-
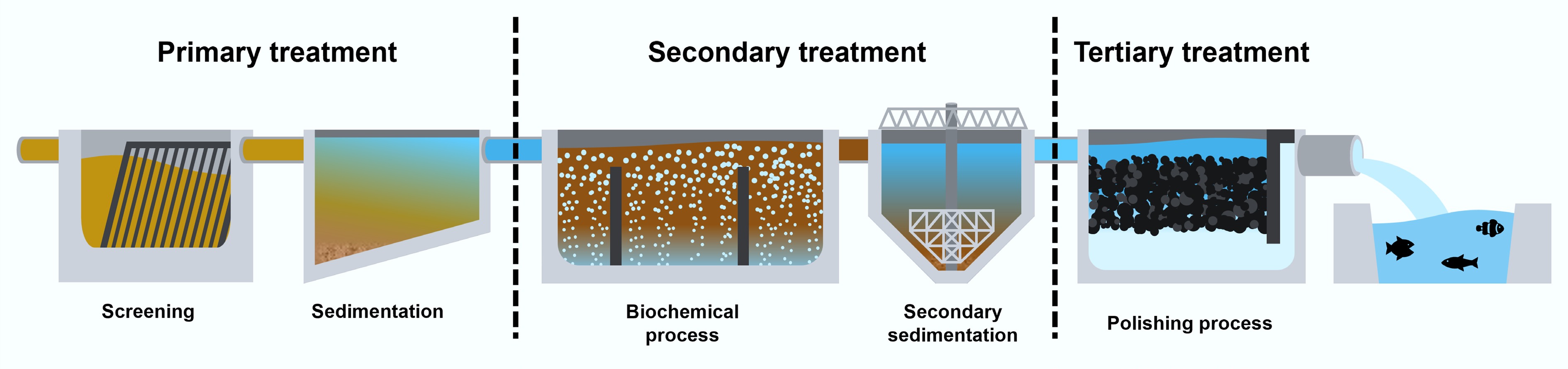
Figure 1.
Schematic diagram of a wastewater treatment plant.
-
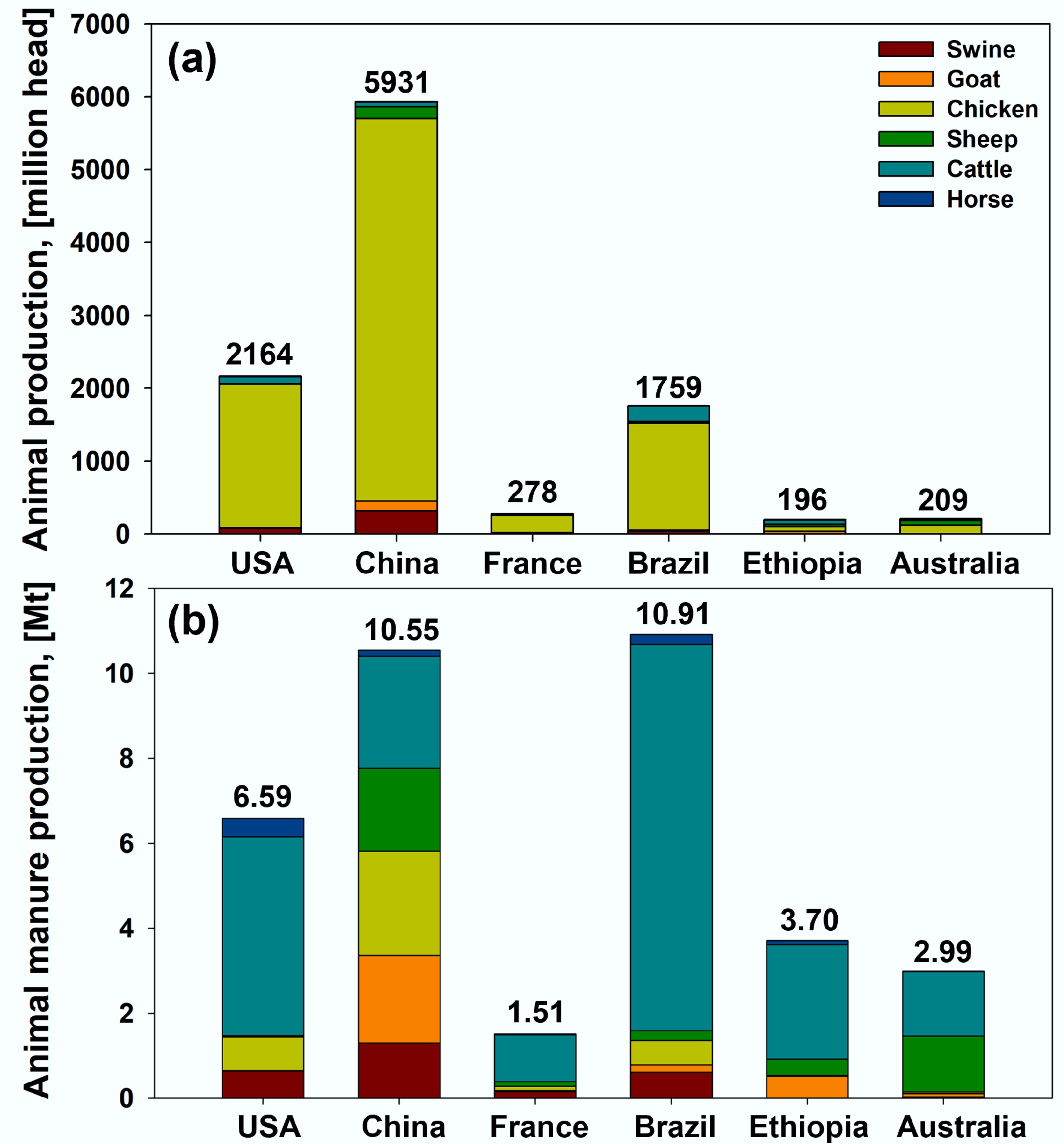
Figure 2.
