-
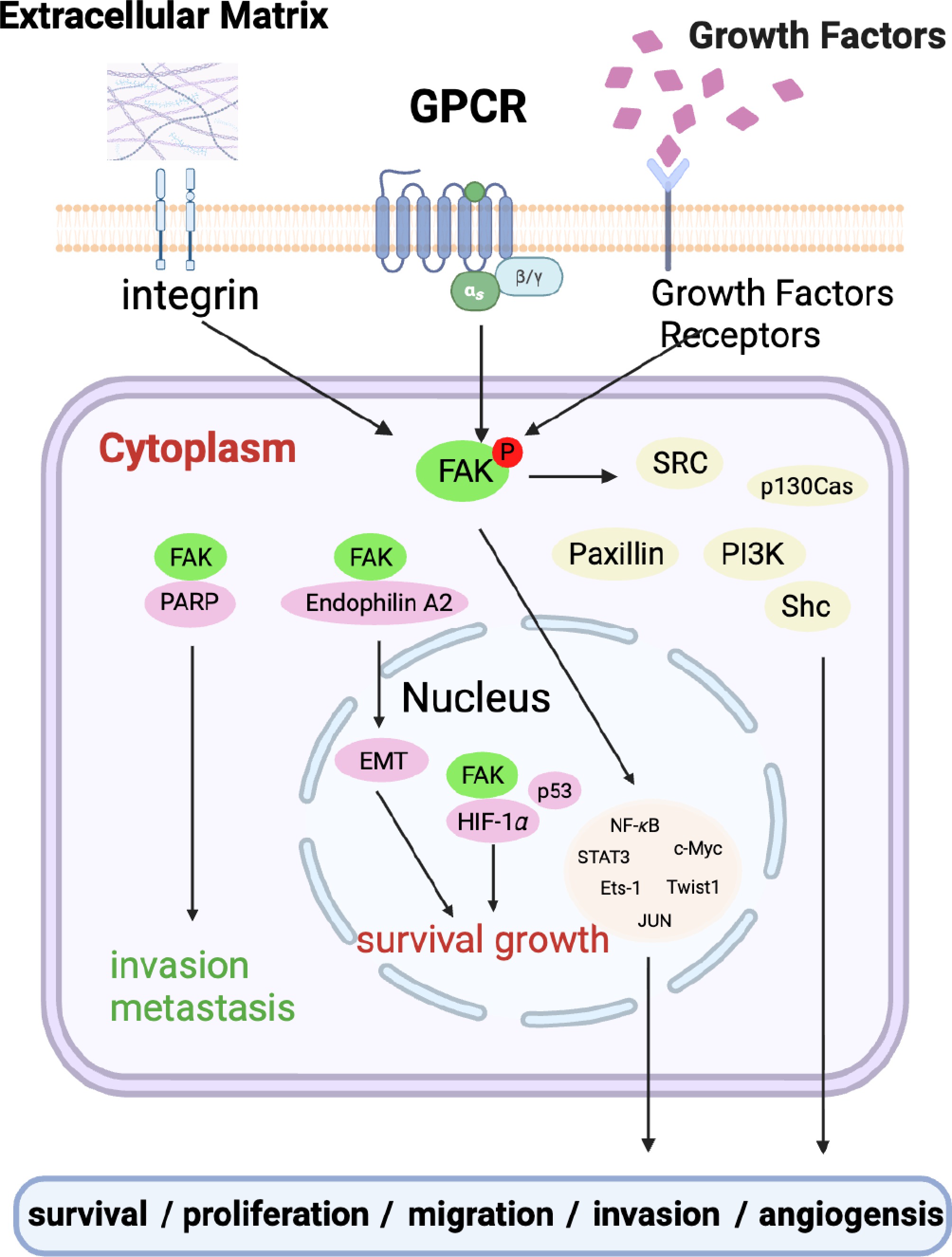
Figure 1.
