-
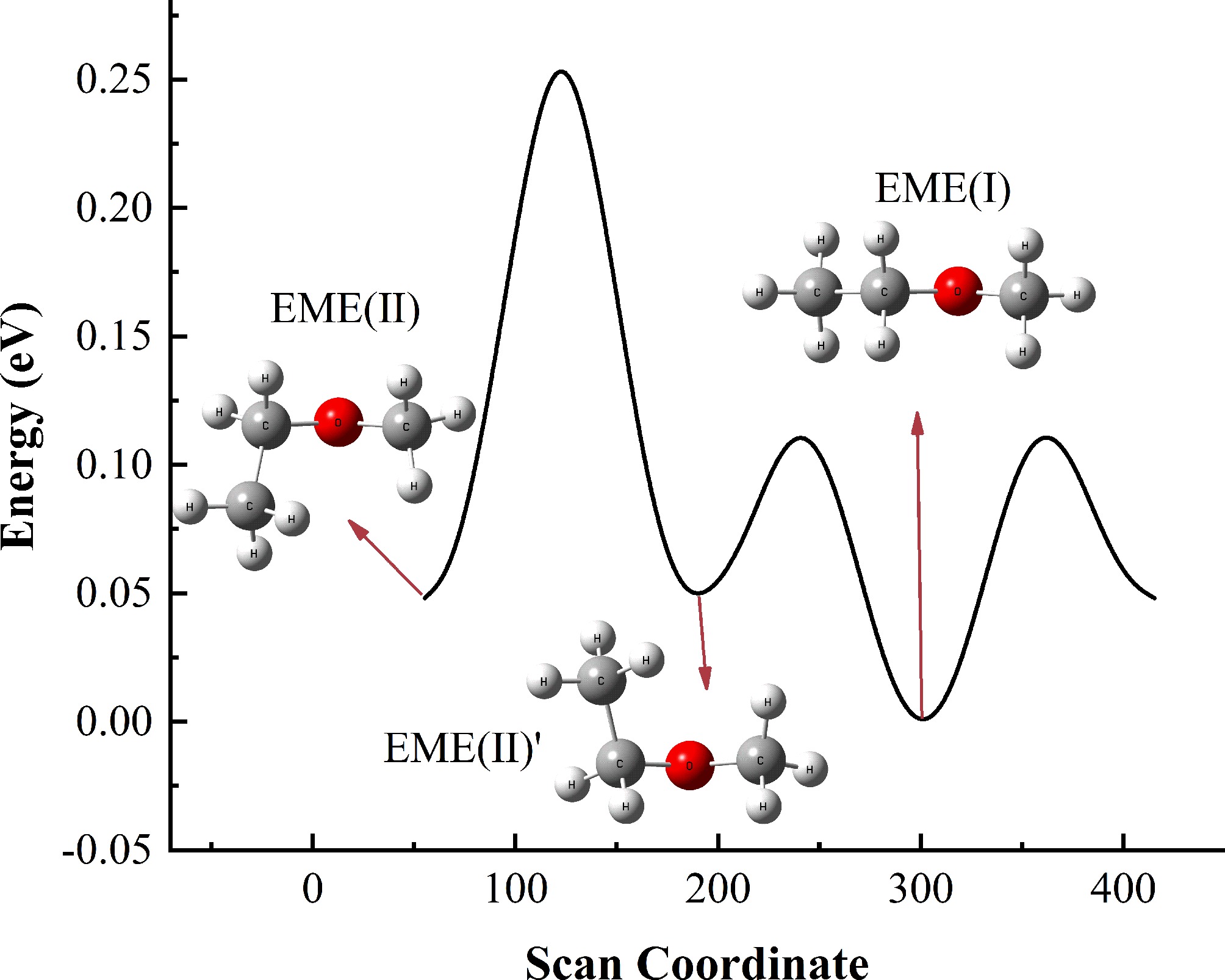
Figure 1.
Potential energy scan of EME conformers, with energies referenced to EME(I).
