-
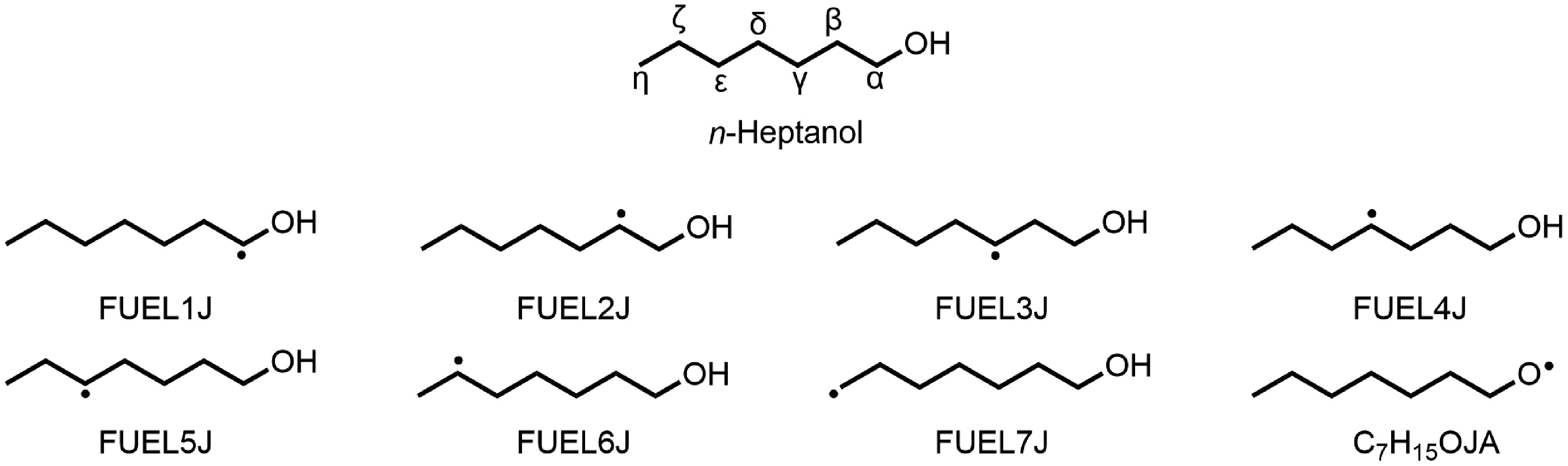
Figure 1.
H-abstraction reactions via H, O, OH, HO2, CH3, etc yield eight fuel radicals (C7H15O, R).
-
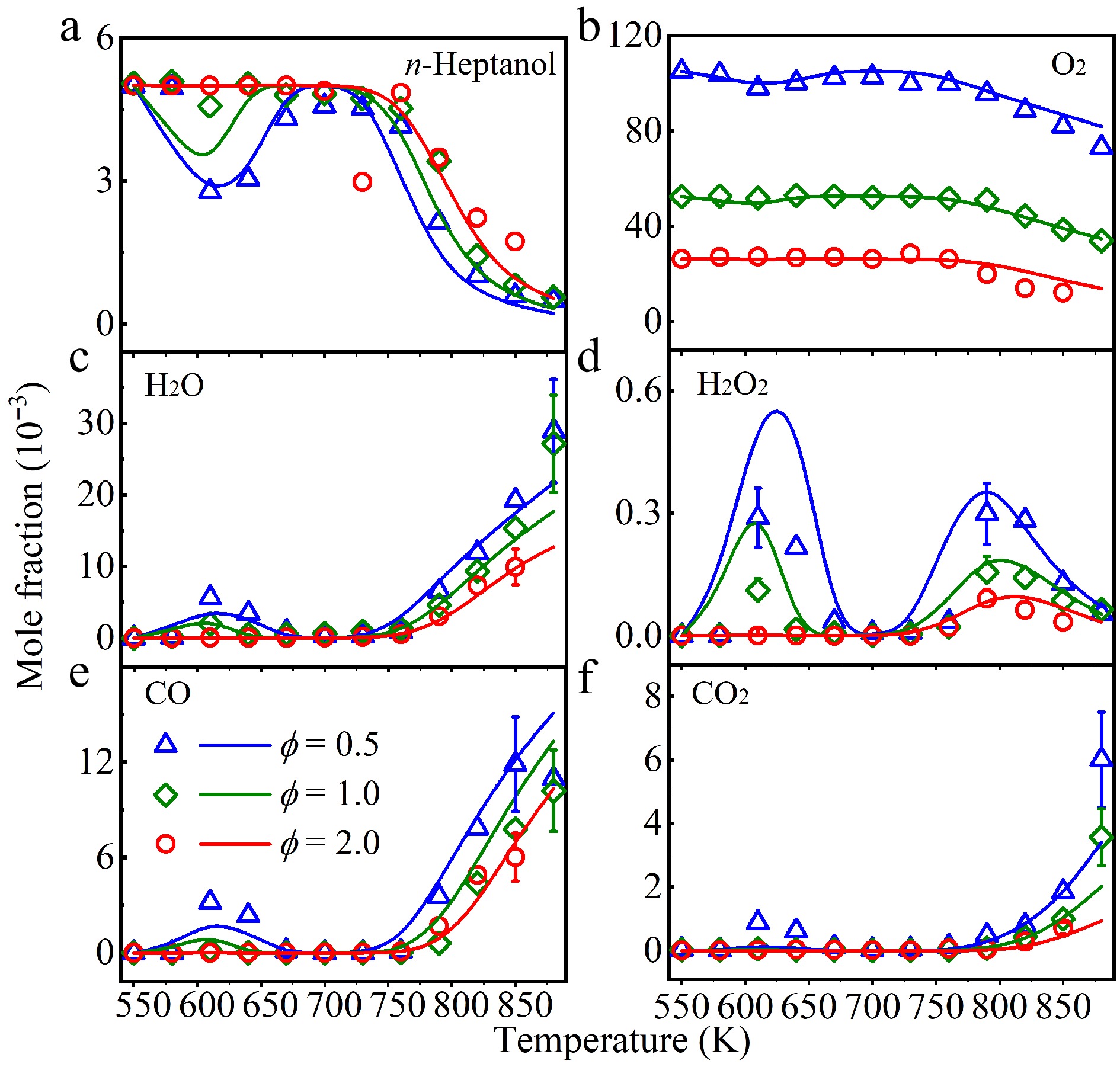
Figure 2.
Experimental (symbols), and simulated (lines) mole fraction profiles of (a) n-heptanol, (b) oxygen (O2), (c) water (H2O), (d) hydrogen peroxide (H2O2), (e) carbon monoxide (CO), and (f) carbon dioxide (CO2) in a jet-stirred reactor (JSR) at ϕ = 0.5 (triangle), 1.0 (diamond), and 2.0 (circle), and at atmospheric pressure.
-
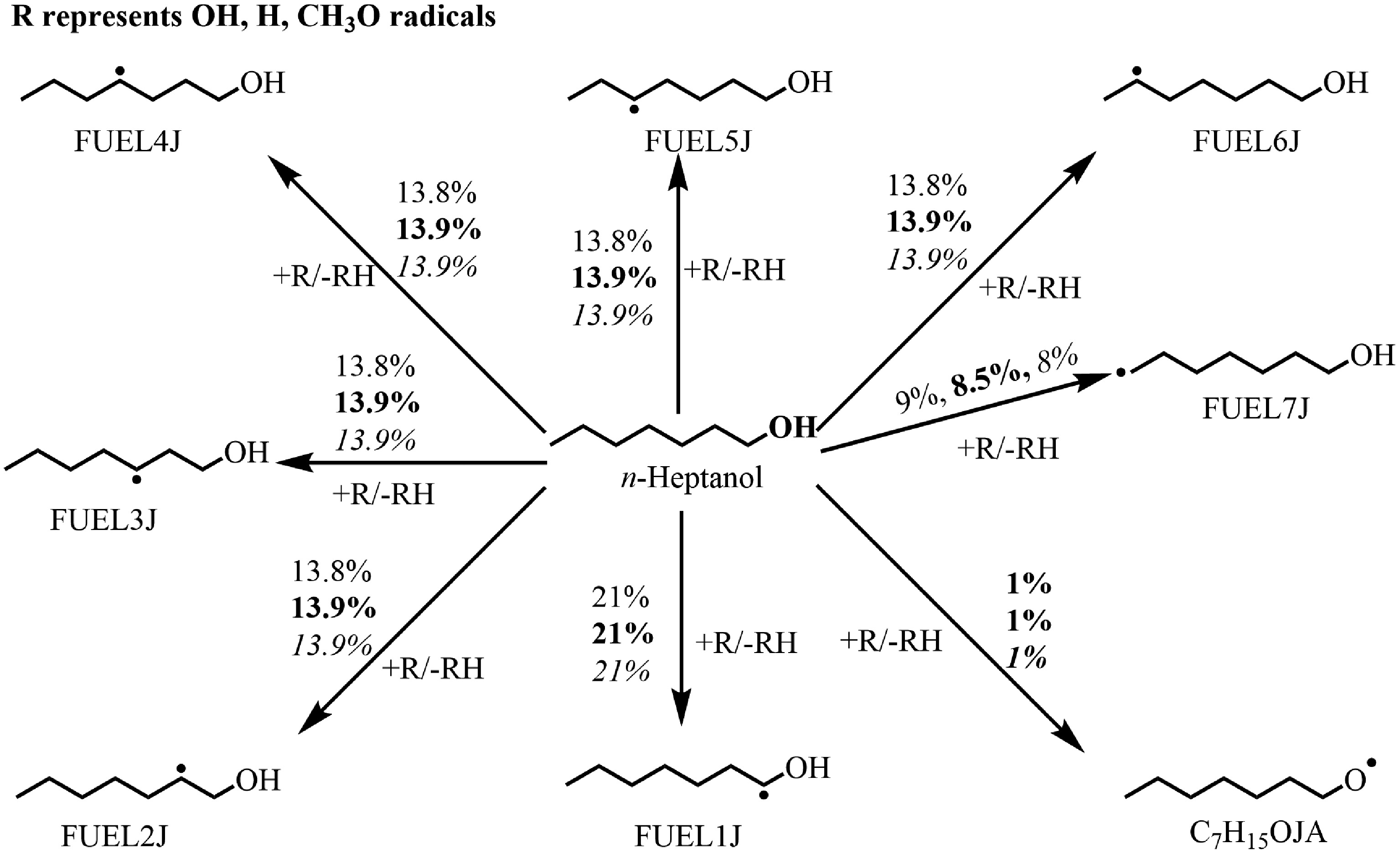
Figure 3.
