-
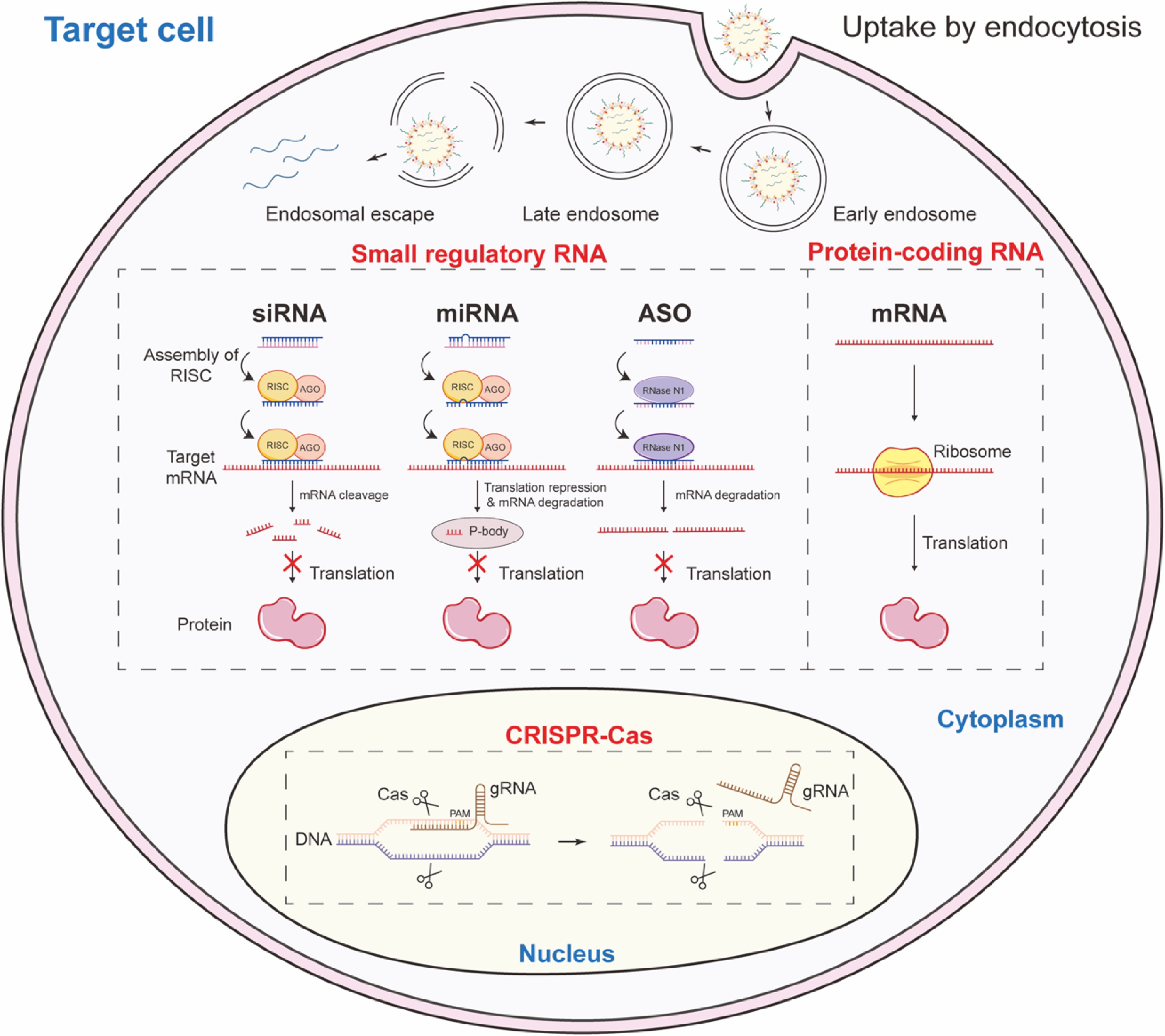
Figure 1.