Schematic showing the redundant signaling pathway employed by tumor cells for survival. In the diagram, extracellular matrix, GPCR, growth factors, etc. activate FAK, triggering a series of intrinsic adaptive regulatory mechanisms such as intracellular signal transduction, which in turn regulate processes like cell survival, proliferation, and migration.
-
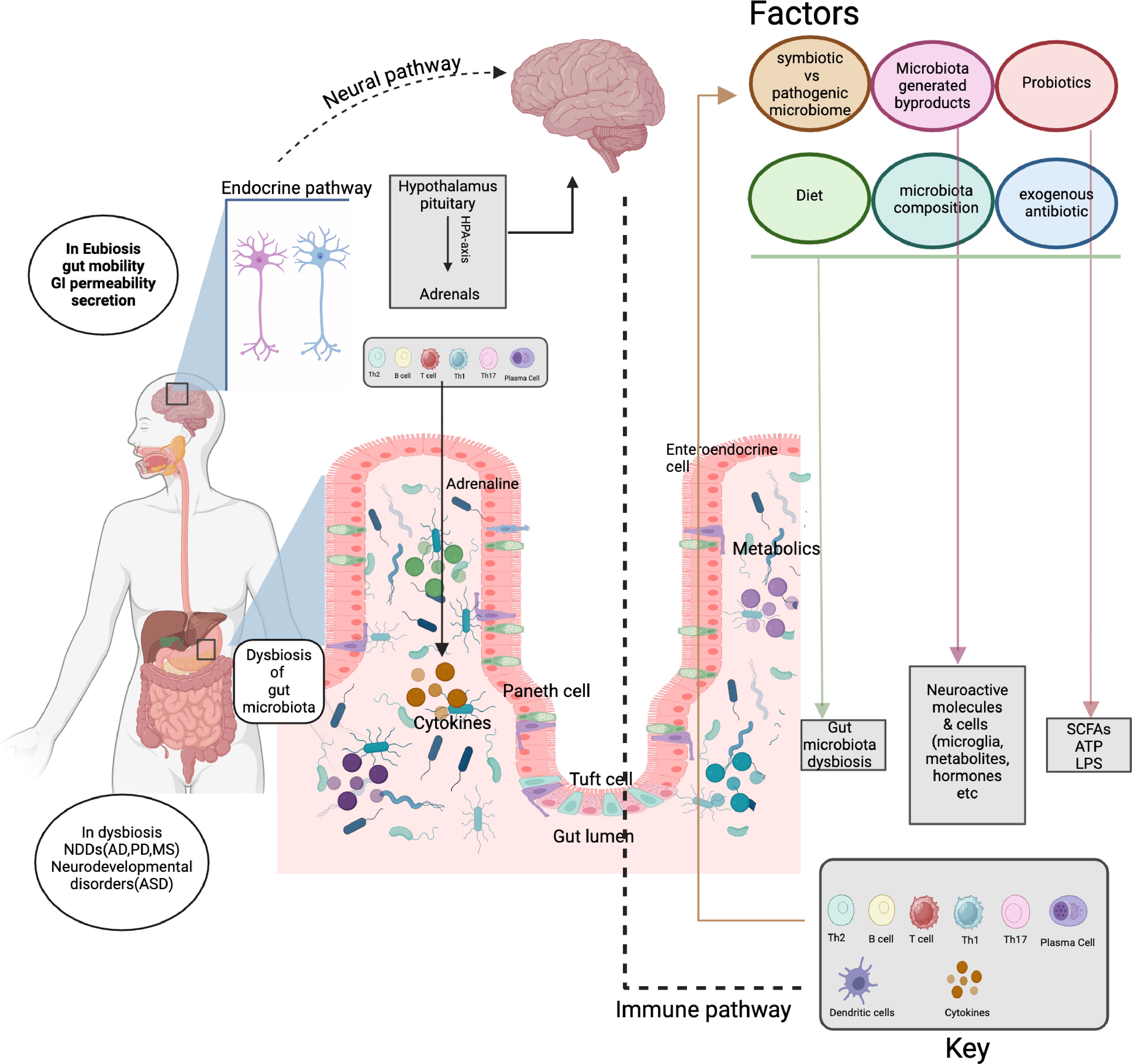
Figure 2.