-
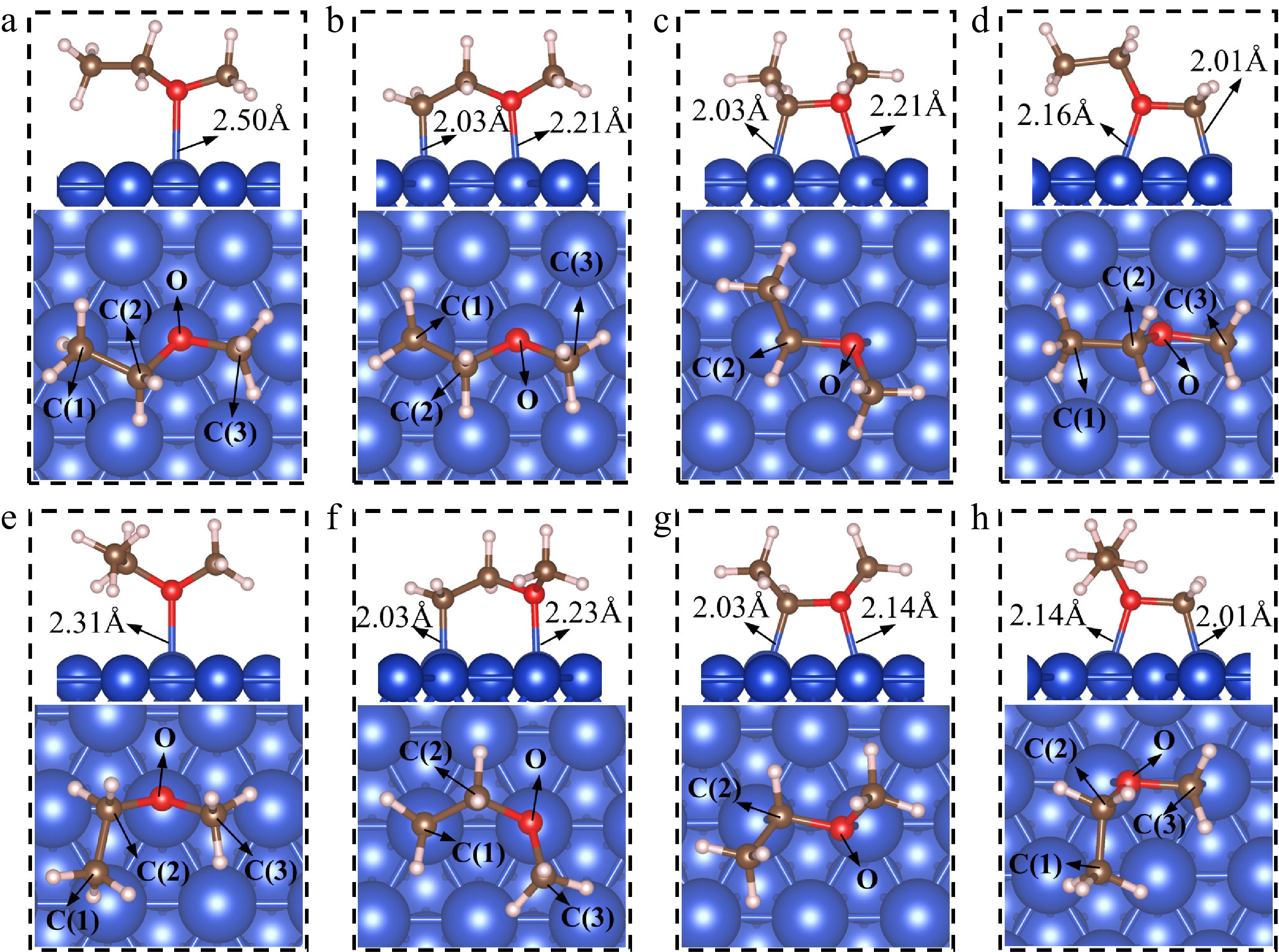
Figure 2.
The adsorption structures of EME(I)* and EME(II)*, and their dehydrogenation products. (a) EME(I)*; (b) CH2CH2OCH3(I)*; (c) CH3CHOCH3(I)*; (d) CH3CH2OCH2(I)*; (e) EME(II)*; (f) CH2CH2OCH3(II)*; (g) CH3CHOCH3(II)*; (h) CH3CH2OCH2(II)*.