(a) Annual animal (swine, goat, chicken, sheep, cattle, and horse) populations and (b) their manure production in the USA, China, France, Brazil, Ethiopia, and Australia (2019)[22].
-
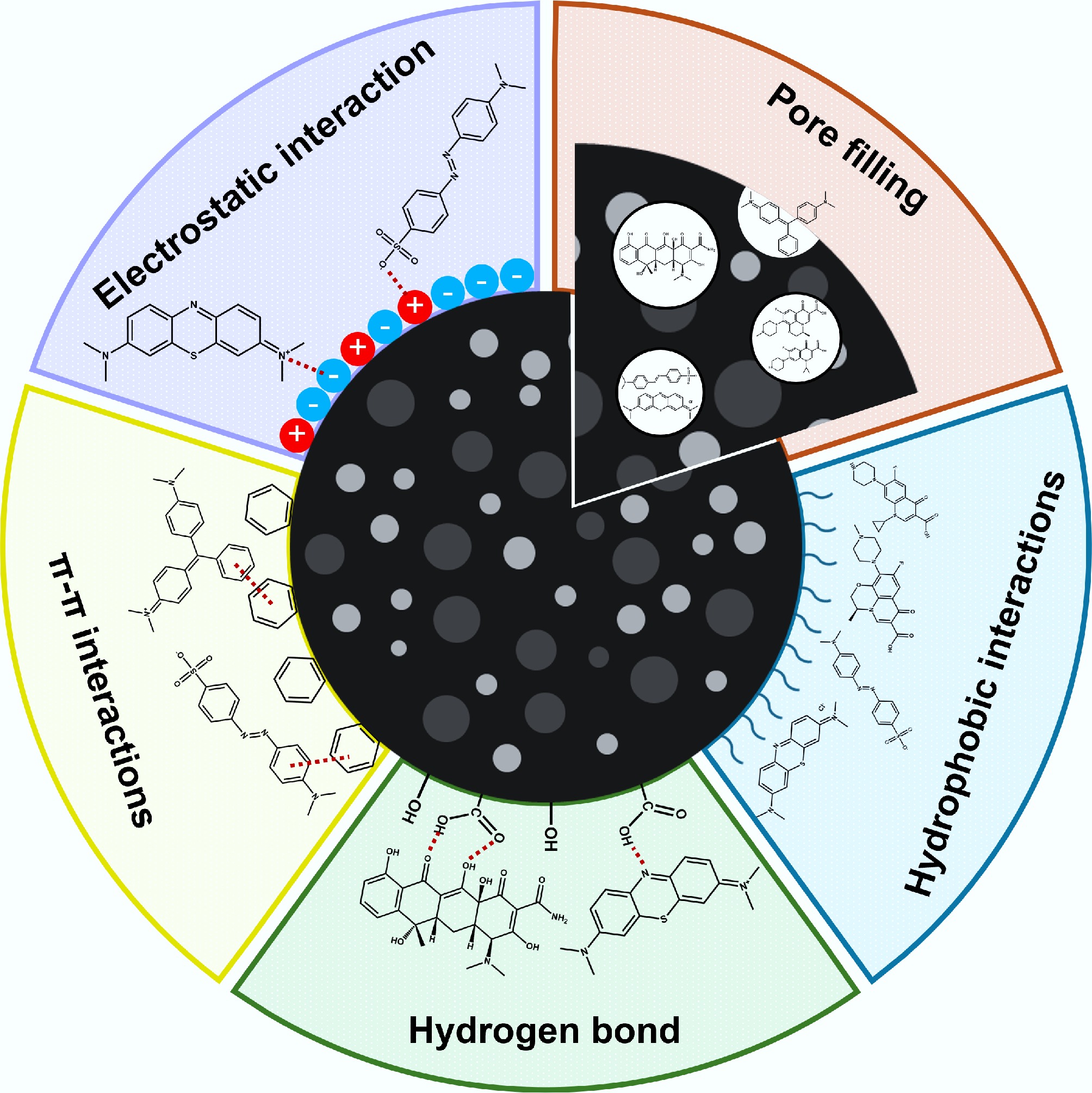
Figure 3.
Mechanisms of the adsorption of organic pollutants by biochar.