Schematic showing gut microbiota as a signaling hub in the bidirectional crosstalk between the gut and brain. Gut microbiota: Interacts with factors (diet, probiotics, antibiotics) to impact composition, byproducts, metabolism, and immunity. Immune system: uses immune cells (T cells, B cells, and plasma cells) and cytokines to mediate gut-body communication. Endocrine and nervous systems: enable gut-brain communication via the hypothalamus-pituitary-adrenal (HPA) axis and neural pathways, influencing gut and neural functions.
-
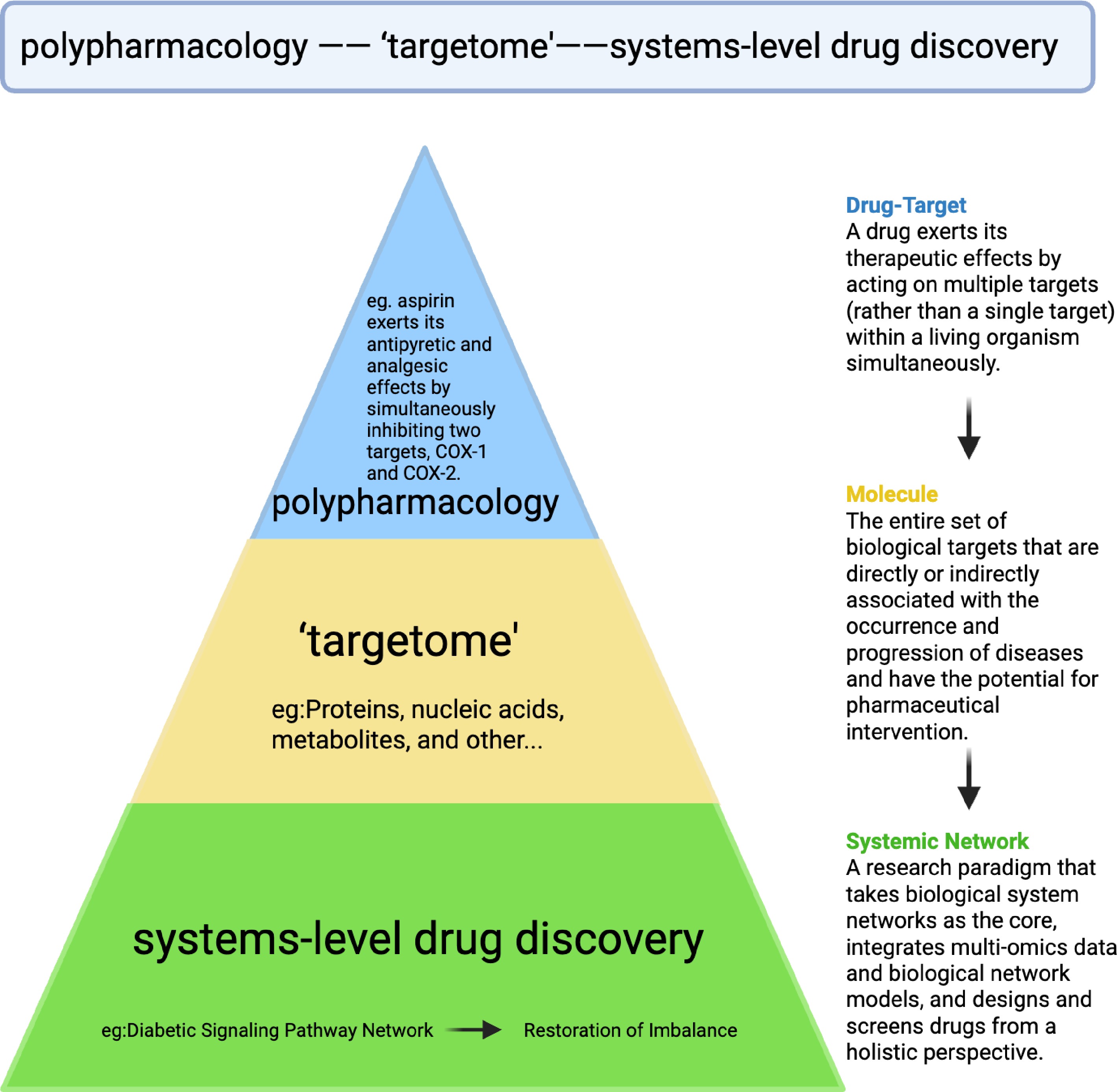
Figure 3.
