-
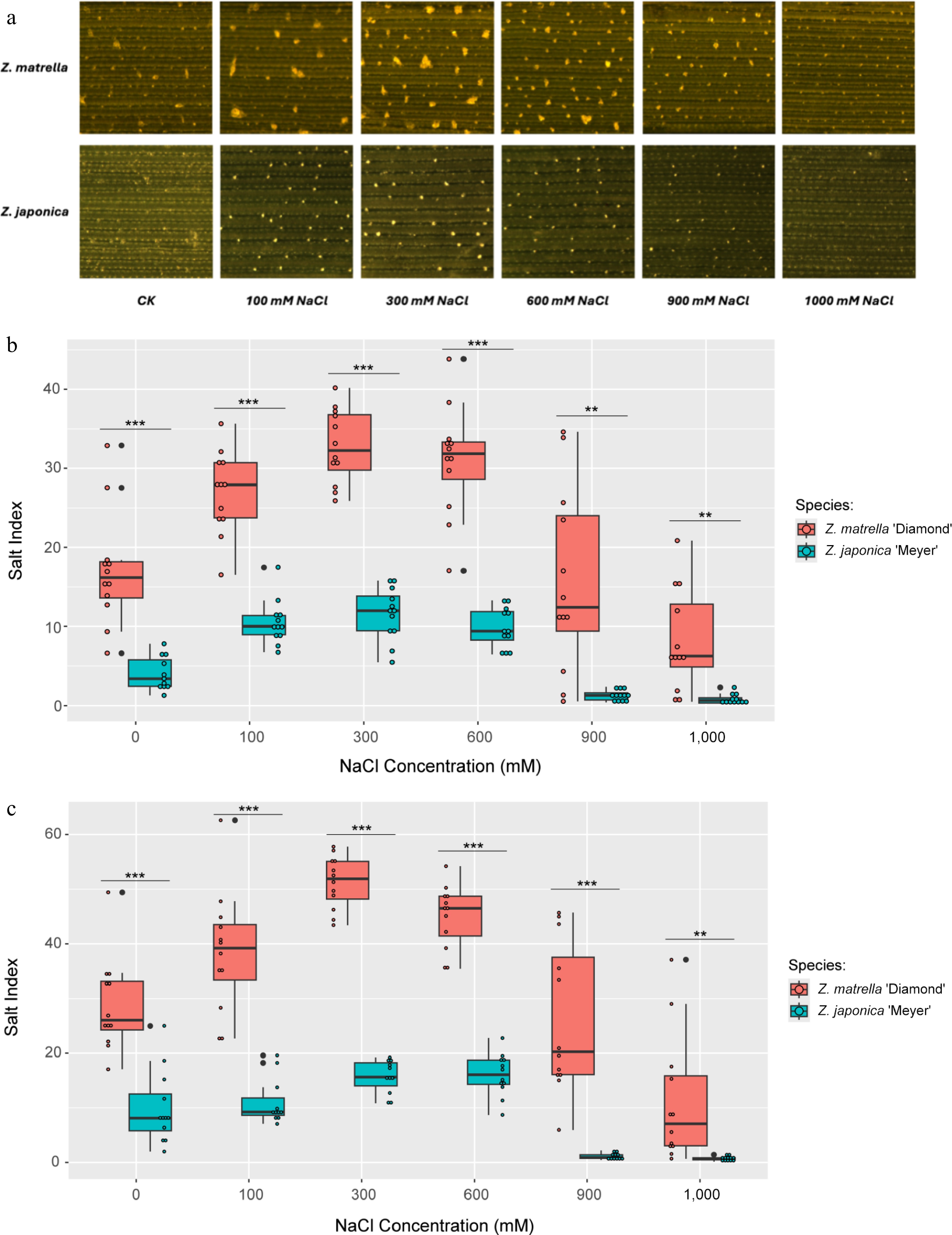
Figure 1.
Salt secretion in Z. matrella and Z. japonica under in vitro conditions with ambient NaCl. (a) Adaxial leaf surface after 24 h of salt treatment, illustrating the quantity of salt secreted. Boxplots comparing salt secretion rates per unit area for the two species measured at (b) 24 h, and (c) 48 h of salt treatment. (* p < 0.05, ** p < 0.01, *** p < 0.001).
