 19.8 0.992 0.00055 29.6 0.997 1.146 6.4 0.978 328 24.9 0.013 22.1 0.974 0.00056 29.9 0.994 1.154 6.7 0.966 Table 5.
Kinetic parameters for the removal of DBT by adsorbents.
-
Adsorbent Thermodynamic parameters T (K) ∆G° (kJ·mol−1) ∆H° (kJ·mol−1) ∆S° (J·mol−1) Co/GNM 278 −4.25 −13.37 −80 308 −3.31 −13.37 −80 328 −2.65 −13.37 −80 Co/GNU 278 −3.70 −12.35 −78 308 −2.79 −12.35 −78 328 −1.86 −12.35 −78 Table 6.
Thermodynamic parameters for the DBT adsorption by adsorbents.
Figures
(6)
Tables
(6)