-
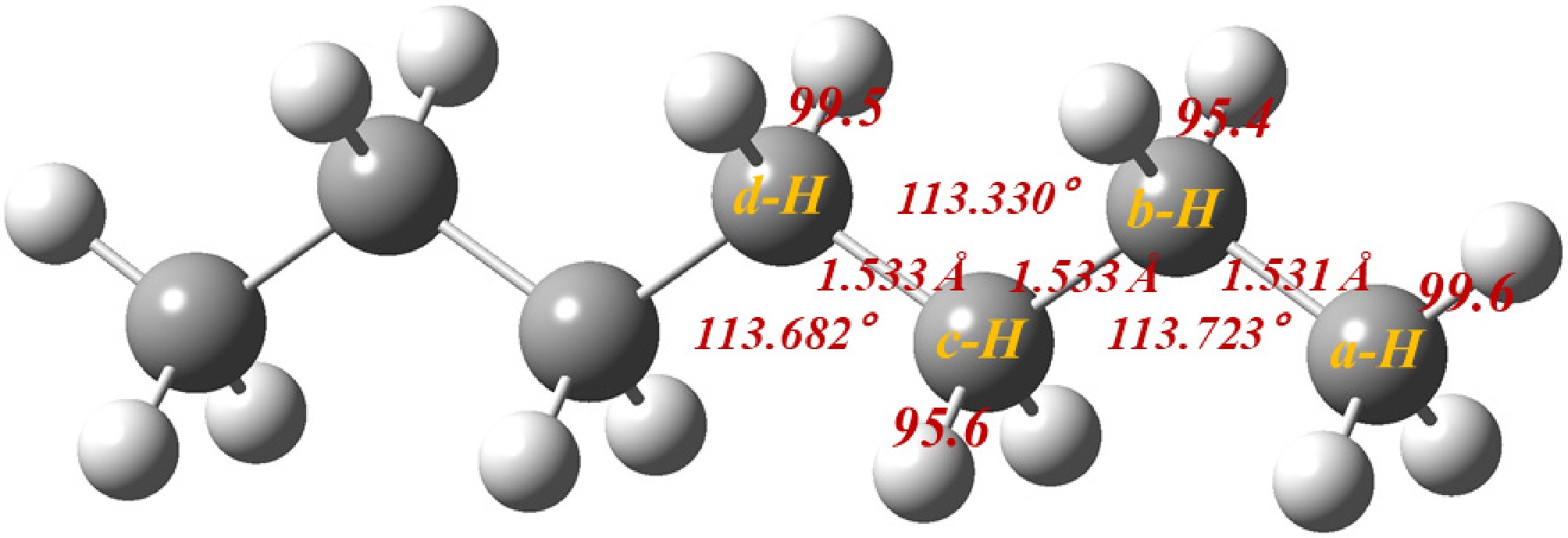
Figure 1.
Optimized n-heptane structure with corresponding BDEs (kcal/mol).
-