Rate of production (ROP) analysis for n-heptanol at T = 610 K at ϕ = 0.5 (regular), 1.0 (bold), and 2.0 (italic) and at atmospheric pressure. The numbers reflect the percentages of corresponding pathways in total reaction flux.
-
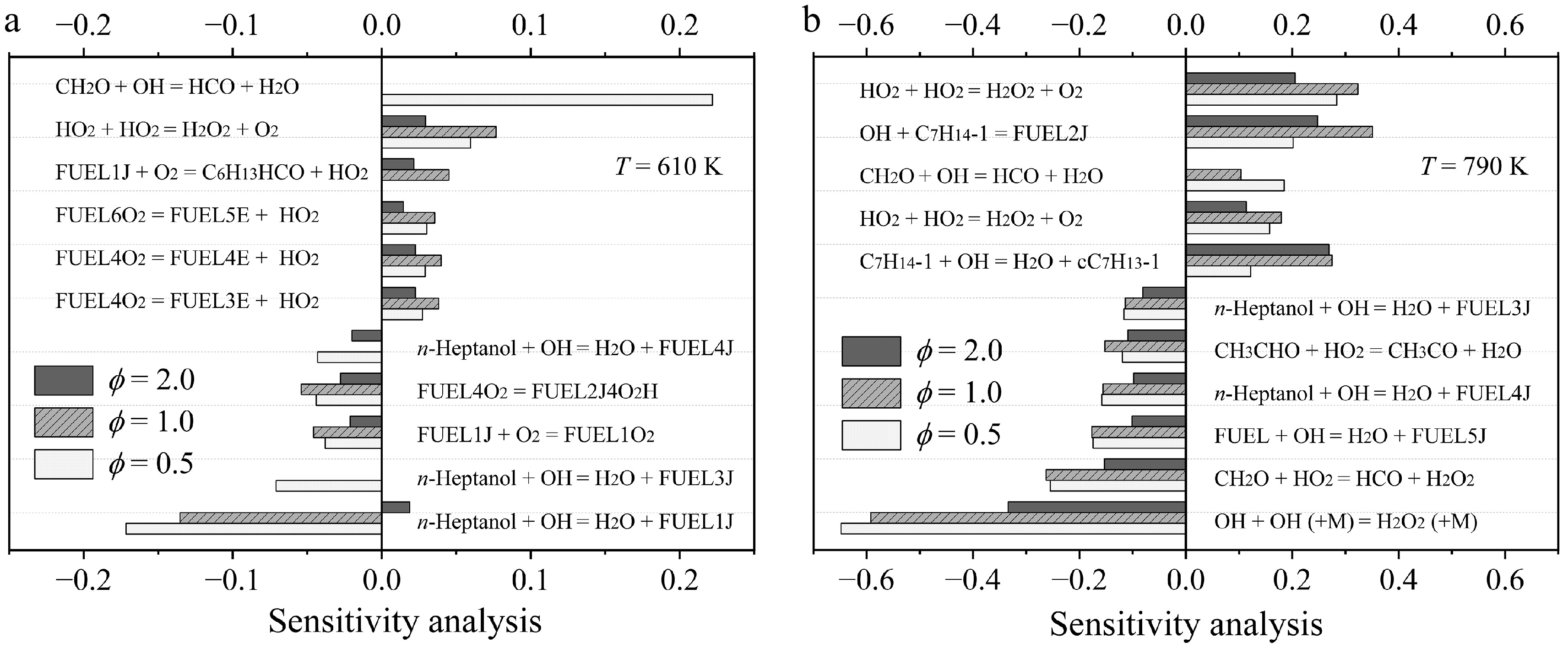
Figure 4.