Categories and therapeutic mechanisms of RNA therapeutics.
-
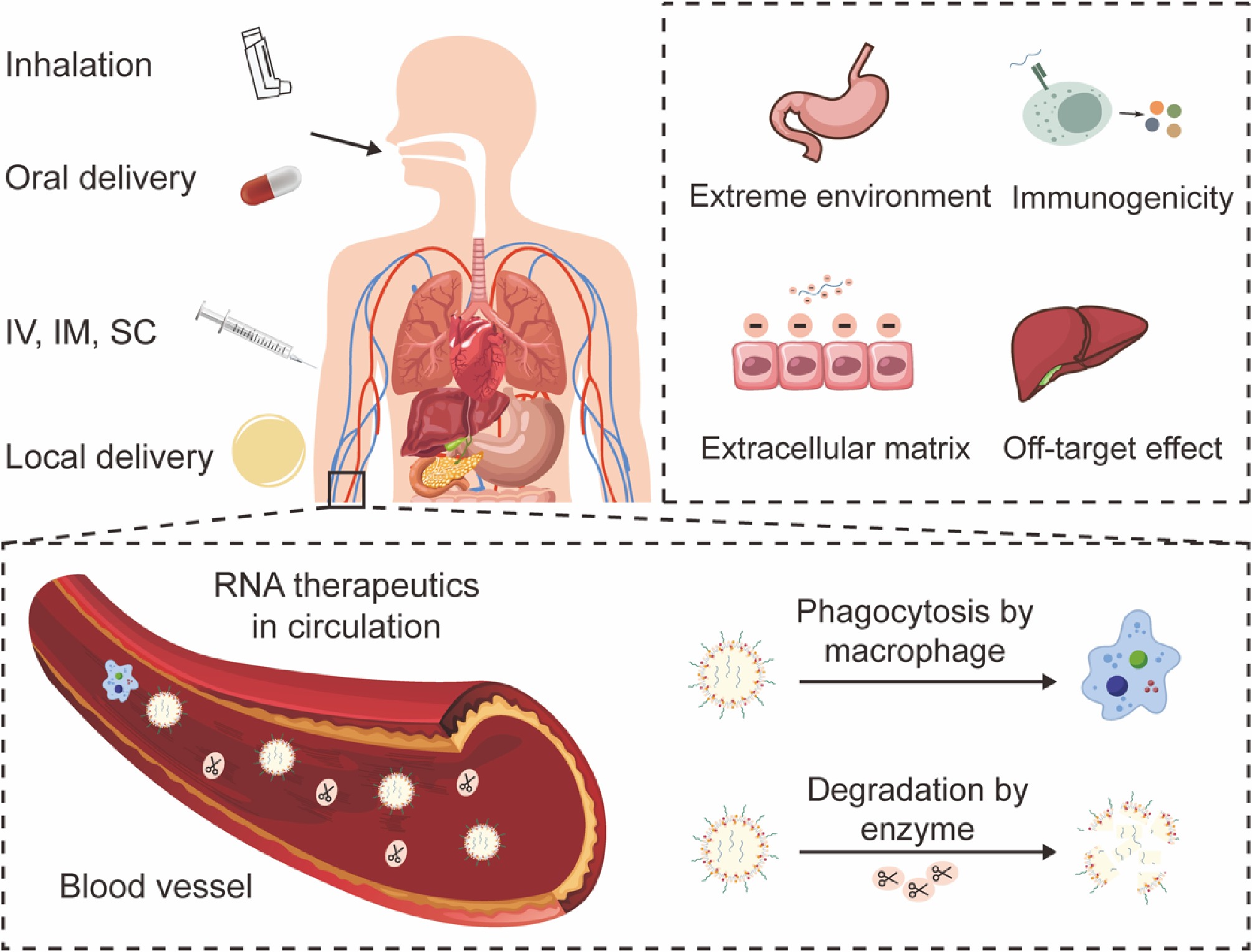
Figure 2.
Administration routes and delivery challenges faced by RNA therapeutics.