-
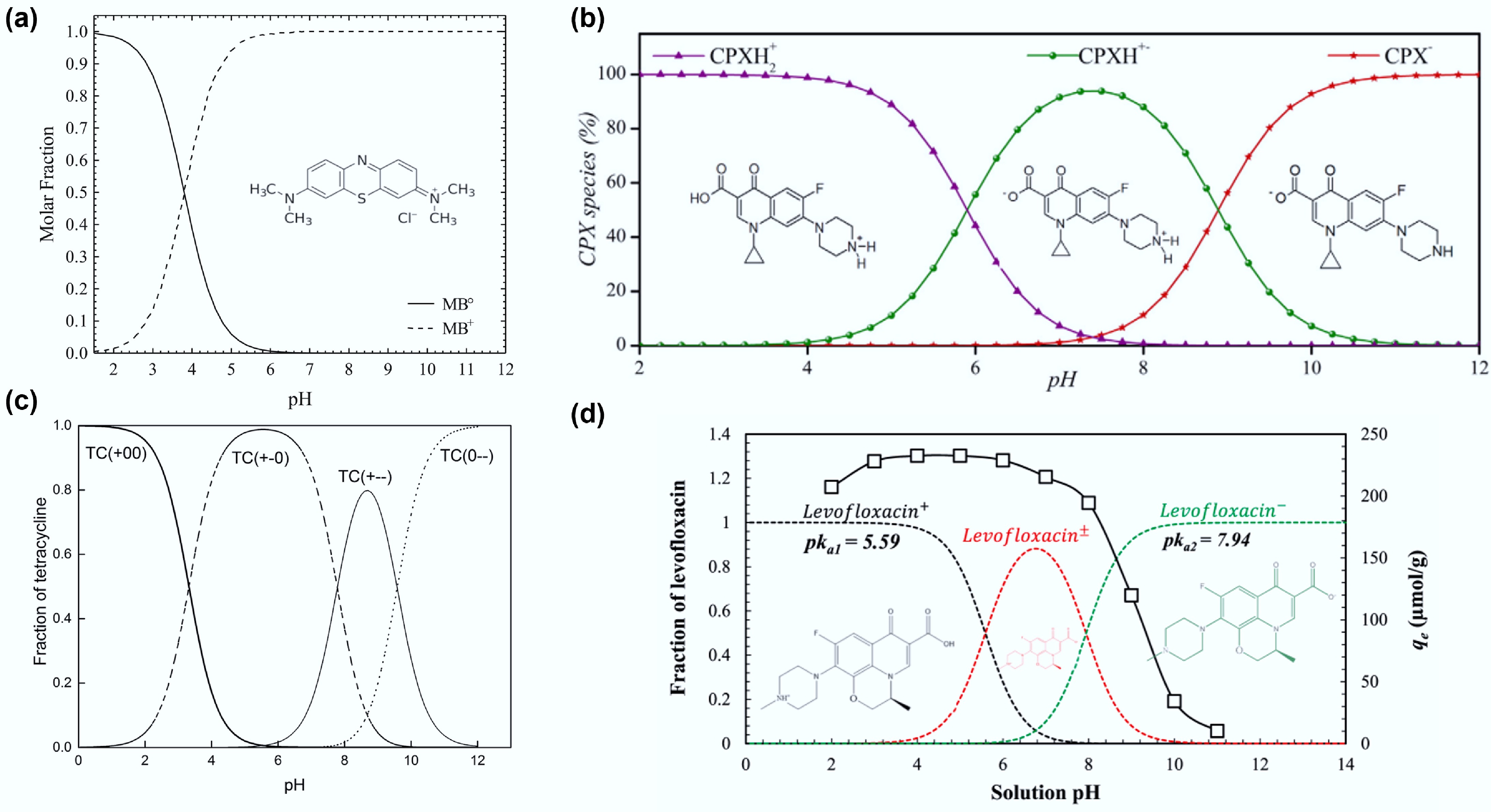
Figure 4.
Distribution of (a) methylene blue (reprinted from Salazar-Rabago et al.[119] with the permission of Elsevier), (b) ciprofloxacin (reprinted from Roca Jalil et al.[122] with the permission of Elsevier), (c) tetracycline (reprinted from Wang et al.[120] with permission; copyright 2018 Royal Society of Chemistry), and (d) levofloxacin (reprinted from Shaha et al.[121] with the permission of Elsevier) at different pH values.
-
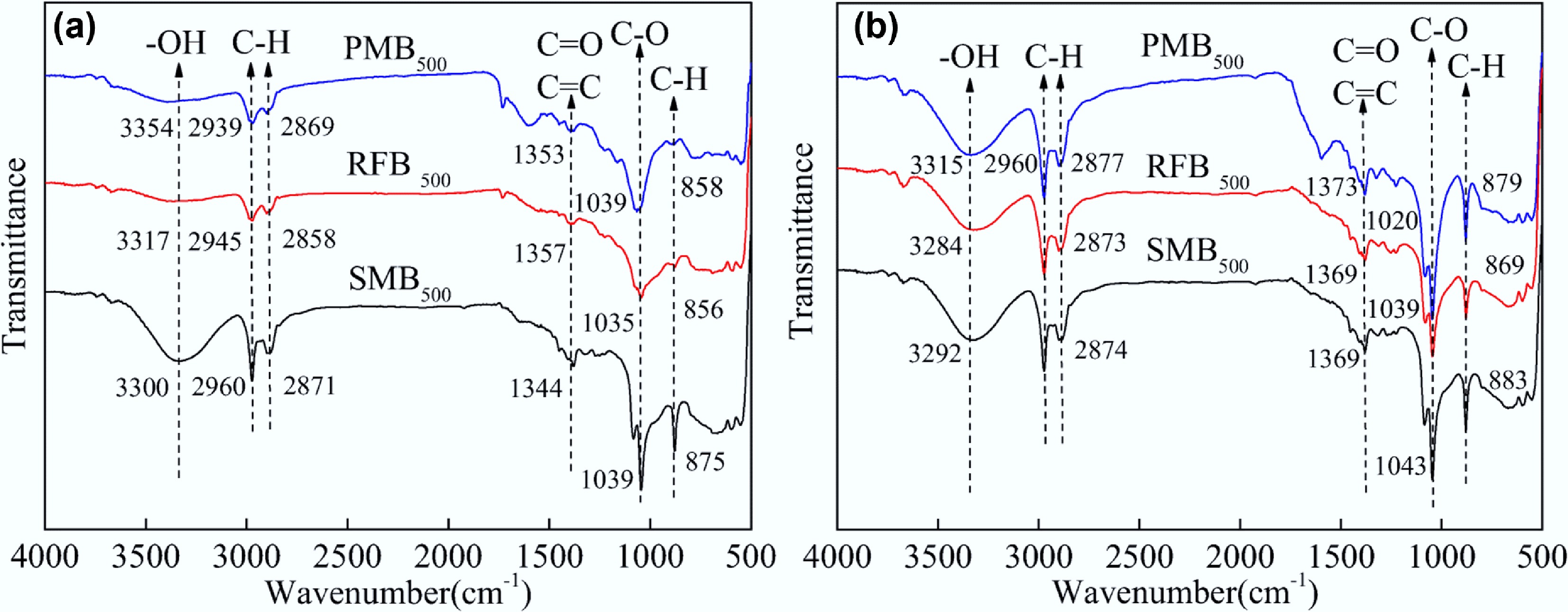
Figure 5.