Sensitivity analysis of n-heptanol oxidation at (a) 610 K, and (b) 790 K at ϕ = 0.5, 1.0, and 2.0, and atmospheric pressure.
-
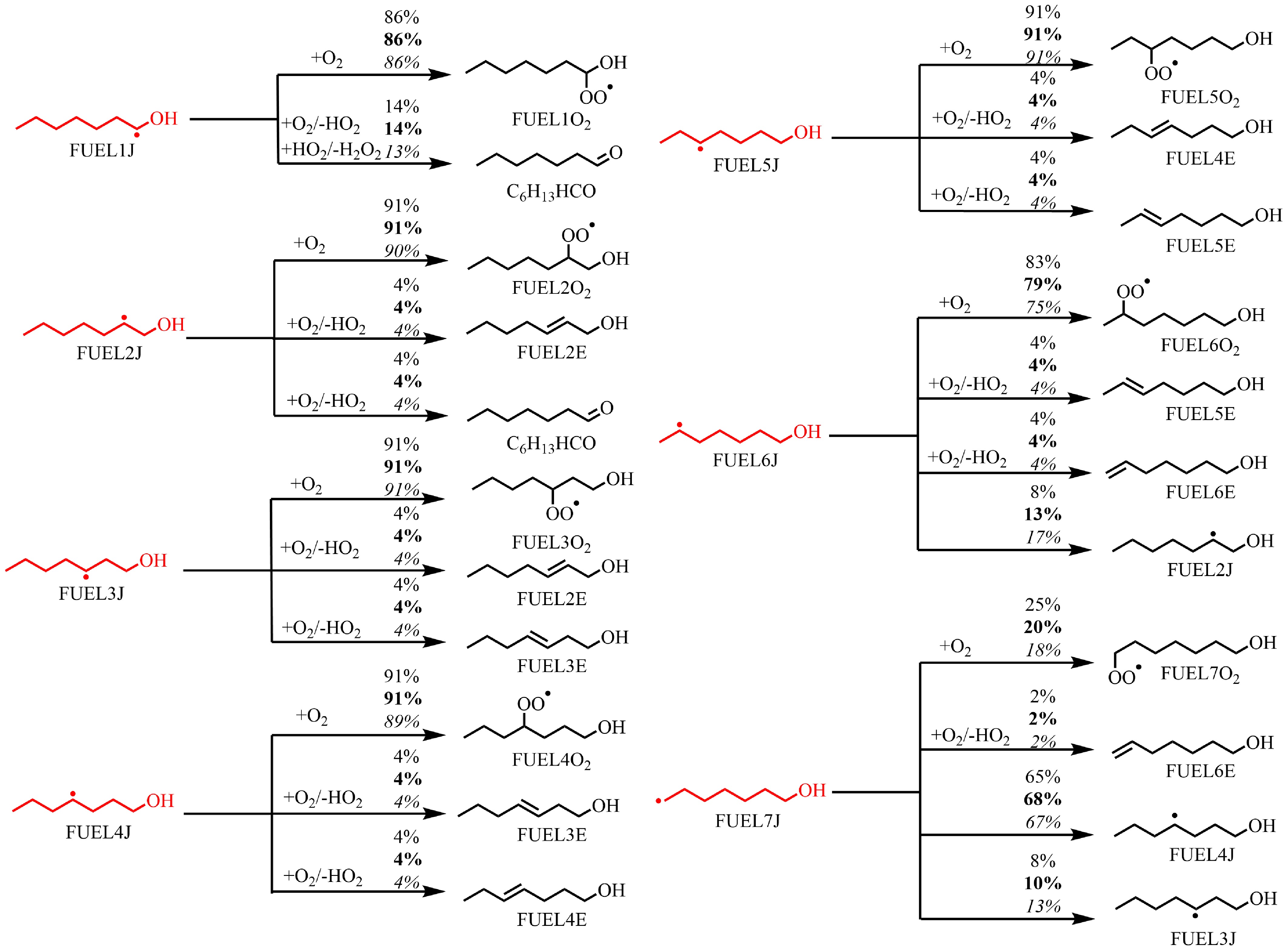
Figure 5.