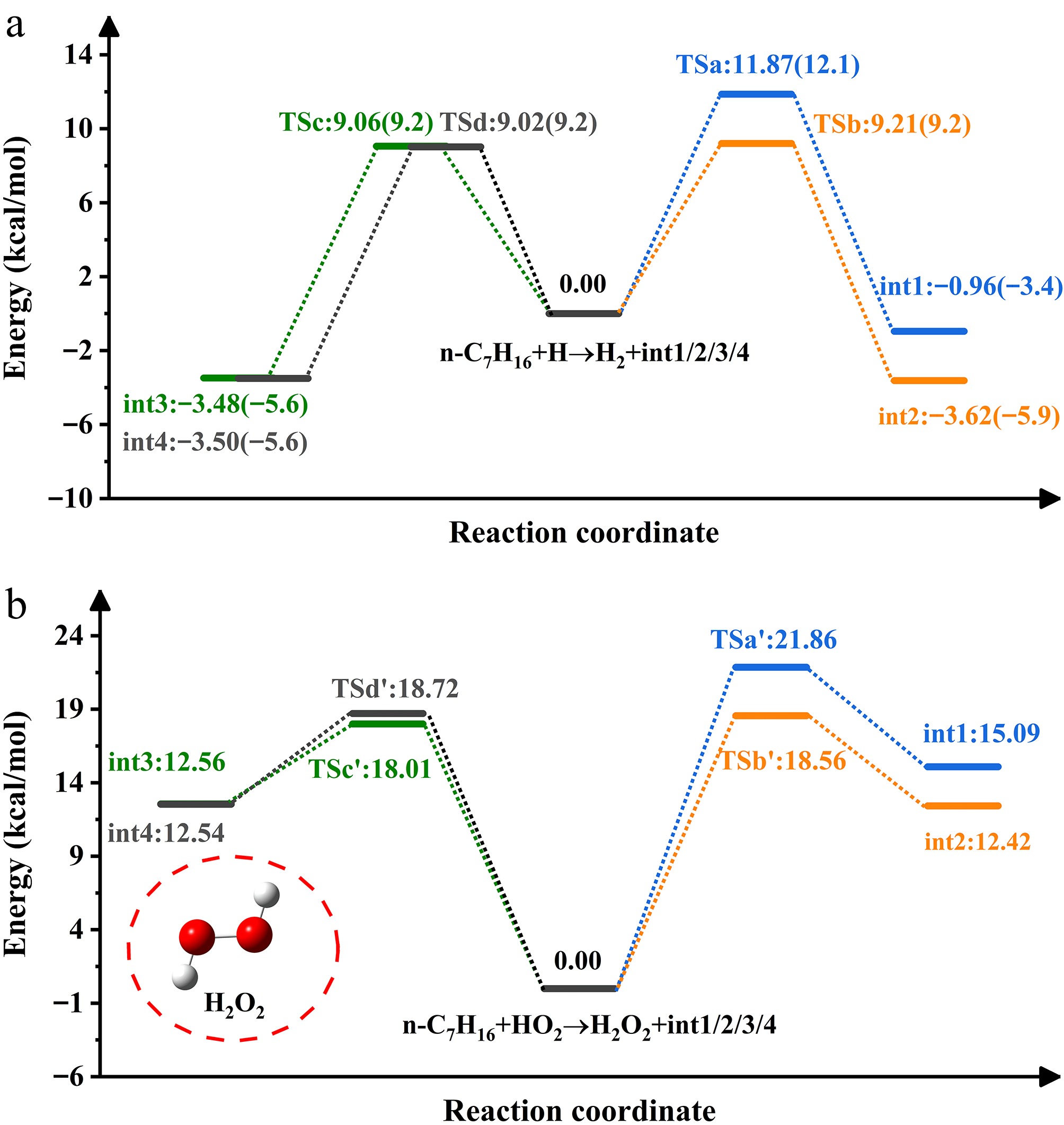
Figure 2.
PES of H- and HO2-initiated H-abstraction from n-heptane.
-
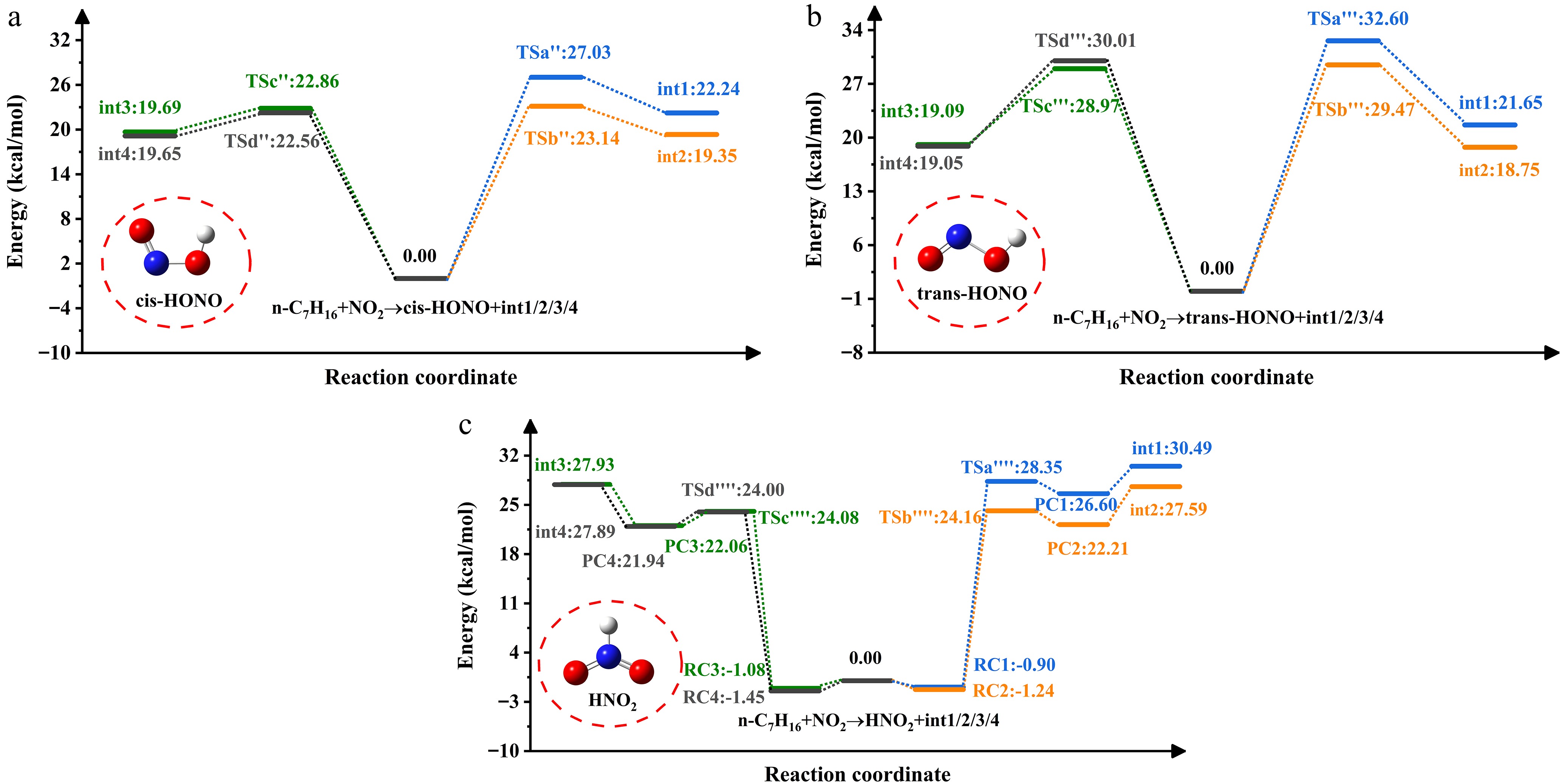
Figure 3.