FT-IR spectra of pig (blue), rabbit (red), and sheep (black) manure biochar (a) before and (b) after adsorption of methylene blue. Reprinted from Huang et al.[127] with the permission of Springer Nature.
-
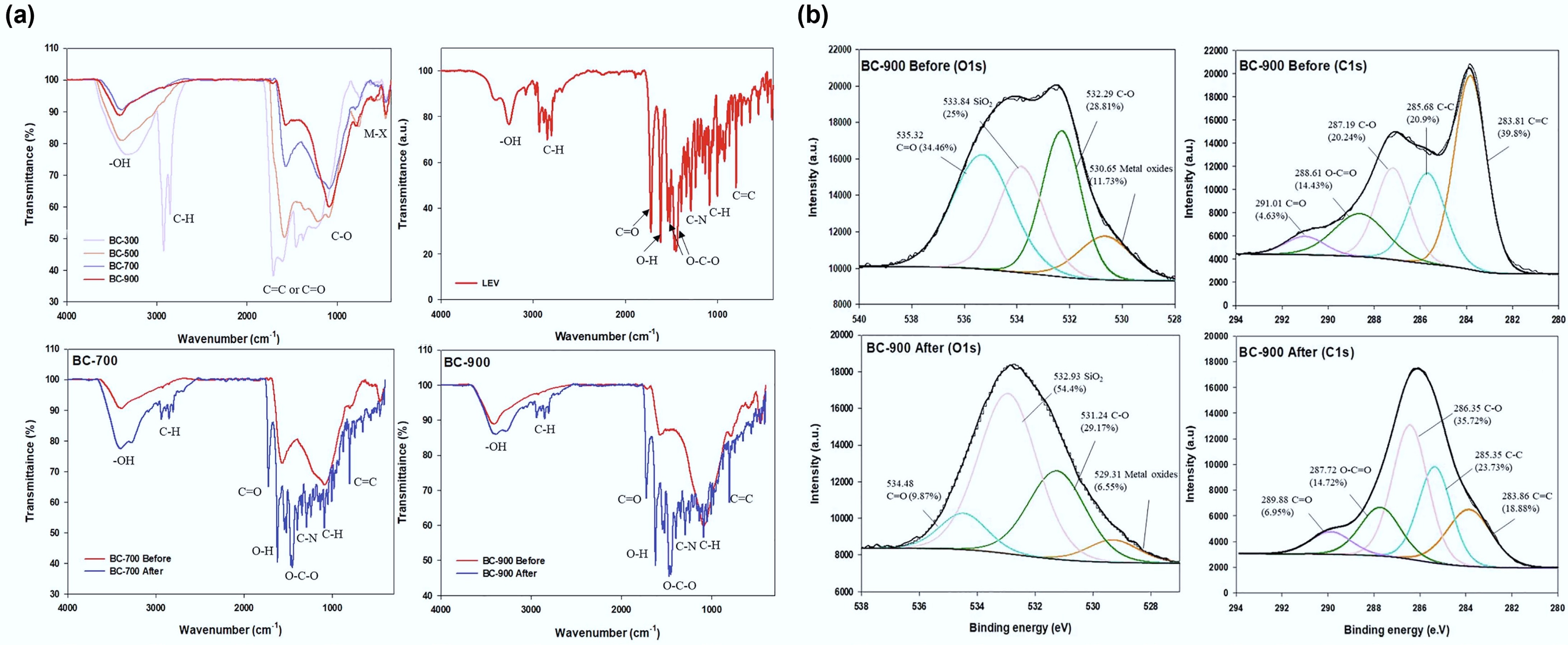
Figure 6.
(a) FT-IR spectra and (b) X-ray photoelectron spectroscopy (XPS) spectra (O1s and C1s) of swine manure biochar before and after adsorption of levofloxacin. Reprinted from Wang & Jang[107] with the permission of Elsevier.