Rate of production (ROP) analysis for FUEL1J–FUEL7J radicals at T = 610 K at ϕ = 0.5 (regular), 1.0 (bold), and 2.0 (italic), and at atmospheric pressure. The numbers reflect the percentages of corresponding pathways in relative reaction flux.
-
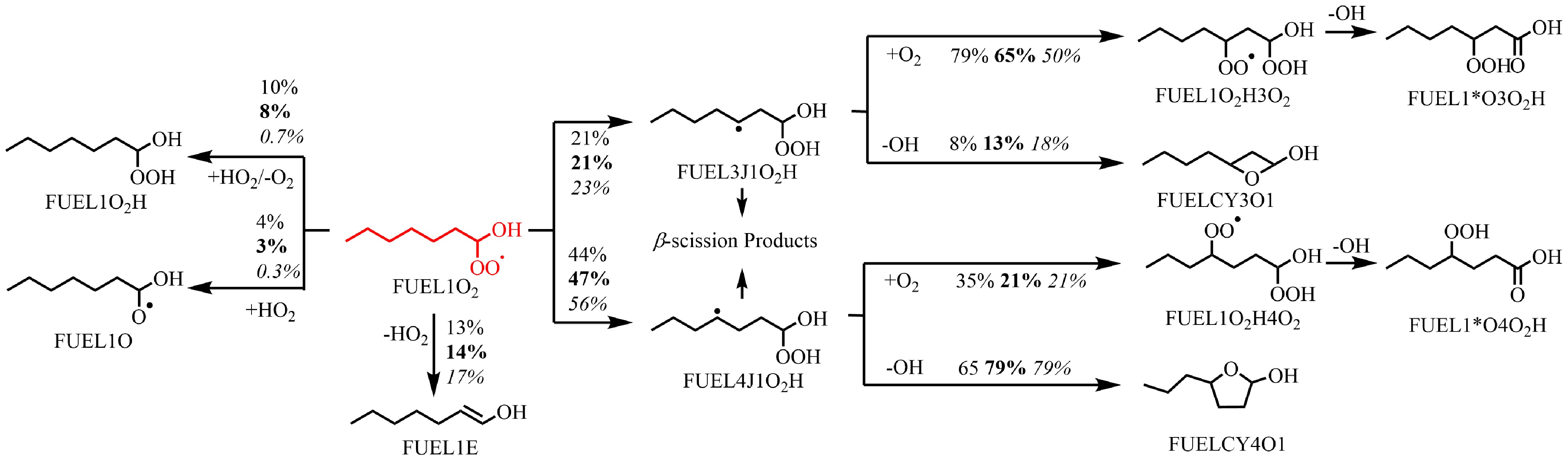
Figure 6.
Rate of production (ROP) analysis of FUEL1O2 radicals at T = 610 K at ϕ = 0.5 (regular), 1.0 (bold), and 2.0 (italic), and at atmospheric pressure. The numbers reflect the percentages of corresponding pathways in relative reaction flux.