PES of NO2-initiated H-abstraction from n-heptane.
-
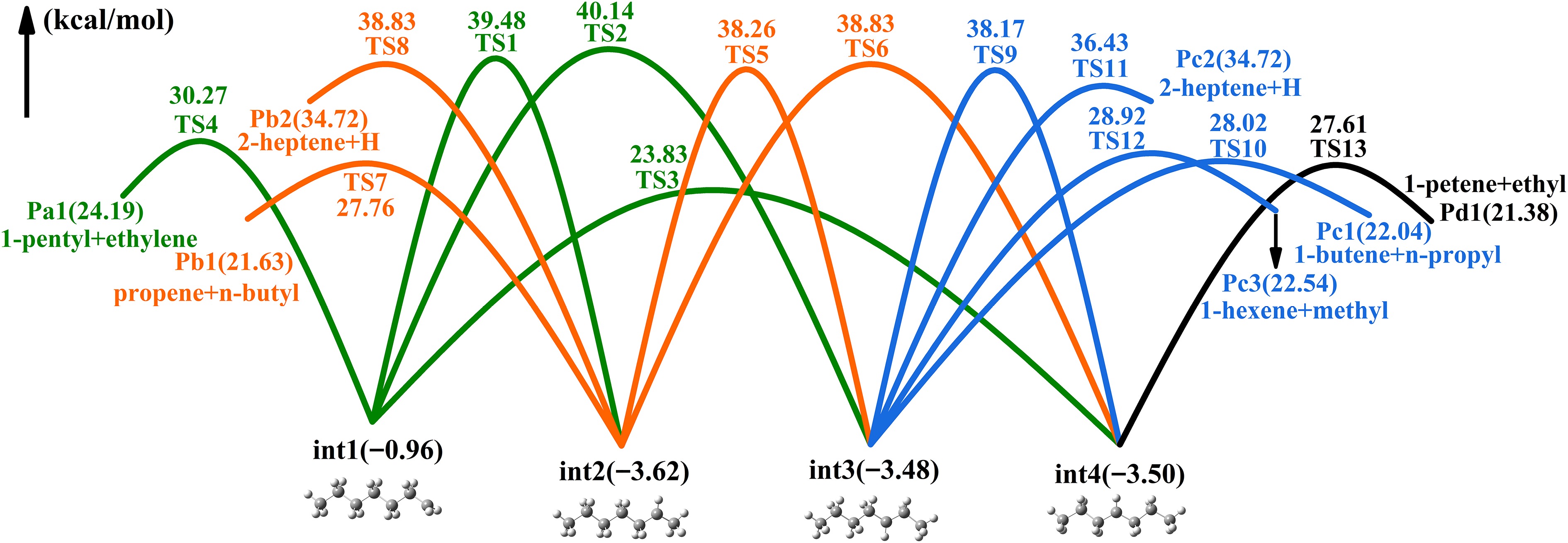
Figure 4.
PES of heptyls' isomerization and decomposition reactions.