Schematic illucidation of the relationship between targetome and polypharmacology. Within the triangular systems-level drug discovery framework, 'targetome' represents a much broader concept in comparison with polypharmacology (multitarget drug action, e.g., aspirin on COX-1/2), both of which fit into the system-level strategies in dissecting the disease pathway networks, and holistic drug development.
-
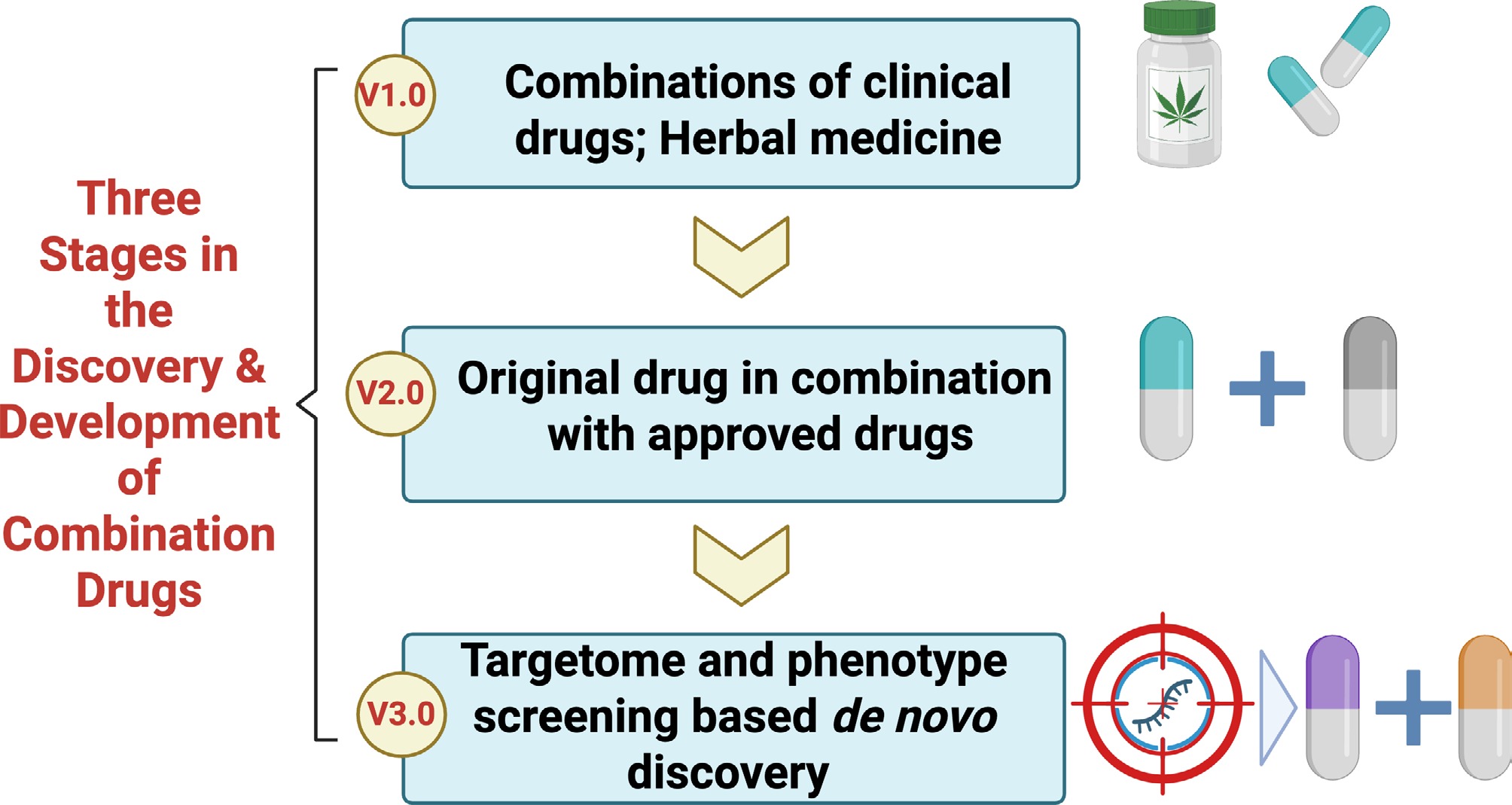
Figure 4.
Proposal of three stages in the discovery and development of combination drugs. Step 1: combinations of clinical drugs and herbal medicine are adopted for the exploration of combination therapies. Step 2: original drugs are combined with approved ones. Step 3: de novo discovery of combination drugs is carried out based on targetome and phenotype screening.
-
Trade name Time to market
(FDA approval)Ingredient Indication Targets Widaplik 2025 Telmisartan, Tmlodipine, Indapamide Hypertension Angiotensin II receptor, L-type calcium channel, NCC Cobenfy 2024 Xanomeline, Trospium chloride Schizophrenia M1, M4 Inavolisib 2024 Palbociclib, Fulvestrant Breast cancer PI3Kα, hormone receptor, HER2 Opdualag 2022 Nivolumab, Relatlimab Metastatic melanoma PD-1, LAG-3 Entresto 2015 Sacubitril, Valsartan Heart failure Neprilysin, AT1 Evotaz 2015 Atazanavir, Cobicistat HIV-1 HIV protease Namzaric 2014 Memantine hydrochloride,