-
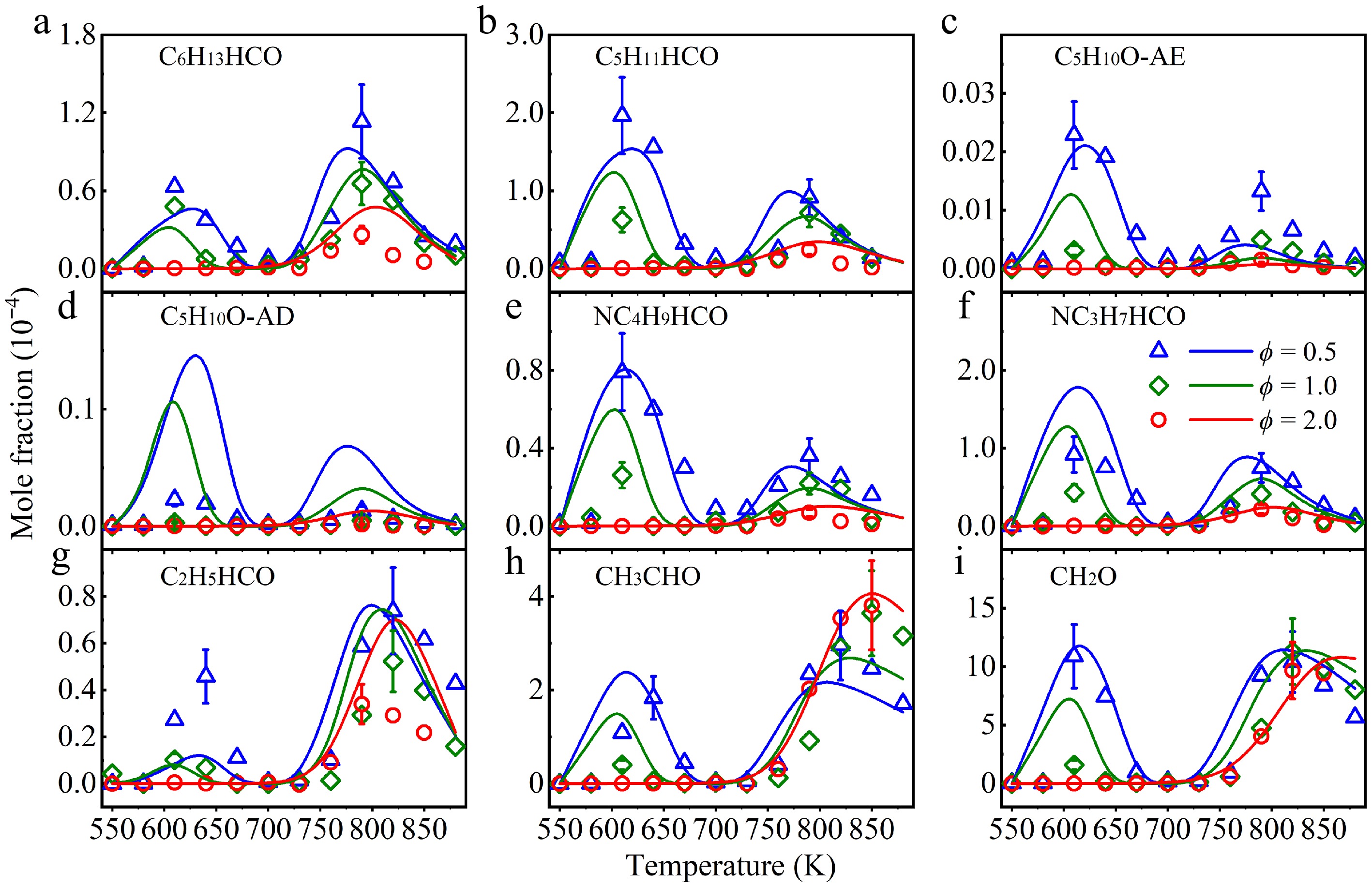
Figure 7.
Experimental (symbols), and simulated (lines) mole fraction profiles of (a) heptanal (C6H13HCO), (b) hexanal (C5H11HCO), (c) tetrahydropyran (C5H10O-AE), (d) 2-methyltetrahydrofuran (C5H10O-AD), (e) valeraldehyde (NC4H9HCO), (f) butanal (NC3H7HCO), (g) propanal (C2H5HCO), (h) acetaldehyde (CH3CHO), and (i) formaldehyde (CH2O) in a jet-stirred reactor (JSR) at atmospheric pressure at ϕ = 0.5 (triangle), 1.0 (diamond), and 2.0 (circle).