-
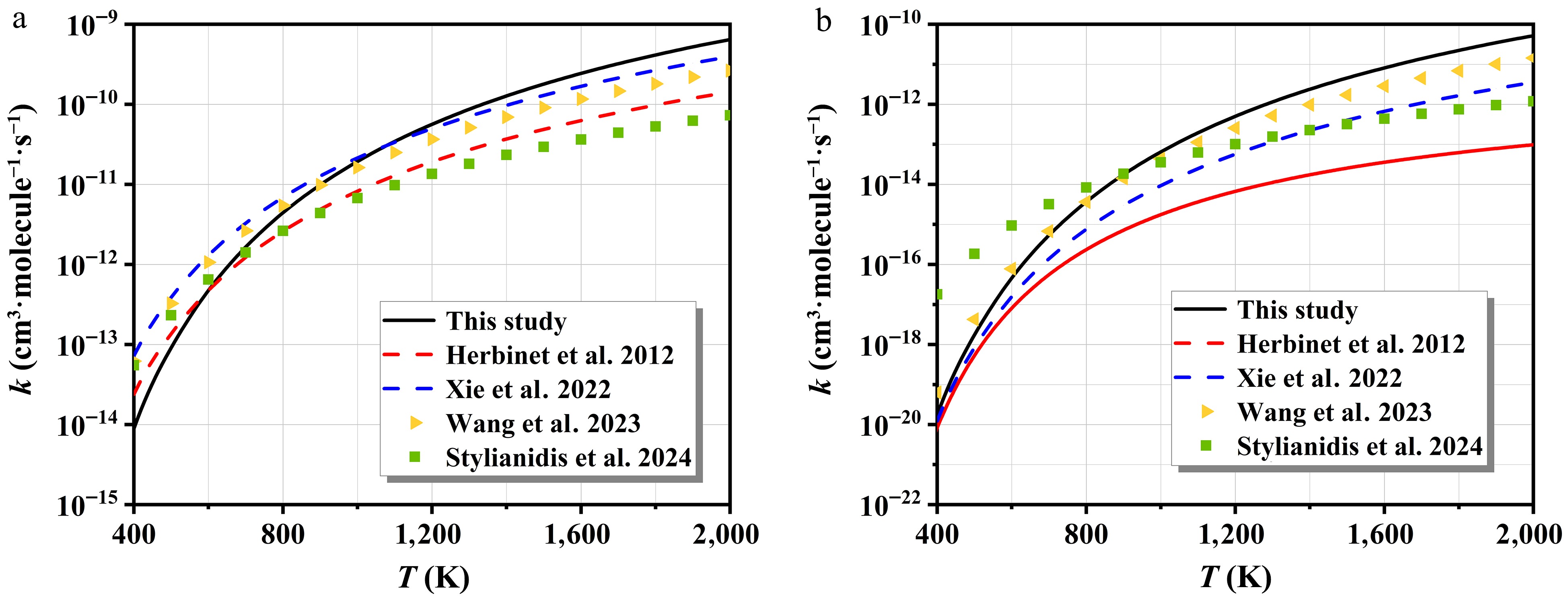
-
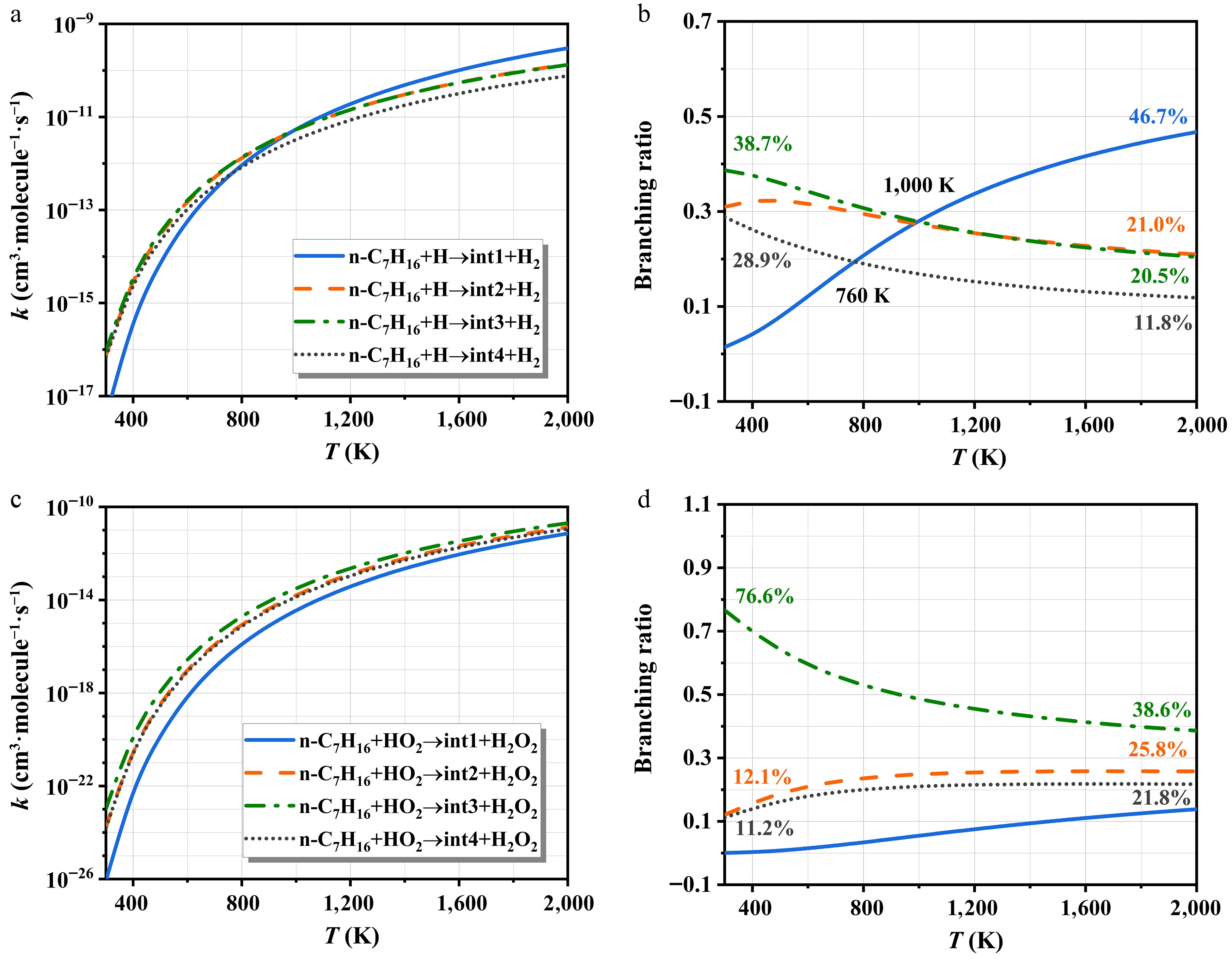
Figure 6.