-
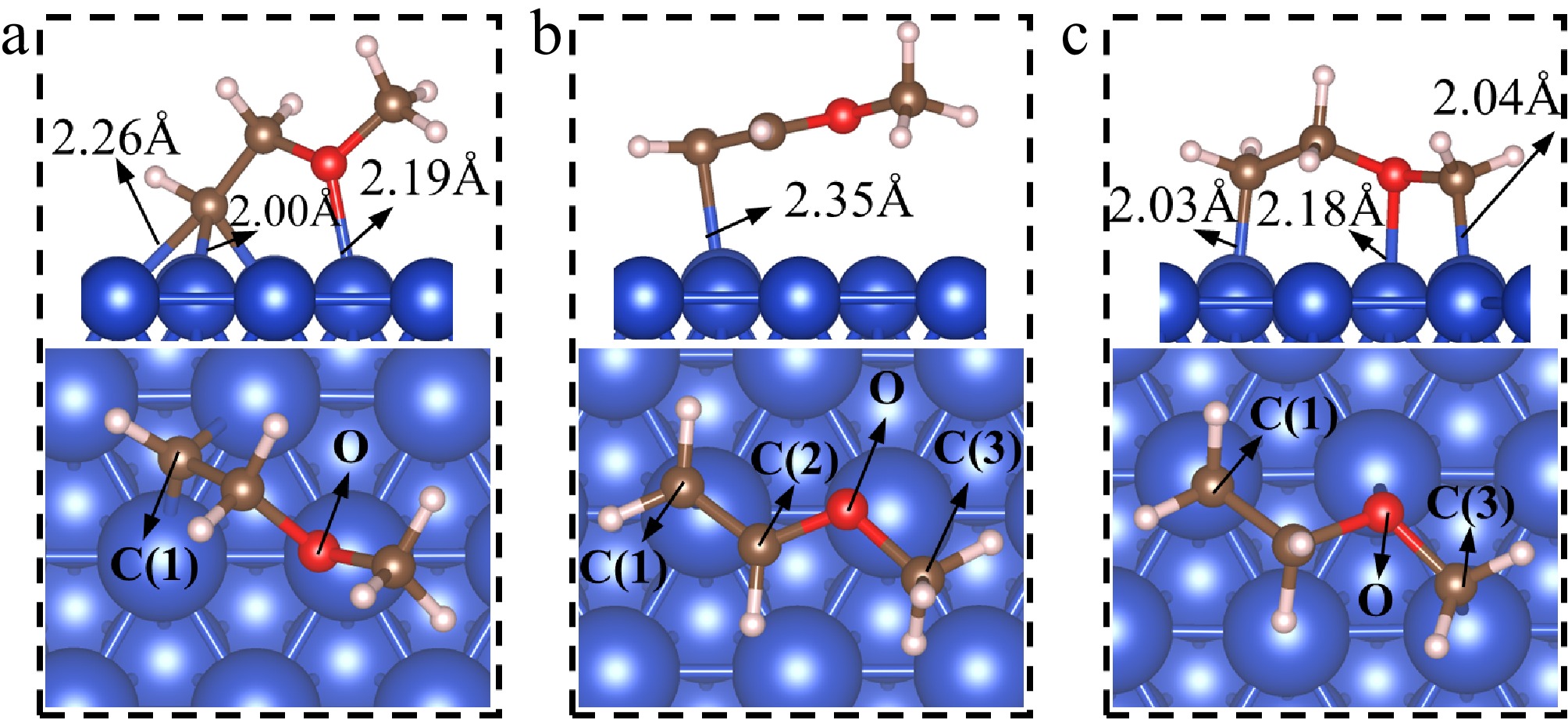
Figure 3.
The adsorption structures of dehydrogenation products of CH2CH2OCH3(I)*. (a) CHCH2OCH3*; (b) CH2CHOCH3*; (c) CH2CH2OCH2*.