-
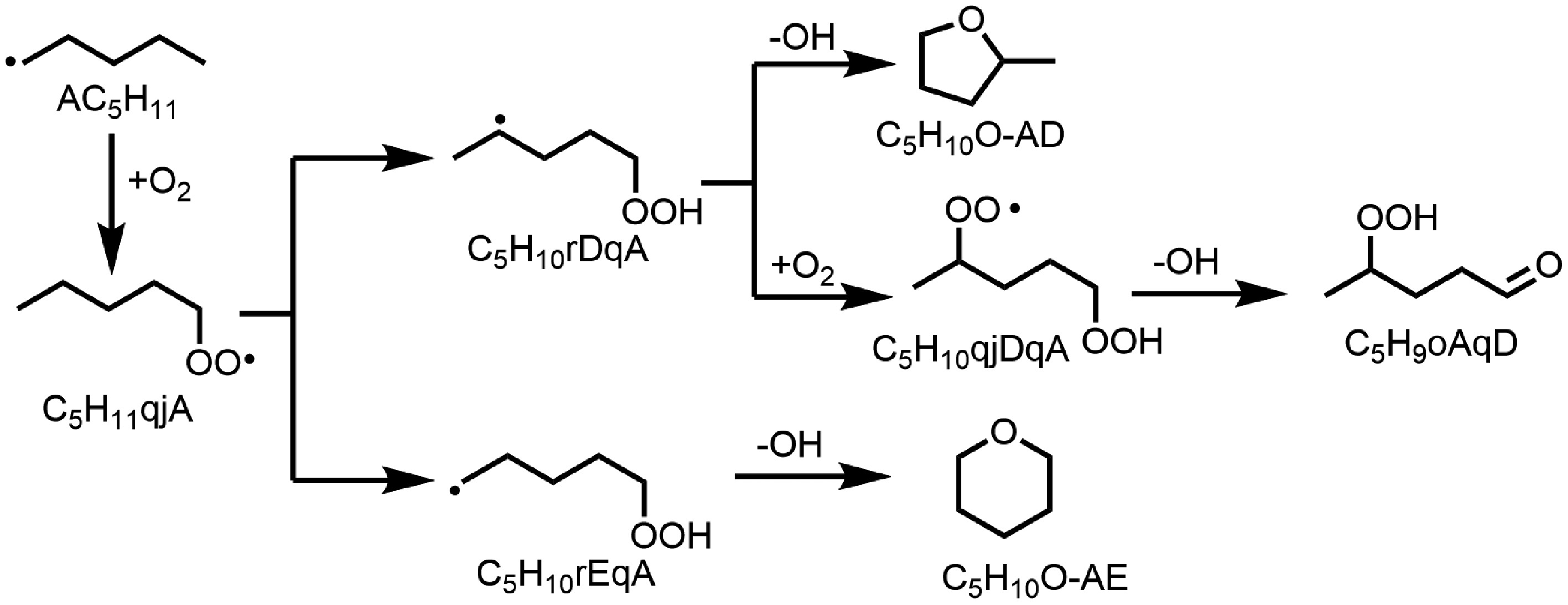
Figure 8.
The main pathway of tetrahydropyran (C5H10O-AE), and 2-methyltetrahydrofuran (C5H10O-AD) in n-heptanol oxidation in a jet-stirred reactor (JSR).
-
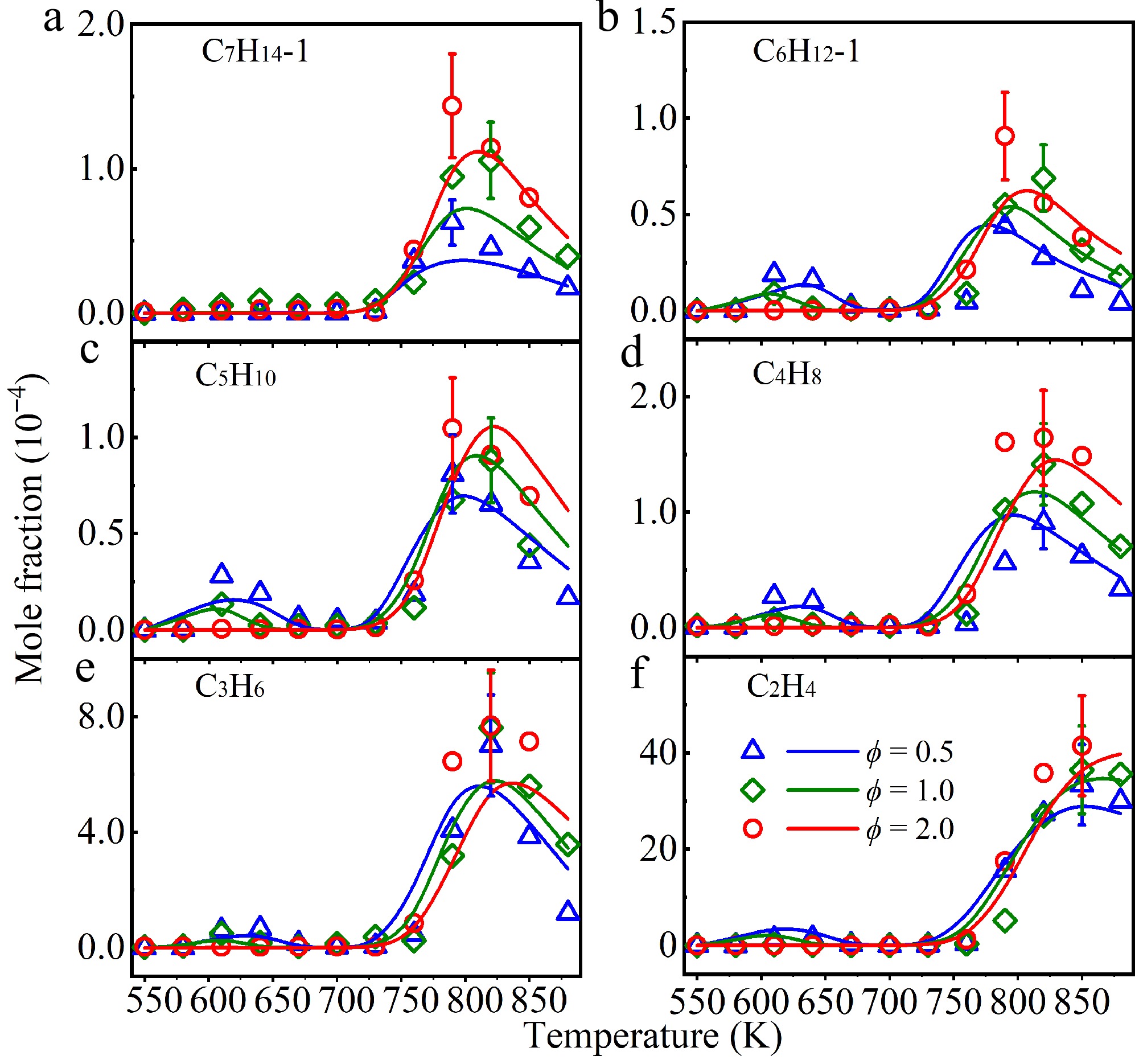
Figure 9.
Experimental (symbols), and simulated (lines) mole fraction profiles of (a) 1-heptene (C7H14−1), (b) 1-hexene (C6H12−1), (c) 1-pentene (C5H10), (d) 1-butene (C4H8), (e) propene (C3H6), and (f) ethylene (C2H4) in a jet-stirred reactor (JSR) at atmospheric pressure and ϕ = 0.5 (triangle), 1.0 (diamond), and 2.0 (circle).
-
Properties Methanol[37] Ethanol[37] n−Propanol[37] n−Butanol[1] n−Pentanol[1] n−Hexanol[1] n−Heptanol[25,26] Gasoline[37] Diesel[37] Molecular formula CH3OH C2H5OH C3H7OH C4H9OH C5H11OH C6H13OH C7H15OH C4−C14 C8−C25 Structural formula 