The rate constants of H- and HO2-initiated H-abstraction and corresponding branching ratios.
-
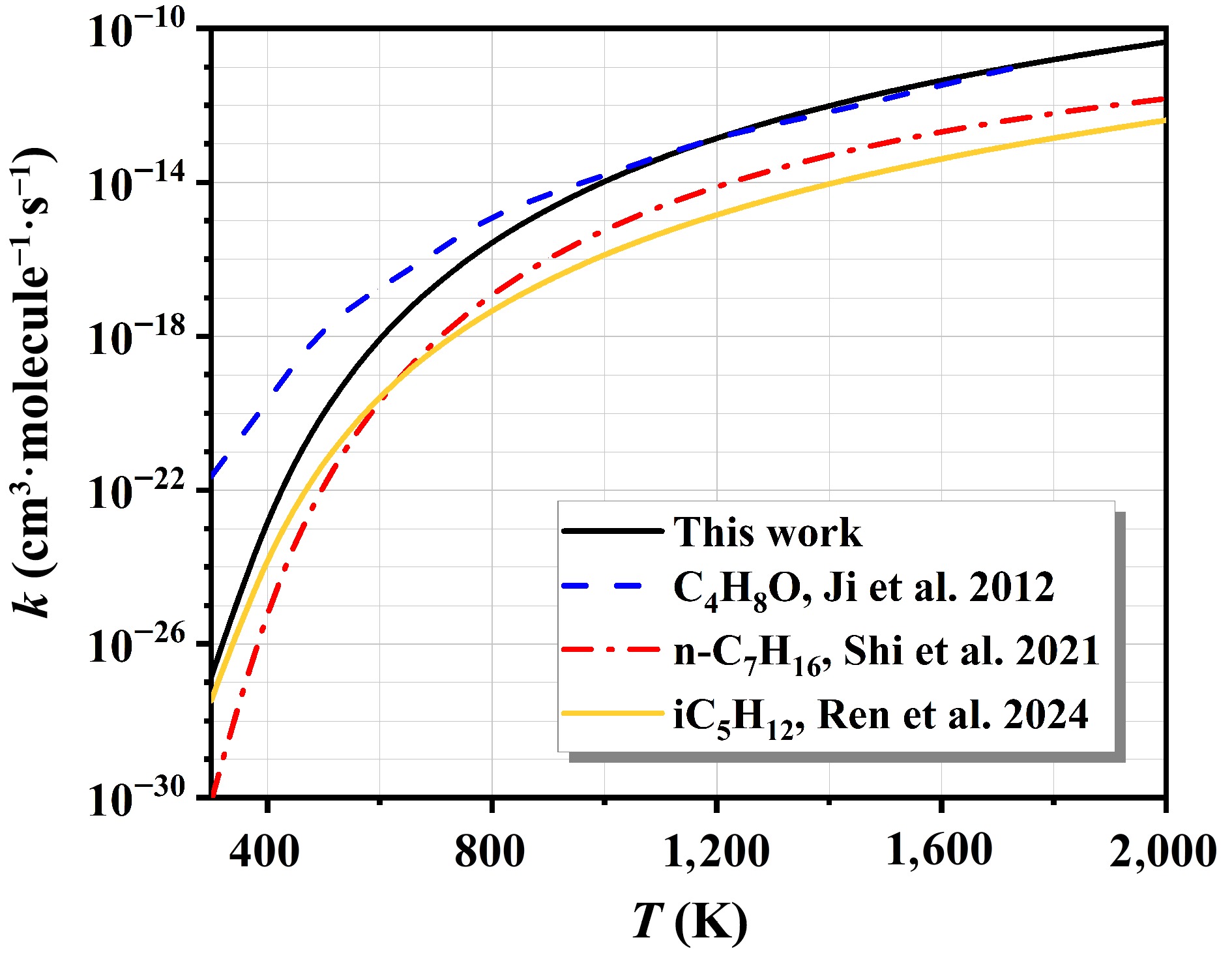
-