-
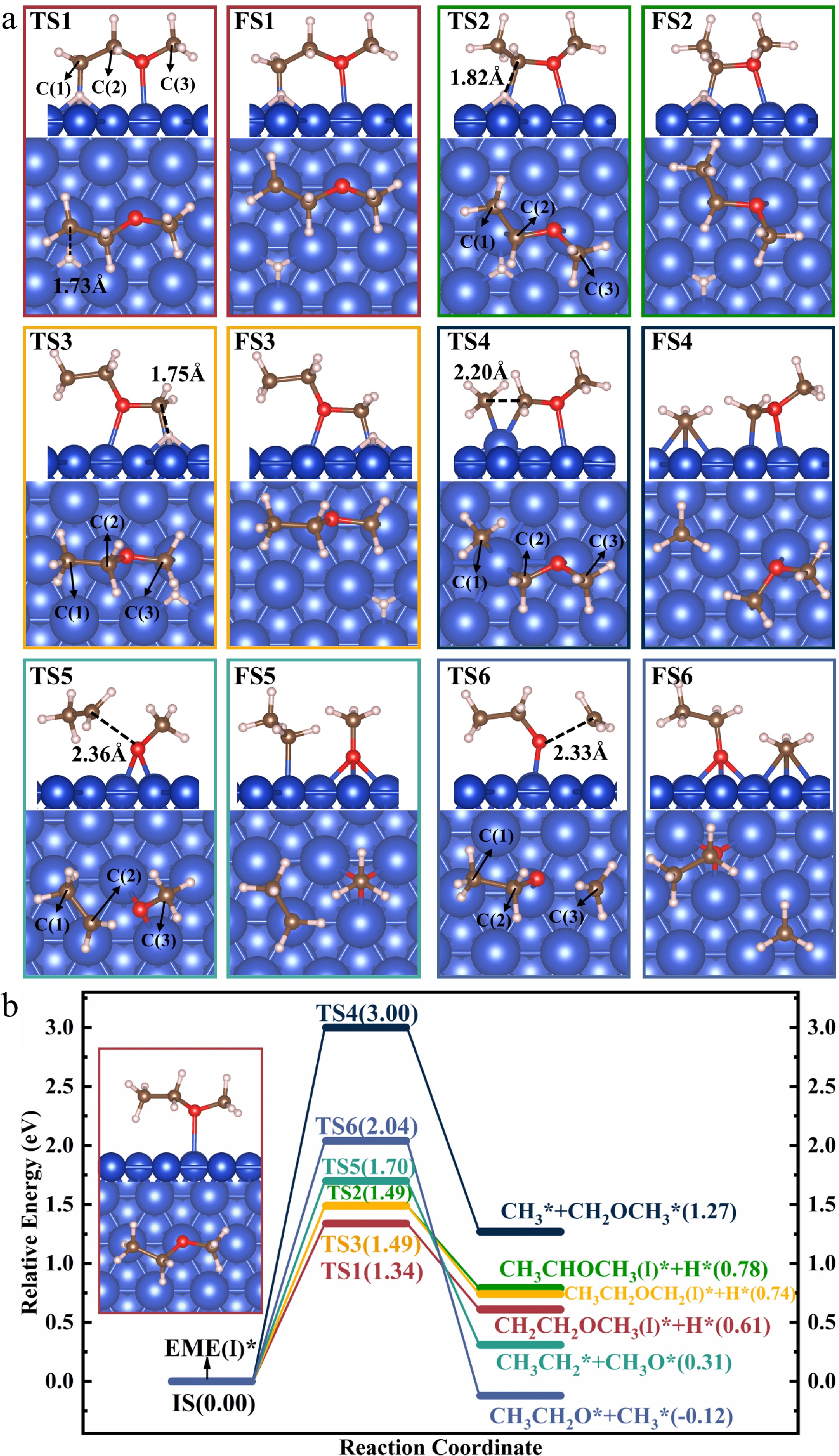
Figure 4.
Structures of the (a) TSs and FSs, and energy profiles with (b) IS structure for the decomposition of EME(I)*.