− − Oxygen content (wt%) 0.50 0.35 0.27 0.22 0.18 0.16 0.14 0 0 Density (g/ml) 0.791 0.794 0.804 0.810 0.816 0.814 0.822 0.737 0.835 Cetane number 3.0 8.0 − 25.0 18.2 23.3 23.0 ~ 23 40–55 Research Octane Number 109 109 104 98 80 56 − 95 − Latent heating (kJ/kg) at 298 K 1,109.0 919.6 792.1 707.9 647.1 603.0 575.0 232.0 351.0 Boiling point (°C) 64.5 78.0 97.0 118.0 138.0 157.0 175.0 27–225 125–400 Lower heating value (MJ/kg) 19.9 26.9 24.7 26.9 28.5 29.3 30.1 42.7 43.0 Solubility in water at 25 °C (wt%) Miscible Miscible Miscible 7.4 2.2 0.6 0.2 Negligible Negligible -
No. Equivalence
ratios (ϕ)Fuel (%)* O2 (%)* Ar (%)* T (K) p (atm) Resident
time (s)1 0.5 0.5 10.50 89.00 550–880 1.0 2.0 2 1.0 0.5 5.25 94.25 550–880 1.0 2.0 3 2.0 0.5 2.625 96.875 550–880 1.0 2.0 * Percentage of species in a molar. Table 2.
Experimental conditions of n-heptanol oxidation.
Figures
(9)
Tables
(2)