-
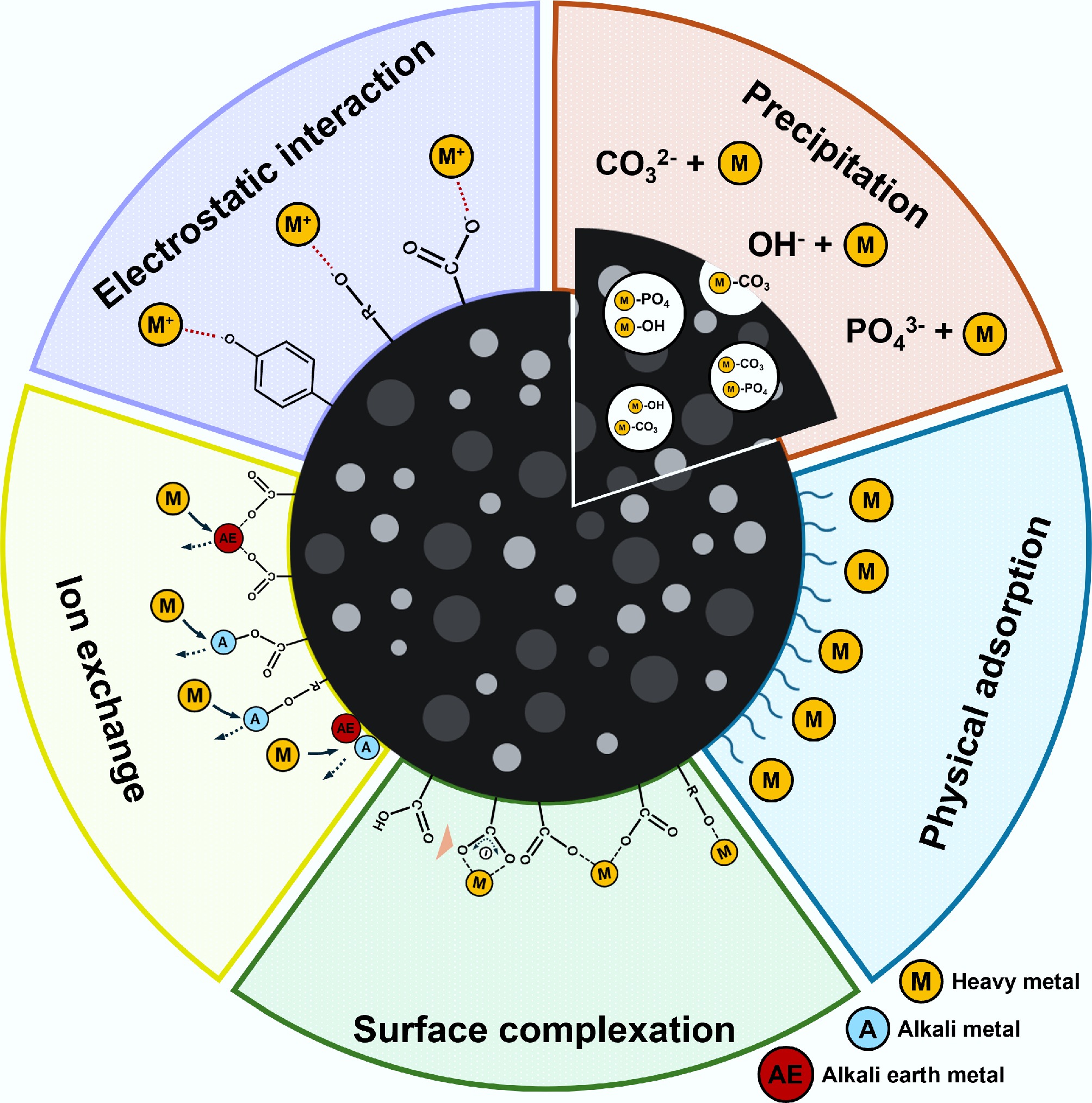
Figure 7.
Mechanisms for adsorption of heavy metals by biochar.
-
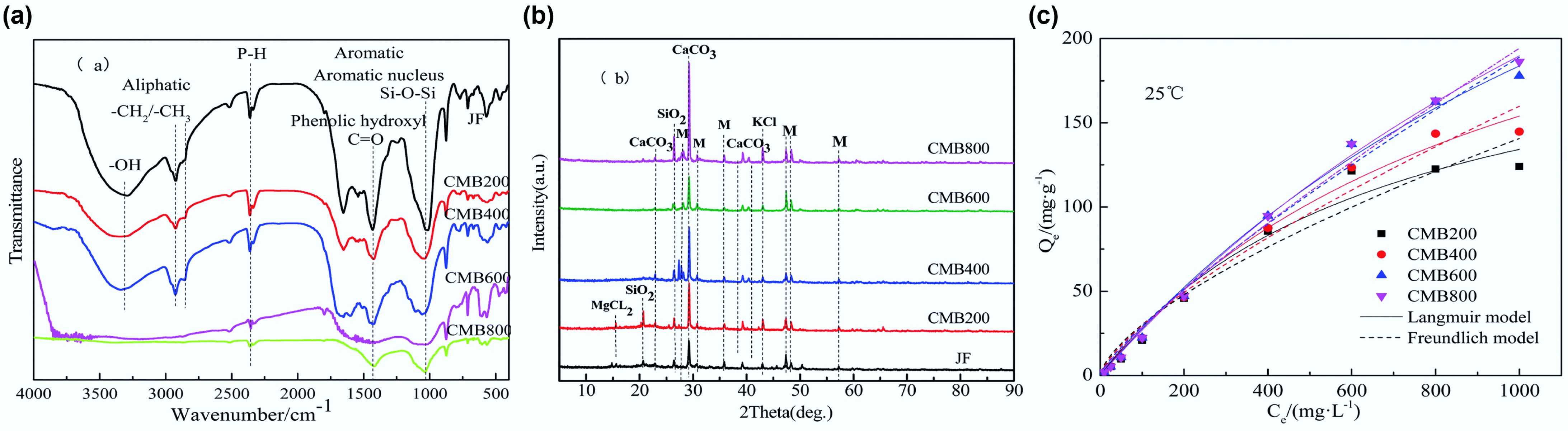
Figure 8.