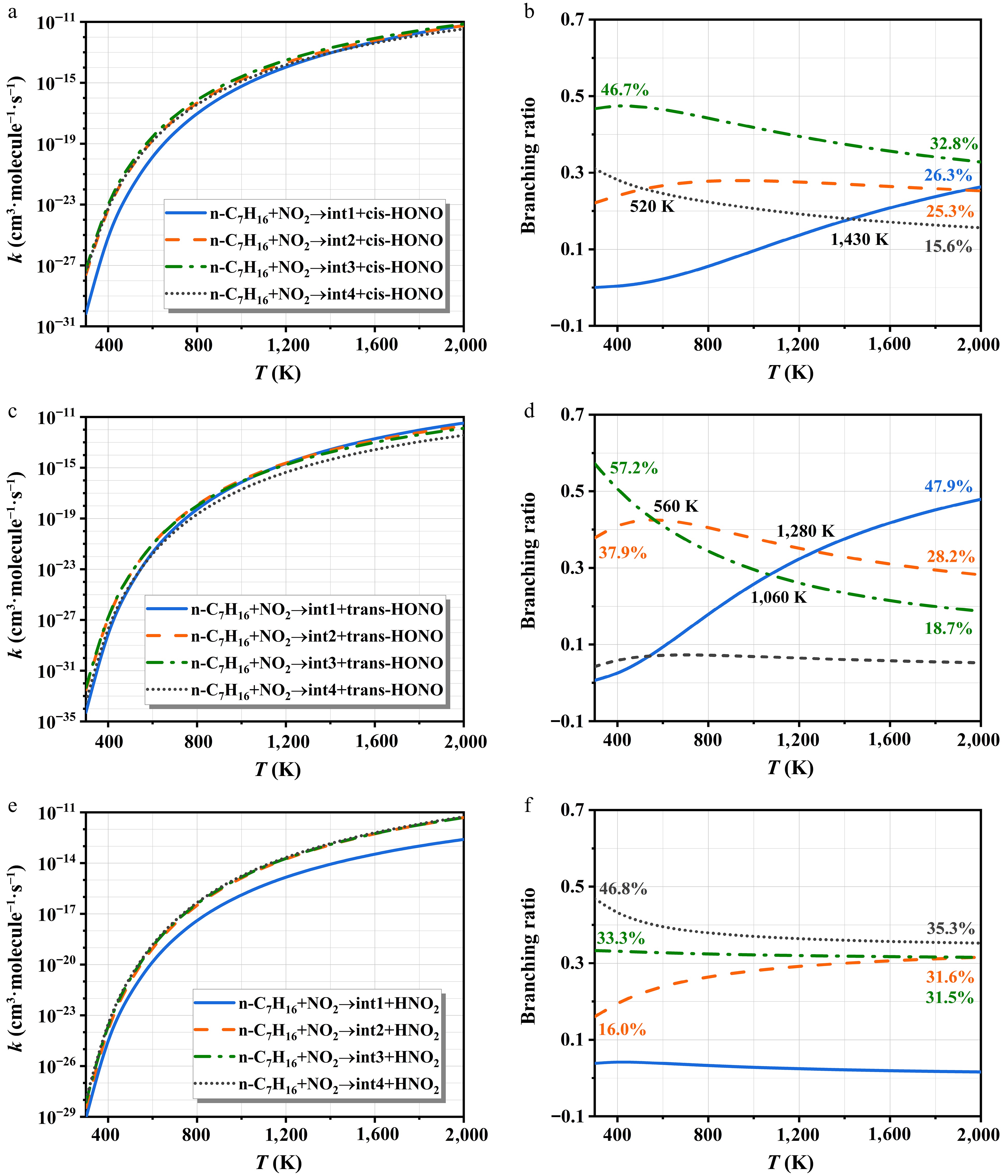
Figure 8.
The rate constants of NO2-initiated H-abstraction and corresponding branching ratios.