-
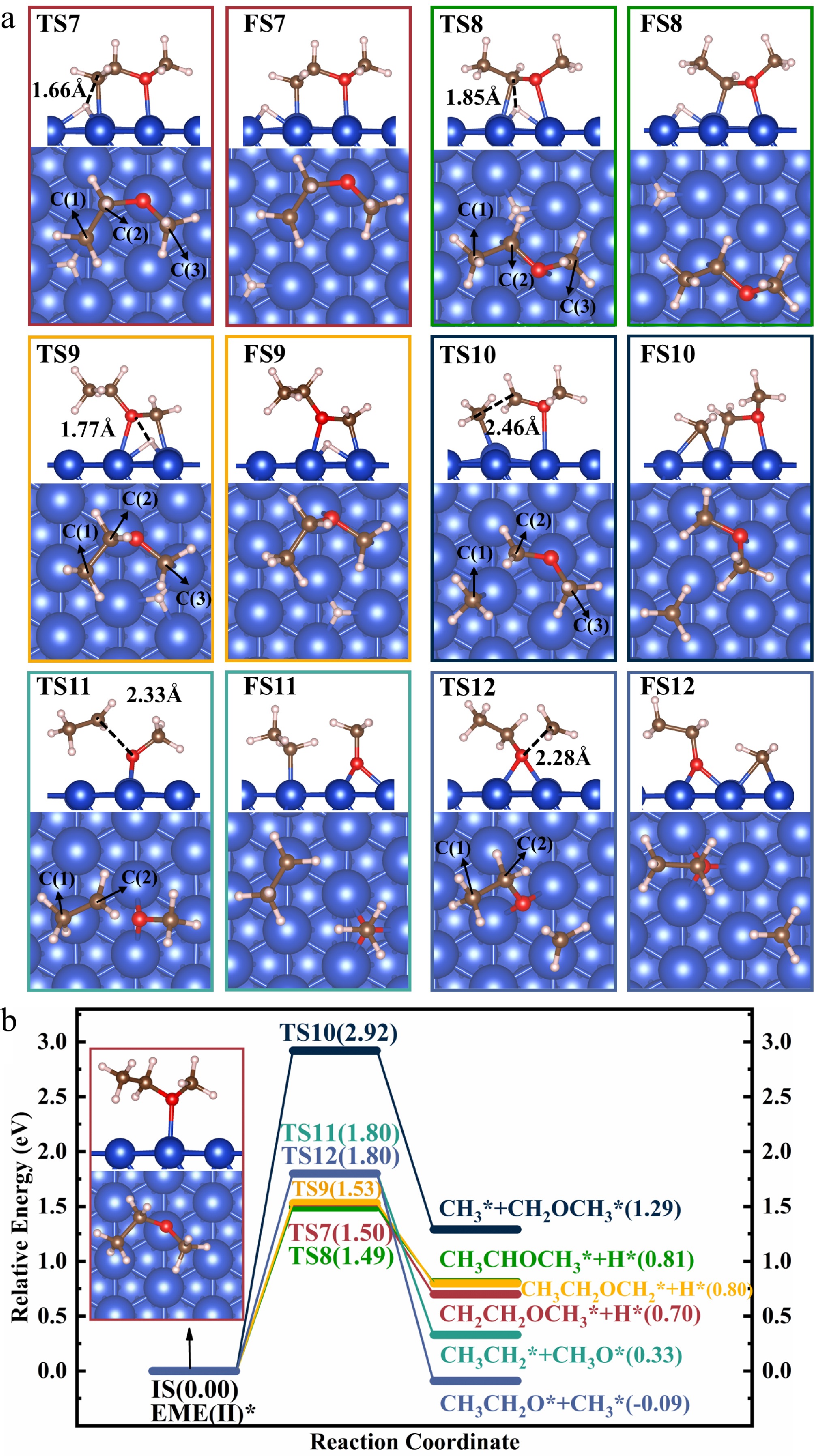
Figure 5.
Structures of (a) the TSs and FSs, and (b) energy profiles with IS structure for the decomposition of EME(II)*.