(a) FT-IR and (b) XRD spectra of chicken manure biochar and as a function of temperature and (c) their adsorption isotherm curves. Reprinted from Yan et al.[166] with permission. Copyright 2019 Royal Society of Chemistry
-
Parameter Lignocellulosic biomass (wt.%) Animal manure waste (wt.%) Wheat straw Rice straw Swine manure Goat manure Chicken manure Sheep manure Cattle manure Proximate analysis (dry sample basis) Moisture 1.8–6.3 Not determined 4.4–13.6 6.0–8.7 5.6–10.0 8 1.6 Volatile matter 61.0–83.3 65.5–76.1 52.1–69.4 62.7–69.5 61.3–74.6 59.98 60.9–73.5 Fixed carbon 10.3–29.5 14.3–19.2 10.5–22.8 4.5–12.9 6.2–22.8 12.79 10.3–16.3 Ash 1.4–17.6 9.6–19.6 10.2–35.8 17.3–29.0 11.6–28.1 16.1–19.2 10.2–31.9 Ultimate analysis (dry sample basis) C 41.0–46.7 30.5–40.4 37.7–52.2 26.5–40.1 28.2–43.9 41.5–42.4 34.4–42.4 H 2.3–6.0 3.9–5.6 3.8–7.8 3.7–5.9 3.5–6.5 5.2–6.1 4.9–5.9 O 40.6–51.4 39.6–47.3 28.9–44.2 37.4–41.2 32.8–35.5 31.4–32.1 28.7–42.3 N 0.1–0.8 0.4–1.4 1.1–5.8 1.5–2.0 3.7–8.1 2.1–2.9 1.9–2.3 S 0.1–0.2 0.6 > 0.2–0.7 0.03 0.2–1.5 0.4–0.6 0.3–0.6 Inorganic material (dry sample basis) P 0.1 > 0.5–0.7 1.6–4.9 1.0–1.9 0.4–2.4 0.4–1.4 0.2–1.5 Ca 0.3–0.4 0.3–0.6 0.2–5.0 2.1–3.9 1.8–5.9 0.8–5.11 0.1–8.3 K 1.8–2.6 1.5–2.3 0.4–7.8 1.6–3.4 0.7–3.1 1.9–3.3 0.9–6.2 Mg 0.1–0.2 0.2–0.5 0.5–2.0 0.8–1.8 0.3–0.7 0.5–0.7 0.1–1.1 Na 0.2–0.3 0.3 > 0.1–0.4 − 0.4–0.9 0.4 0.2–0.4 Fe 0.1 > 0.1 > 0.1–1.3 0.2 0.1 > − 0.3–0.4 Al 0.1 > 0.3 > 0.1–0.4 0.2 0.1 > − 1.6 Si 2.4 5.0–13.9 0.1 > − − − − Table 1.
The proximate, organic, and inorganic contents of animal manure with reference to representative crop residues
Y -
Process Temperature (°C) Heating rate (°C min–1) Reaction medium Residence time Biochar yield (wt.%) Target product Flash pyrolysis 400–1,200 > 1,000 Inert gas (N2, Ar, etc.) < 2 s 10–20 Pyrolytic oil Fast pyrolysis 400–1,000 300–1,200 0.5–10 s 15–35 Pyrolytic oil Slow pyrolysis 300–900 5–60 5 min–12 h 25–50 Biochar Table 2.
Operation conditions, biochar production yields, and target products of different thermo-chemical processes
-
Class Chemical Molecular formular Structure Molecular weight (g mol–1) λmax (nm) Ref. Dye Methylene blue C16H18ClN3S 
319.85 672 [66] Methyl orange C14H14N3NaO3S 
327.33 467 [66] Malachite green C52H56N4O12 
364.91 614 [67] Antibiotics Tetracycline C22H24N2O8 
444.435 359 [68] Levofloxacin C18H20FN3O4 
361.368 289 [69] Ciprofloxacin C17H18FN3O3 
331.346 277 [70] Table 3.
Chemical structures and properties of dyes (methylene blue, methyl orange, and malachite green) and antibiotics (tetracycline, ciprofloxacin, and levofloxacin)
-
Class Chemical Source of pollutants Human health Environmental hazard Ref. Dye Methylene blue Industrial: textile, paint, paper, cosmetics, plastic, leather manufacturing, food processing,
and the pharmaceutical industryCyanosis, Heinz body, eye irritation, tachycardia, delirium, jaundice, tissue necrosis, gastrointestinal disturbances (nausea, vomiting, gastritis, and diarrhoea) Growth inhibition (microalgae), pigment formation (microalgae), aquatic ecosystem imbalance [81] Methyl orange Industrial: textile, paint, paper, leather manufacturing, food processing, printing, and the pharmaceutical industry Teratogenesis, mutagenesis, carcinogenesis, eye irritation, hypersensitivity, allergies, dermatitis, tachycardia, cyanosis, jaundice, quadriplegia, tissue necrosis, gastrointestinal disturbances (vomiting and diarrhoea) Growth inhibition (bacterial), water colouration, sunlight penetration reduction, photosynthesis disturbance, reduction in dissolved oxygen and gas solubility, eutrophication [72,82] Malachite green Industrial: textile, paper, cosmetics, plastic, leather manufacturing, food processing, and the pharmaceutical industry
Aquacultural: fungicide
and antiseptics for aquacultureTeratogenesis, mutagenesis, carcinogenesis, respiratory
issues, chromosomal aberrationsGrowth inhibition (bacterial, plant, and animal), genotoxicity, cytotoxicity, reproduction disturbance, mitochondrial dysfunction [83] Antibiotics Tetracycline Hospital wastewater treatment plants
Industrial: pharmaceutical manufacturing
Agricultural: livestock excreta, agricultural fertiliser, and aquacultureDisturbs intestinal microflora, allergies, kidney and liver dysfunction, chromosomal aberrations, reproductive issues, tooth discolouration, gastrointestinal disturbances, intracranial hypertension, skin infections (rosacea), inhibition
of protein synthesisGenetic mutation, emergence of antibiotic-resistant bacteria, impact on nontarget organisms, aquatic ecosystem imbalance [76−78] Ciprofloxacin Hospital wastewater treatment plants
Industrial: pharmaceutical manufacturing
Agricultural: livestock excreta, agricultural fertiliserGenotoxicity, gastrointestinal disturbances (bleeding), leukopenia, neurological
disorders, allergiesGenotoxicity, cytotoxicity, oxidative stress induction, emergence of antibiotic-resistant bacteria, growth inhibition (plant), toxicity to cyanobacteria [79,84] Levofloxacin Wastewater treatment plants Industrial: pharmaceutical manufacturing Hormonal imbalance, reproductive toxicity, respiratory issues, carcinogenesis Growth inhibition (algal), phytotoxicity, embryotoxicity, enhancement of plasmid transformability, toxicity to aquatic organisms (bacterial, plant, and animal) [75] Table 4.