-
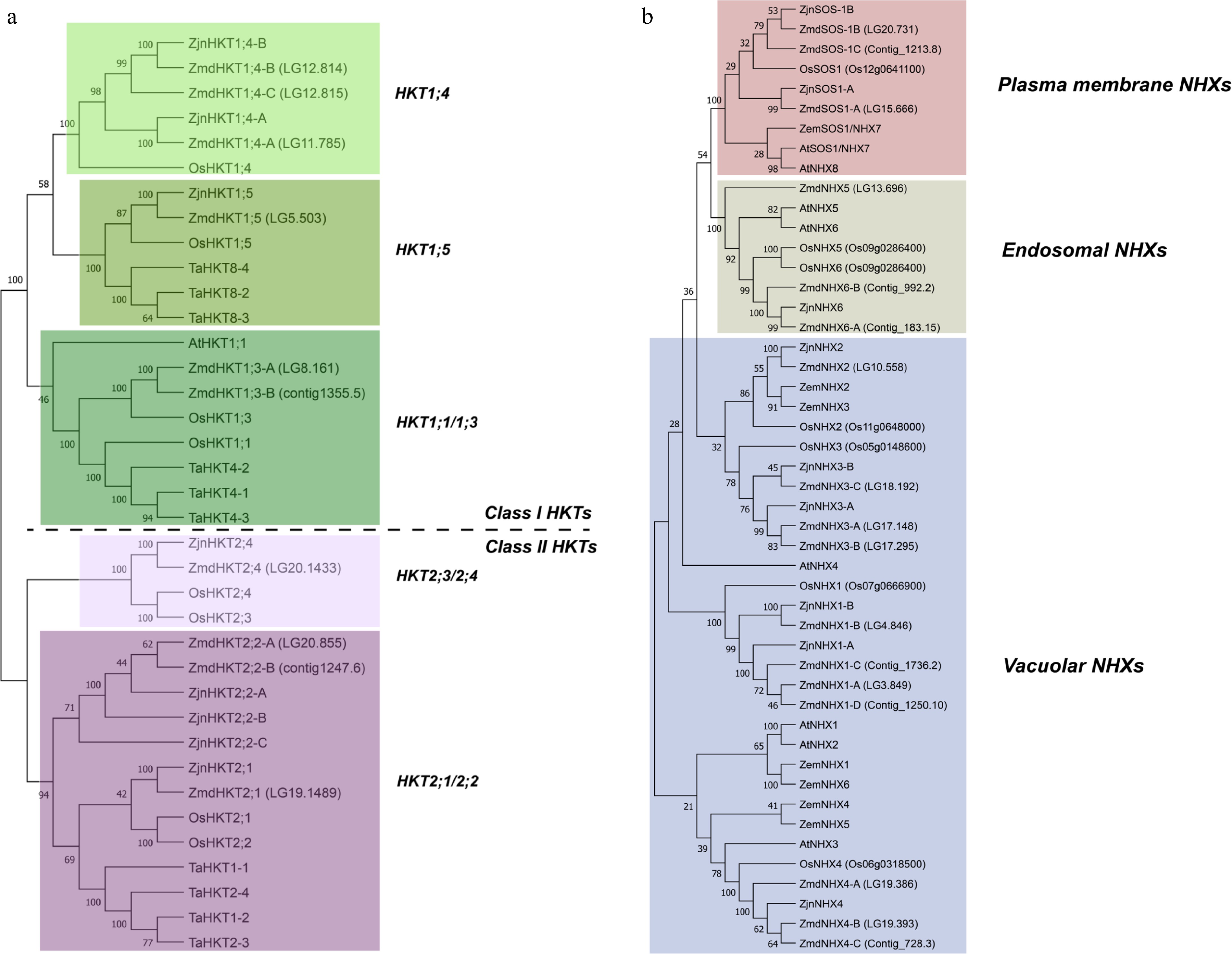
Figure 2.
Phylogenetic analysis of (a) HKT, and (b) NHX gene families. Protein sequences were aligned using the MUSCLE alignment tool, and Neighbor-Joining (NJ) phylogenetic trees were constructed with 1,000 bootstrap replicates using MEGA-11 software. The sequences used in the analysis are listed in Supplementary Tables S2 and S3. Species abbreviations: Zmd, Zoysia matrella cv Diamond; Zjn, Zoysia japonica cv Nagirizaki; At, Arabidopsis thaliana; Os, Oryza sativa; Ta, Triticum aestivum; Zem, Zea mays.