-
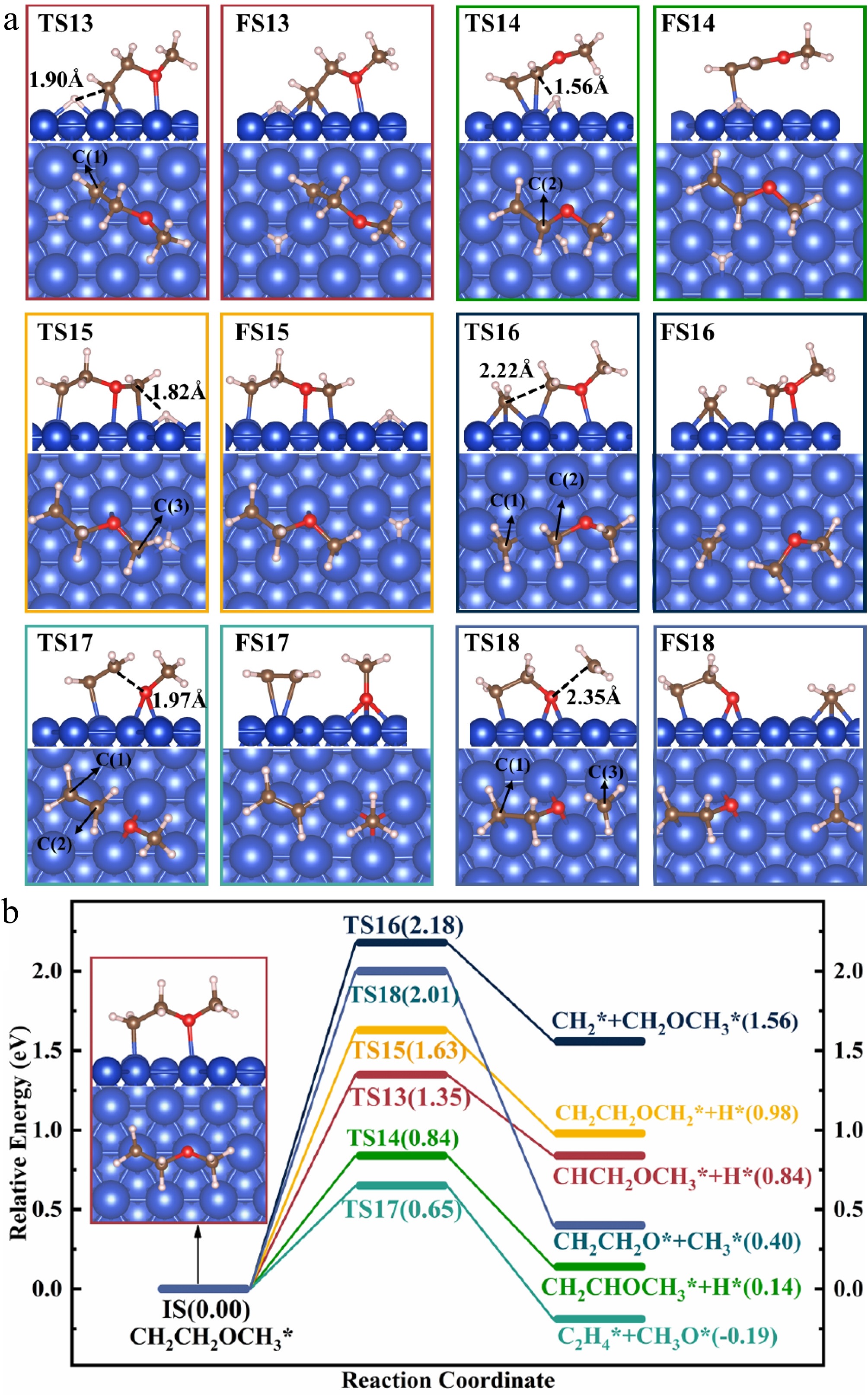
Figure 6.
Structures of (a) the TSs and FSs, and (b) energy profiles with IS structure for the decomposition of CH2CH2OCH3(I)*.