The major pollution sources and adverse effects of representative dyes (methylene blue, methyl orange, and malachite green) and antibiotics (tetracycline, ciprofloxacin, and levofloxacin) on human health and the environment
-
Metal Source of pollutants Human health Environmental hazard Ref. Pb Industrial: steel, battery, pigment, paint, paper manufacturing, mining, smelting, electroplating, and petroleum refining Agricultural: pesticides and fertilisers Gastrointestinal disturbances, kidney and liver dysfunction, neurological disorders (anaemia), reproductive toxicity, cognitive impairment − [87] Cu Industrial: steel, brass, pigments, paint, explosives manufacturing, mining, smelting, metallurgy, metal finishing, electroplating, and petroleum refining
Agricultural: pesticides and fertilisersGastrointestinal disturbances (nausea, vomiting, and bleeding), kidney and liver dysfunction, neurotoxicity, reproductive
issues (Wilson’s disease), headacheInhibition of microbial activity, affects earthworms, slows decomposition of organic matter [88,89] Cd Industrial: electronics, electrode, battery, paint, ceramics manufacturing, mining, metallurgy, electroplating, and welding Gastrointestinal disturbances, kidney and
liver dysfunction, neurological effects (anosmia), reproductive issues (urolithiasis), bone fragility, and carcinogenesisGrowth inhibition (plant), metabolism inhibition, photosynthesis disturbance, reduction of agricultural productivity [90,91] Table 5.
The major pollution sources and adverse effects of representative heavy metals
-
Target Adsorbent Pyrolysis and activation conditions Surface area (m2 g−1) Total pore volume (cm3 g−1) Average pore diameter (nm) Ash content (wt.%) H/C ratio O/C ratio Qmax
(mg g−1)Ref. Methylene blue Bovine Biochar: 500 °C for 3 h 17.50 [126] Methylene blue Sheep Biochar: 500 °C for 2 h 160.53 0.172 10.03 17.82 0.037 0.244 202.72 [127] Methylene blue Rabbit Biochar: 500 °C for 2 h 21.14 0.041 8.64 15.38 0.064 0.165 86.85 [127] Methylene blue Swine Biochar: 500 °C for 2 h 13.36 0.025 7.33 13.14 0.128 0.079 46.95 [127] Methylene blue Bovine Biochar: 200 °C 0.3276 0.00613 75.02 192.31 [133] Methyl orange Chicken Biochar: 600 °C for 2 h 39.47 [128] Methyl orange Sheep Biochar: 600 °C for 2.5 h 181.76 0.245 0.035 0.191 42.51 [129] Malachite green Sheep Biochar: 450 °C 11.731 208.33 [130] Methylene blue Swine Biochar: 700 °C for 2 h;
Activation: alkali fusion pretreatment of fly ash209.1 0.369 7.10 0.43 142.86 [135] Table 6.
Application of animal manure biochar for dye adsorption
-
Target Adsorbent Pyrolysis and activation conditions Surface area (m2 g–1) Total pore volume (cm3 g–1) Average pore diameter (nm) Ash content
(wt.%)H/C ratio O/C ratio Qmax
(mg g–1)Ref. Tetracycline Swine Biochar: 600 °C for 2 h 10.56 0.044 12.36 0.05 0.250 8.125 [120] Tetracycline Bovine Biochar: 700 °C 5.82 [117] Tetracycline Swine Biochar: 700 °C for 2 h 319.04 0.25 43.9 0.01 0.06 160.3 [142] Activation: 14% H3PO4 solution for 24 h at 25 °C Levofloxacin Swine Biochar: 900 °C for 2 h 512.11 0.51 4.008 18.39a 0.014 0.093 158.07 [107] Ciprofloxacin Rabbit Biochar: 700 °C for 2.5 h 91.52 0.237 12.64 19.06 0.032 0.276 57.626 [140] a After acid washing. Table 7.