-
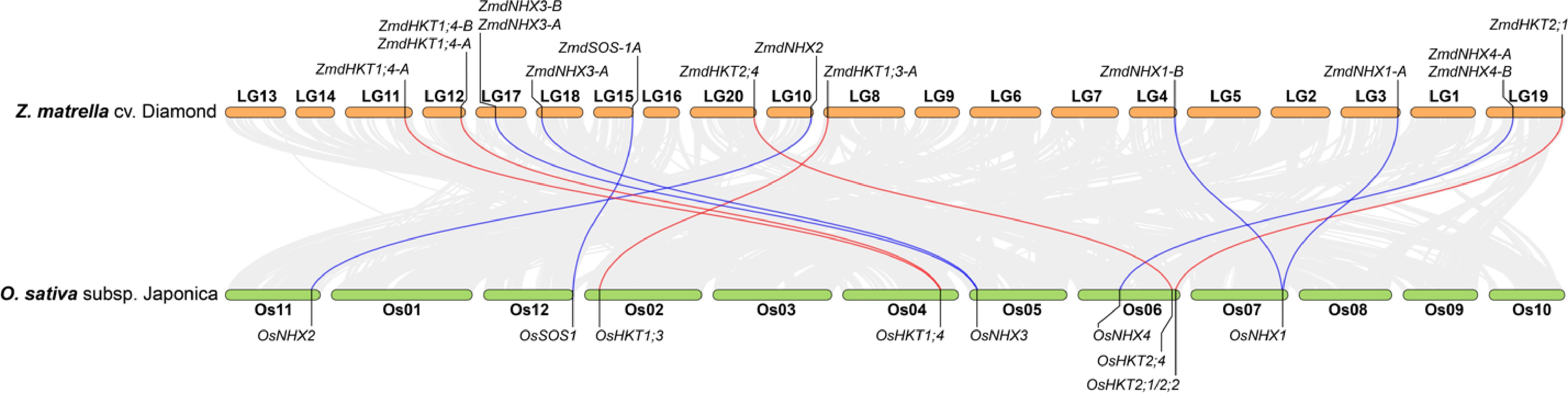
Figure 3.
Genome collinearity analysis between Z. matrella cv. Diamond and O. sativa subsp. japonica, highlighting the relationships of HKT and NHX genes between the two species. Genes located on unanchored contigs in Z. matrella were excluded from the analysis.
-

Figure 4.
Dynamics of relative expression levels of HKT and NHX genes in zoysia leaf blades under in vitro conditions treated with varying NaCl concentrations. (a)–(d) Expression profiles of Z. matrella genes: ZmdHKT1;4-A, ZmdHKT1;4-B, ZmdHKT2;4, and ZmdNHX6-D. (e), (f) Expression profiles of Z. japonica genes: ZjnHKT1;4-A, ZjnHKT1;4-B, ZjnHKT2;4, and ZjnNHX6. Statistical differences among NaCl treatments were analyzed using Tukey's HSD test (p < 0.05).
-
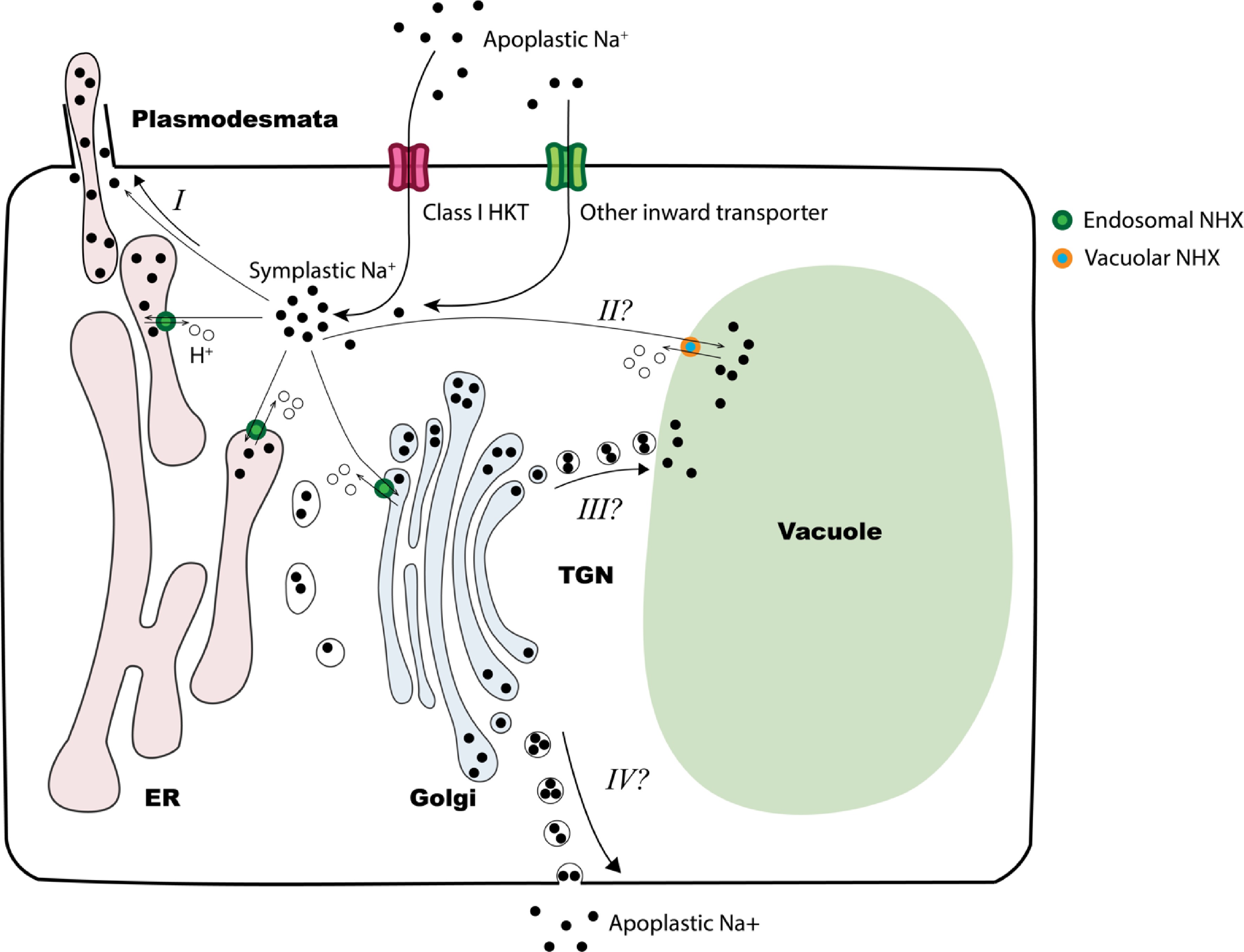
Figure 5.
Proposed model of zoysiagrass response to salinity. Under salinity stress, apoplastic Na+ enters leaf cell through class I HKT transporters or other inward transporters. At high salinity stress levels, the cytosol may act as a temporary sink for Na+. The accumulated Na+ can be loaded to vascular system (not depicted in the figure), transported into endomembrane system via endosomal NHX transporters, or sequestered into the vacuole by vacuolar NHXs (option II). Excess Na+ within endomembrane system may be transported to salt glands via plasmodesmata (option I), to the vacuole (option III) or excreted back into the apoplast (option IV). In Z. matrella, the upregulation of endosomal NHXs under high levels of salinity stress suggests that options III and IV may serve as alternative responses to salt stress.