Donepezil hydrochlorideAlzheimer's disease AChE, NMDA receptor Dapagliflozin/metformin 2014 Dapagliflozin, Metformin hydrochloride Type 2 diabetes mellitus SGLT2, AMPK Symbicort 2006 Budesonide, Formoterol fumarate COPD, Asthma GR, β2-AR Table 1.
Summary of clinically approved combination drugs.
-
Name Time to market
(FDA approval)Launch year Indication Targets Ref. Letrozole 1997 2025 Alzheimer's disease APOE, SYT1, TREM2, GFAP [75] Irinotecan 1996 Prazosin 1976 2023 Traumatic
brain injuryα1, α2, β1, β2 [54] Atipamezole 1966 Propranolol 1967 Rapamycin 1999 2024 Neuroblastoma mTOR,
BCR-ABL, Topoisomerase I[28] Dasatinib 2006 Irinotecan 1996 Temozolomide 1999 Navitoclax / 2024 lymphoblastic leukemia MDM2, Bcl-2, Bcl-xL, Bcl-w [9] Idasanutlin / Durvalumab 2017 2024 NSCLC CTLA4, PD-L1 [130] Tremelimumab 2022 Azacitidine 2004 2024 Peripheral T
cell lymphomas (PTCLs)DNMT, HDAC [33] Chidamide / Ipilimumab 2011 2025 Non-clear
cell renal cell cancers (nccRCCs)CTLA-4, PD-1 [10] Nivolumab 2014 Ibrutinib 2013 2025 CNS lymphoma PD-1, BTK [23] Nivolumab 2014 Table 2.
Representative example of combination drugs under clinical trial (2023–2025).
Figures
(4)
Tables
(2)