-
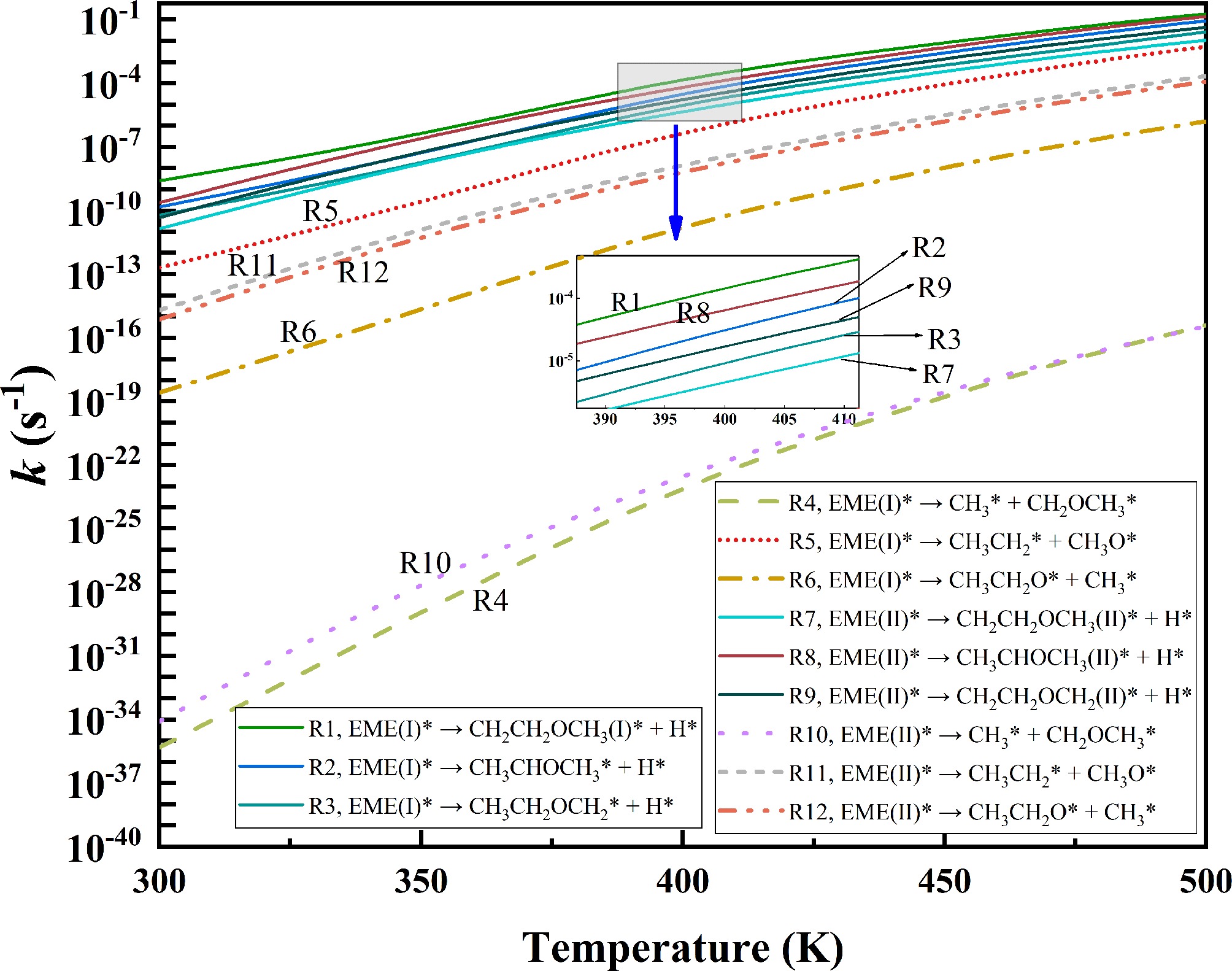
Figure 7.
Rate constants for the initial decomposition of EME(I)* and EME(II)*.