-
Transporter Species Gene ID Gene name Type Chr/contig Start End Protein High-affinity K+ transporters (HKTs) Zoysia matrella evm.TU.chrLG5.503 ZmdHKT1;5 Class I HKT LG5 1E+07 1E+07 399 evm.TU.chrLG8.161 ZmdHKT1;3-A LG8 1E+06 1E+06 694 evm.TU.chrLG11.785 ZmdHKT1;4-A LG11 2E+07 2E+07 856 evm.TU.chrLG12.814 ZmdHKT1;4-B LG12 1E+07 1E+07 966 evm.TU.chrLG12.815 ZmdHKT1;4-C LG12 1E+07 1E+07 613 evm.TU.contig_1355.5 ZmdHKT1;3-B Contig1355 47032 60625 1369 evm.TU.chrLG19.1489 ZmdHKT2;1 Class II HKT LG19 3E+07 3E+07 447 evm.TU.chrLG20.855 ZmdHKT2;2-A LG20 2E+07 2E+07 541/4871 evm.TU.chrLG20.1433 ZmdHKT2;4 LG20 2E+07 2E+07 519 evm.TU.contig_1247.6 ZmdHKT2;2-B Contig1247 40514 42319 541 Zoysia japonica Zjn_sc00004.1.g09630 ZjnHKT1;4-A Class I HKT Zjn_sc00004.1 420993 4E+06 925 Zjn_sc00011.1.g08720 ZjnHKT1;5 Zjn_sc00011.1 5E+06 5E+06 479 Zjn_sc00023.1.g02580 ZjnHKT1;4-B Zjn_sc00023.1 1E+06 1E+06 672 Zjn_sc00008.1.g01340 ZjnHKT2;4 Class II HKT Zjn_sc00008.1 560759 566679 876 Zjn_sc00068.1.g02380 ZjnHKT2;1 Zjn_sc00068.1 1E+06 1E+06 420 Zjn_sc00068.1.g02390 ZjnHKT2;2-C Zjn_sc00068.1 1E+06 1E+06 253 Zjn_sc00107.1.g01070 ZjnHKT2;2-A Zjn_sc00107.1 564336 566416 436 Zjn_sc00107.1.g01080 ZjnHKT2;2-B Zjn_sc00107.1 570341 571277 290 Na+/K+ antiporters (NHXs) Zoysia matrella evm.TU.chrLG13.696 ZmdNHX5 Endosomal NHX LG13 2E+07 2E+07 265 evm.TU.contig_183.15 ZmdNHX6-A Contig183 324272 333257 553 evm.TU.contig_992.2 ZmdNHX6-B Contig992 27765 37237 530 evm.TU.chrLG15.666 ZmdSOS1-A Plasma membrane NHX LG15 1E+07 1E+07 1144/10461 evm.TU.chrLG20.731 ZmdSOS1-B LG20 1E+07 1E+07 1154/10571 evm.TU.contig_1213.8 ZmdSOS1-C Contig1213 59774 68887 975 evm.TU.chrLG3.849 ZmdNHX1-A Vacuolar NHX LG3 2E+07 2E+07 540/3911 evm.TU.chrLG4.846 ZmdNHX1-B LG4 2E+07 2E+07 429/4013 evm.TU.chrLG10.558 ZmdNHX2 LG10 1E+07 1E+07 544 evm.TU.chrLG17.148 ZmdNHX3-A LG17 2E+06 2E+06 811 evm.TU.chrLG17.295 ZmdNHX3-B LG17 6E+06 6E+06 392 evm.TU.chrLG18.192 ZmdNHX3-C LG18 2E+06 2E+06 583 evm.TU.chrLG19.386 ZmdNHX4-A LG19 9E+06 9E+06 440 evm.TU.chrLG19.393 ZmdNHX4-B LG19 9E+06 9E+06 523 evm.TU.contig_738.3 ZmdNHX4-C Contig738 15126 19095 524/448/395/4444 evm.TU.contig_1250.10 ZmdNHX1-D Contig1250 58727 63951 540/391/4582 evm.TU.contig_1736.2 ZmdNHX1-C Contig1736 7 4443 429 Zoysia japonica Zjn_sc00085.1.g00950 ZjnNHX6 Endosomal NHX Zjn_sc00085.1 966404 976204 634 Zjn_sc00035.1.g02750 ZjnSOS1-B Plasma membrane NHX Zjn_sc00035.1 3E+06 3E+06 1057 Zjn_sc00105.1.g00050 ZjnSOS1-A Zjn_sc00105.1 32266 47024 1073 Zjn_sc00001.1.g01510 ZjnNHX-1A Vacuolar NHX Zjn_sc00001.1 634926 639121 540 Zjn_sc00016.1.g04350 ZjnNHX3-B Zjn_sc00016.1 3E+06 3E+06 525 Zjn_sc00044.1.g04210 ZjnNHX1-B Zjn_sc00044.1 2E+06 2E+06 546 Zjn_sc00096.1.g00980 ZjnNHX2 Zjn_sc00096.1 445565 450176 452 Zjn_sc00146.1.g00380 ZjnNHX4 Zjn_sc00146.1 294475 297777 245 Zjn_sc00166.1.g00230 ZjnNHX3-A Zjn_sc00166.1 148408 157386 507 1Two isoforms; 2three isoforms; 3five isoforms: one isoform of 429 aa and four isoforms of 401 aa; 4six isoforms: three isoforms of 395 aa, one isoform of 528 aa, one isoform of 448 aa, one isoform of 444 aa. Table 1.
Summary of HKT and NHX genes in Zoysia matrella and Zoysia japonica.
Figures
(5)
Tables
(1)