Applications of animal manure biochar for the adsorption of antibiotics
-
Adsorbent Pyrolysis and activation conditions Surface area (m2 g–1) Total pore
volume (cm3 g–1)Average pore diameter (nm) Ash content (wt.%) pH H/C ratio O/C ratio Qmax
(mg g–1)Ref. Bovine Biochar 500 °C for 150 101 [164] Chicken Biochar: 800 °C for 2 h 64.63 10.1 0.04 0.83 242.57 [166] Chicken Biochar: 550 °C for 2 h 7.09 7.09 87.2 9.95 0.06 0.49a 120.383b [167] Yak Biochar: 350 °C for 2 h 6.36 23.91 1.2 0.55 155.36 [170] Activation: 10% H2O2 solution at room temperature Bovine Biochar: 300 °C for 4 h 25.94 0.055 27.13 9.98 0.15 0.92 175.53 [92] Activation: 2 M NaOH (shaking for 12 h at 65 °C) Swine Biochar: 500 °C;
Activation: Fe2+ and Fe3+ solutions (stirred for 20 min at 20–25 °C)225.08 [52] a Calculated from the results of a proximate analysis of biochar; b The molecular weight of Pb(II) was assumed to be 207 g mol–1. Table 8.
Applications of animal manure biochar for adsorption of Pb(II)
-
Adsorbent Pyrolysis and activation conditions Surface area (m2 g–1) Total pore volume (cm3 g–1) Ash content (wt.%) pH H/C ratio O/C ratio Qmax
(mg g–1)Ref. Bovine Biochar: 300 °C for 1 h 3.42 0.0005 34.54 8.62 1.07 0.37 61.68 [173] Bovine Biochar: 400 °C for 1 h 3.66 0.0008 39.98 9.86 0.82 0.4 64.23 [173] Bovine Biochar: 500 °C for 1 h 4.34 0.0007 46.97 10.75 0.54 0.37 58.86 [173] Bovine Biochar: 600 °C for 1 h 6.11 0.0019 48.84 10.79 0.33 0.3 70.42 [173] Bovine Biochar: 700 °C for 1 h 10.61 0.004 50.45 10.83 0.29 0.26 66.77 [173] Bovine Biochar: 500 °C for 4 h 195.1 0.09 7.16a 9.86 0.05 49.57b [153] Yak Biochar: 350 °C for 2 h 1.18 40.8 1.88 0.23 46.71 [170] Yak Biochar: 350 °C for 2 h;
Activation: 10% H2O2 solution at room temperature6.36 23.91 1.2 0.55 64.98 [170] a Sum of inorganic elements such as Ca, K, Mg, and Na in the biochar; b The molecular weight of Cu(II) was assumed to be 63.55 g mol–1. Table 9.
Applications of animal manure biochar for the adsorption of Cu(II)
-
Adsorbent Pyrolysis and activation conditions Surface area (m2 g–1) Ash content (wt. %) pH H/C ratio O/C ratio Qmax (mg g–1) Ref. Chicken Biochar: 300 °C for 4 h 4.51 40.09 9.68 0.94 0.38 62.55 [156] Chicken Biochar: 500 °C for 4 h 8.08 50 11.02 0.37 0.39 64.62 [156] Chicken Biochar: 700 °C for 4 h 10.89 54.78 11.81 0.17 0.36 123.16 [156] Farmyard Biochar: 450 °C for 5 h 10.11 27.18 8.2 0.056 0.37 60.94 [176] Poultry Biochar: 450 °C for 5 h 8.61 31.44 7.8 0.076 0.35 90.09 [176] Yak Biochar: 350 °C for 2 h 1.18 40.8 1.88 0.23 47.181 [170] Yak Biochar: 350 °C for 2 h;
Activation: 10% H2O2 solution at room temperature6.36 23.91 1.2 0.55 69.08 [170] Table 10.
Applications of animal manure biochar the for adsorption of Cd(II)
-
Heavy metal ion Pb(II) Cu(II) Cd(II) Zn(II) Hydration energy (kJ mol–1) –1,481 –2,100 –1,807 –2,046 Hydrated ionic radius (nm) 0.401 0.419 0.426 0.430 Electronegativity 2.330 1.900 1.690 1.650 -
Pollutant type Dominant adsorption mechanism Performance-enhancing factor Optimisation strategy Dyes • Cationic species: electrostatic attraction above pHpzc • High surface area and porosity • Enhance O-containing groups via targeted modification • Anionic species: protonation-driven attraction under acidic conditions • High O-containing groups and O/C ratio • π–π EDA interactions, hydrogen bonding • Alkali metal modification for ion exchange Antibiotics • TC: pH 3.5–8.0 (zwitterionic form), hydrogen bonding in addition to π–π EDA interactions
• LEV: Multilayer (physical) adsorption involving electrostatic attraction, hydrogen bonding, cation–π,and π–π EDA interactions, and pore filling
• CIP: monolayer (chemical) adsorption by hydrogen bonding between –O, –F, and -N of CIP and O-containing sites; π–π EDA interactions enhanced by higher aromaticity• TC: surface area, enriched O-containing functional groups
• LEV: porosity and surface area
• CIP: surface area and O-containing sites• Enhance O-containing groups via targeted modification for TC and CIP
• Increase the pyrolysis temperature to enhance the surface area for LEVHeavy metals • Precipitation with carbonate and phosphate species
• Ion exchange with alkali/alkaline earth metals
• Surface complexation with O-containing functional groups• Ash content with carbonate and phosphate species
• O-containing functional groups• Raise the pyrolysis temperature
• Establish a balance between adsorption performance and energy–economic trade-offs for the activation treatmentsTable 12.
Summary of adsorption mechanisms, performance-enhancing factors, and optimisation strategies of animal manure-derived biochar for dyes, antibiotics, and heavy metals
Figures
(8)
Tables
(12)