-
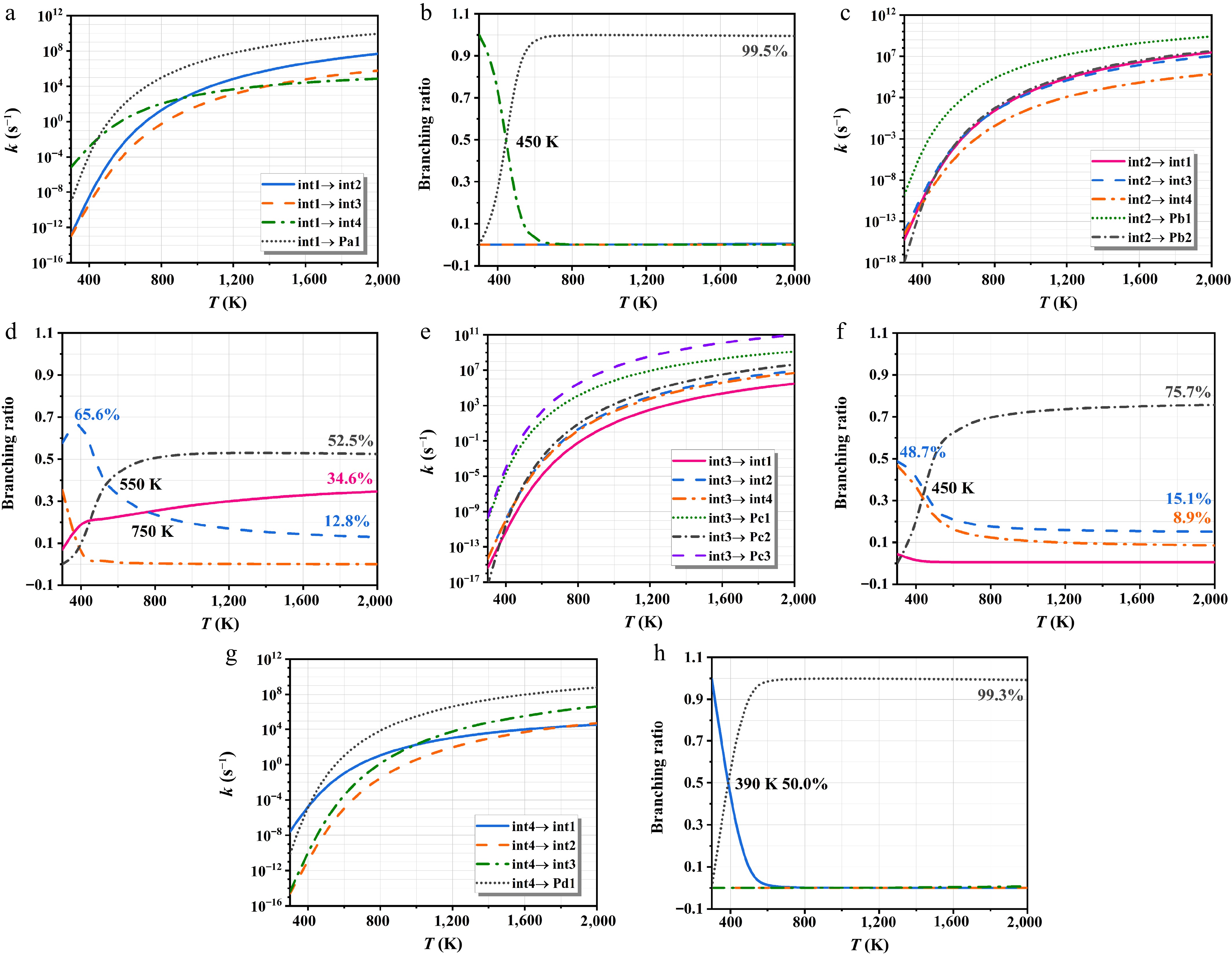
Figure 9.
The rate constants of heptyls' isomerization and decomposition of at HPL.
-
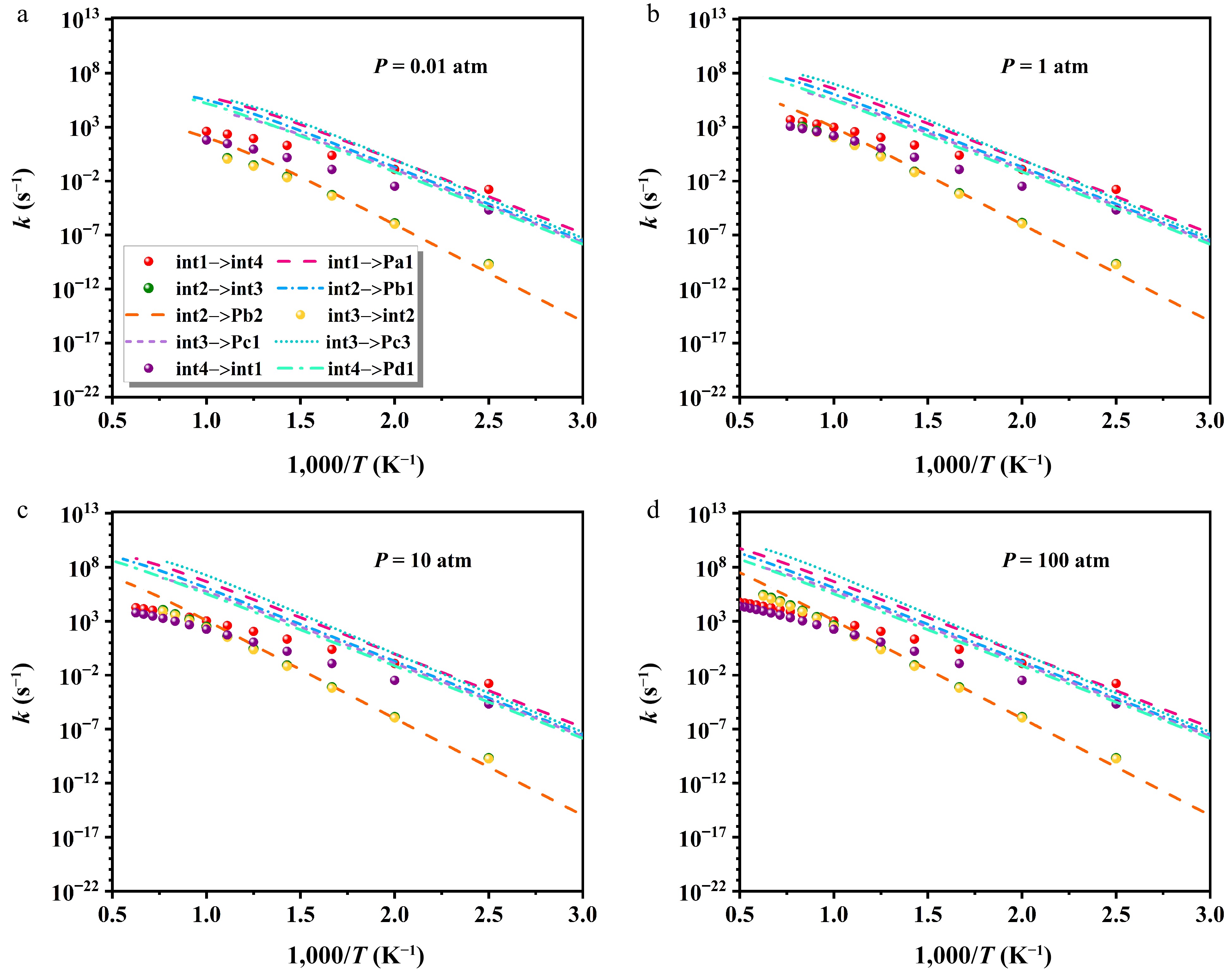
Figure 10.
Impact of varying pressures on isomerization and decomposition rate constants.
-