-
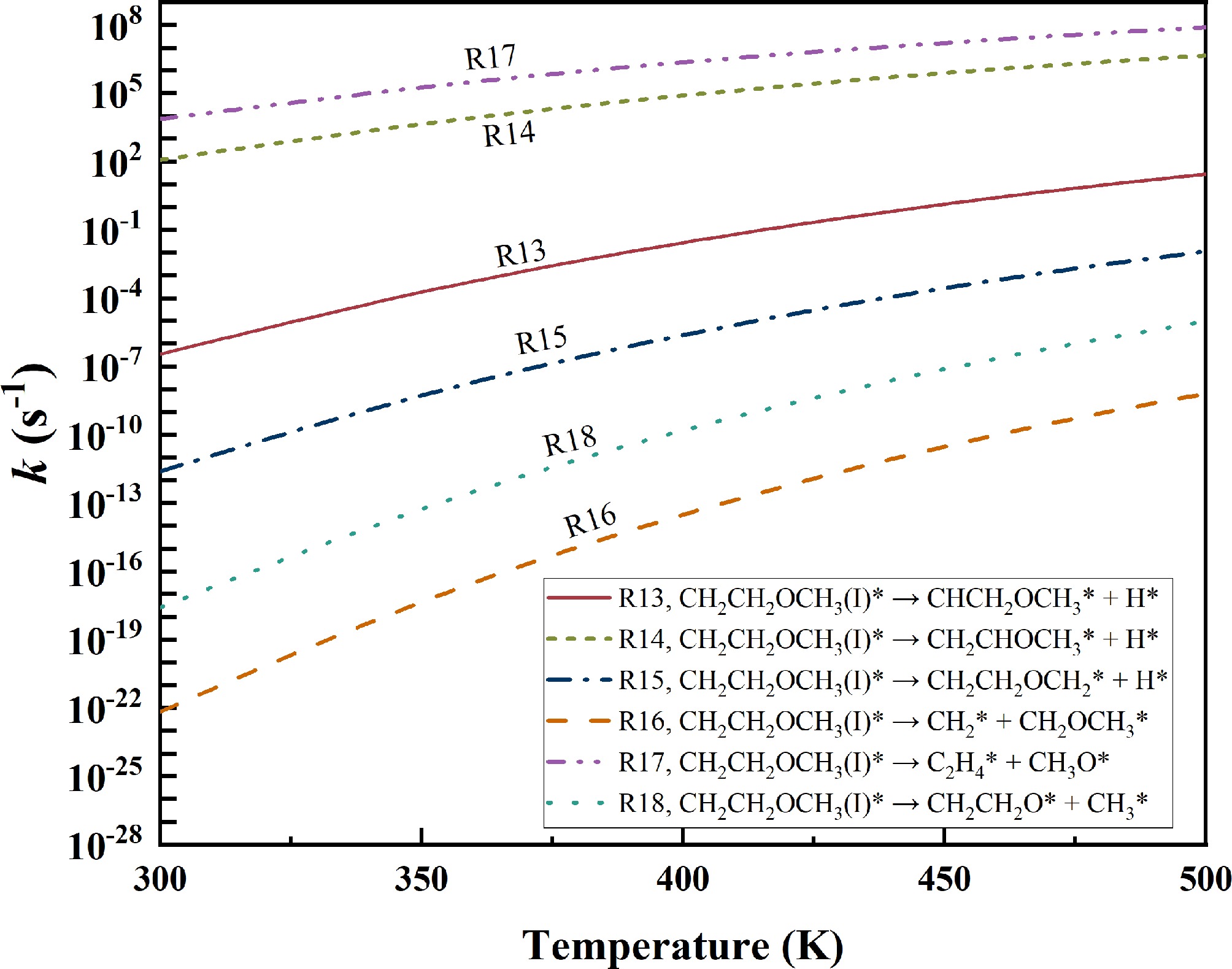
Figure 8.
Rate constants for the decomposition of CH2CH2OCH3(I)*.
-
Species Adsorption manner Adsorption
energy (Eads/eV)EME(I)* Via O atom at top −0.72 CH2CH2OCH3(I)* Via C(1) and O atoms at top −2.12 CH3CHOCH3(I)* Via C(2) and O atoms at top −1.52 CH3CH2OCH2(I)* Via O and C(3) atoms at top −1.66 EME(II)* Via O atom at top −0.80 CH2CH2OCH3(II)* Via C(1) and O atoms at top −2.11 CH3CHOCH3(II)* Via C(2) and O atoms at top −1.61 CH3CH2OCH2(II)* Via O and C(3) atoms at top −1.64 CH3CH2O* Via O atom at fcc −2.77 CH3CH2* Via C atom at top −1.54 CHCH2OCH3* Via C(1) atom at top and O atom at fcc −3.58 CH2CHOCH3* Via C(1) atom at top −0.88 CH2CH2OCH2* via C(1), O and C(3) atoms at top −2.83 CH2CH2O* Via C atom at bridge and O atom at top −3.65 C2H4* Via two C atoms at top −0.67 Table 1.
The adsorption manner and energies of EME(I)* and EME(II)*, along with their intermediates.
Figures
(8)
Tables
(1)