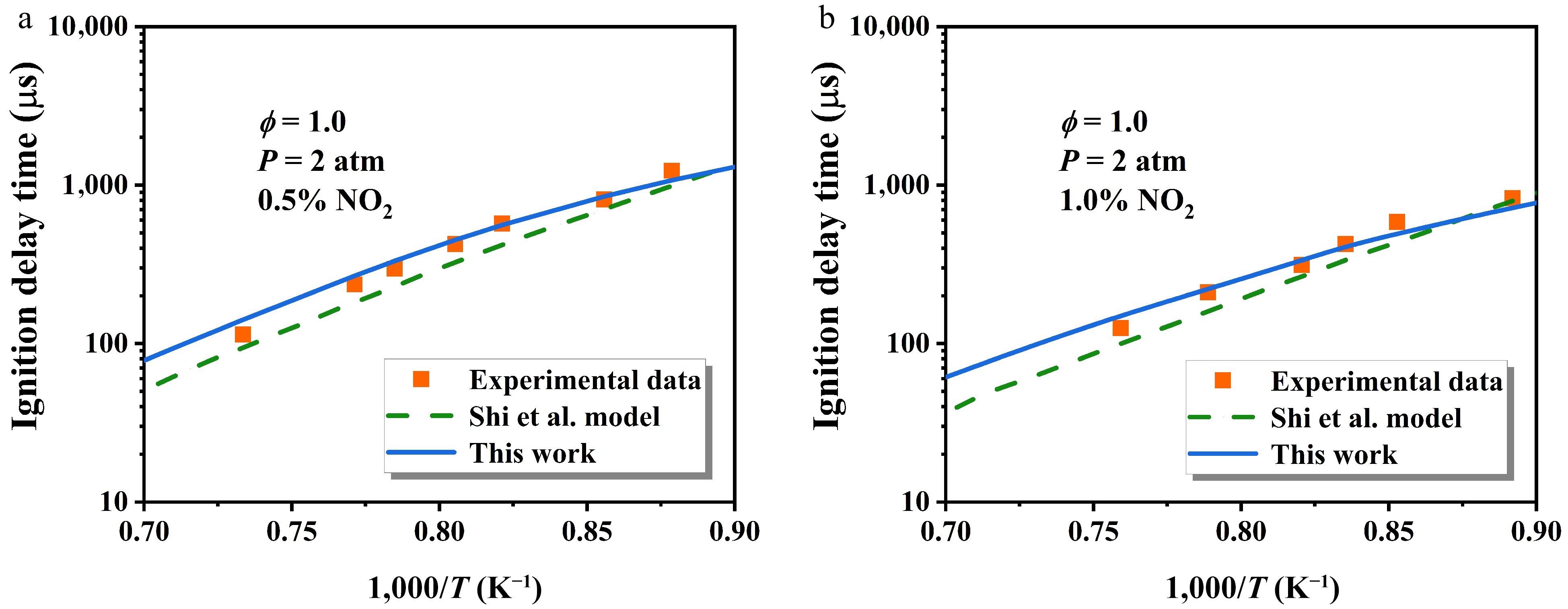
Figure 11.
Comparison of the influence of NO2 addition on n-heptane IDTs at ϕ = 1.0 and P = 2 atm[48]. The symbol, dashed line, and solid line represent experimental data, the original model, and the updated model, respectively.
Figures
(11)
Tables
(0)