-
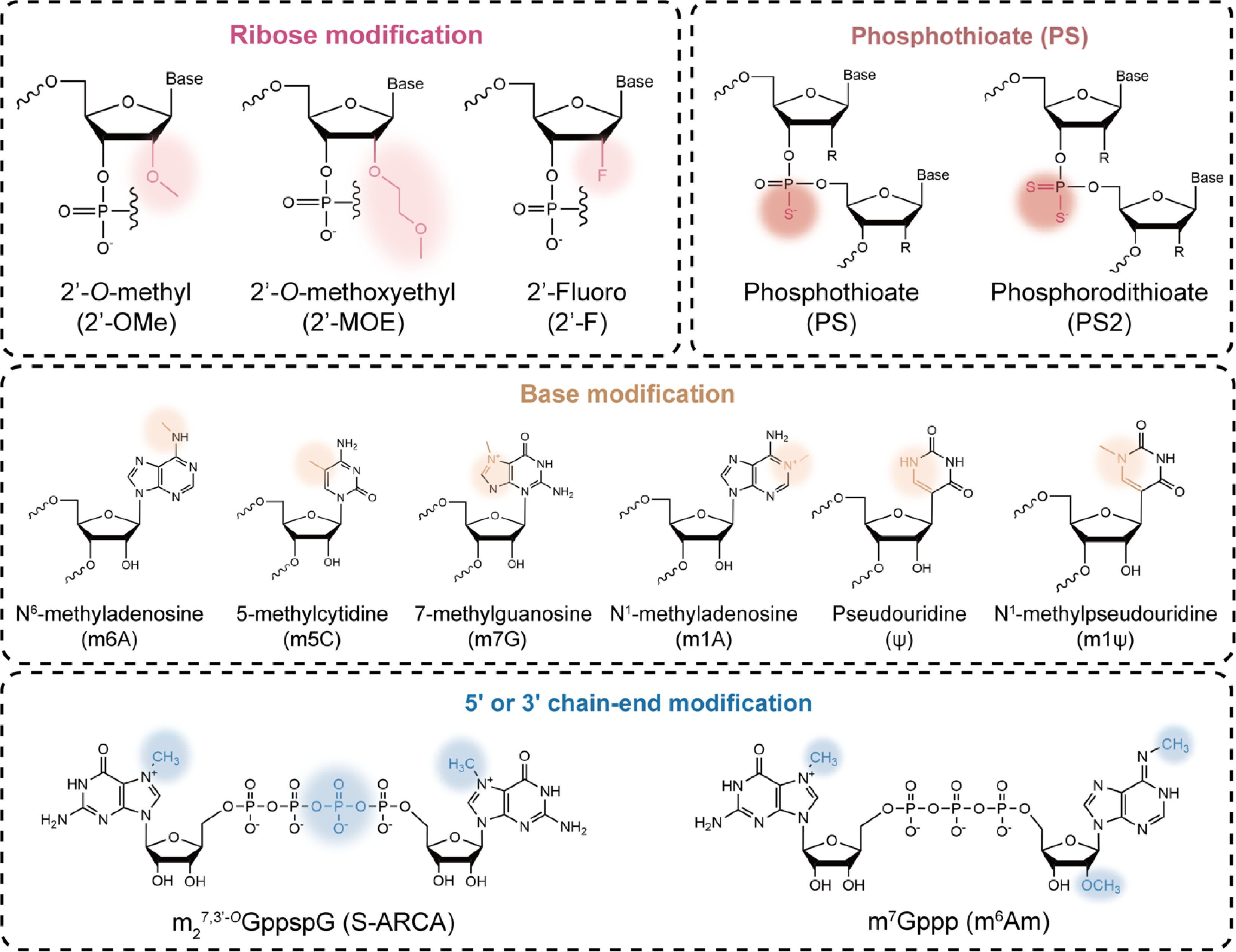
Figure 3.
Representative structural modification strategies for RNA therapeutics.
-
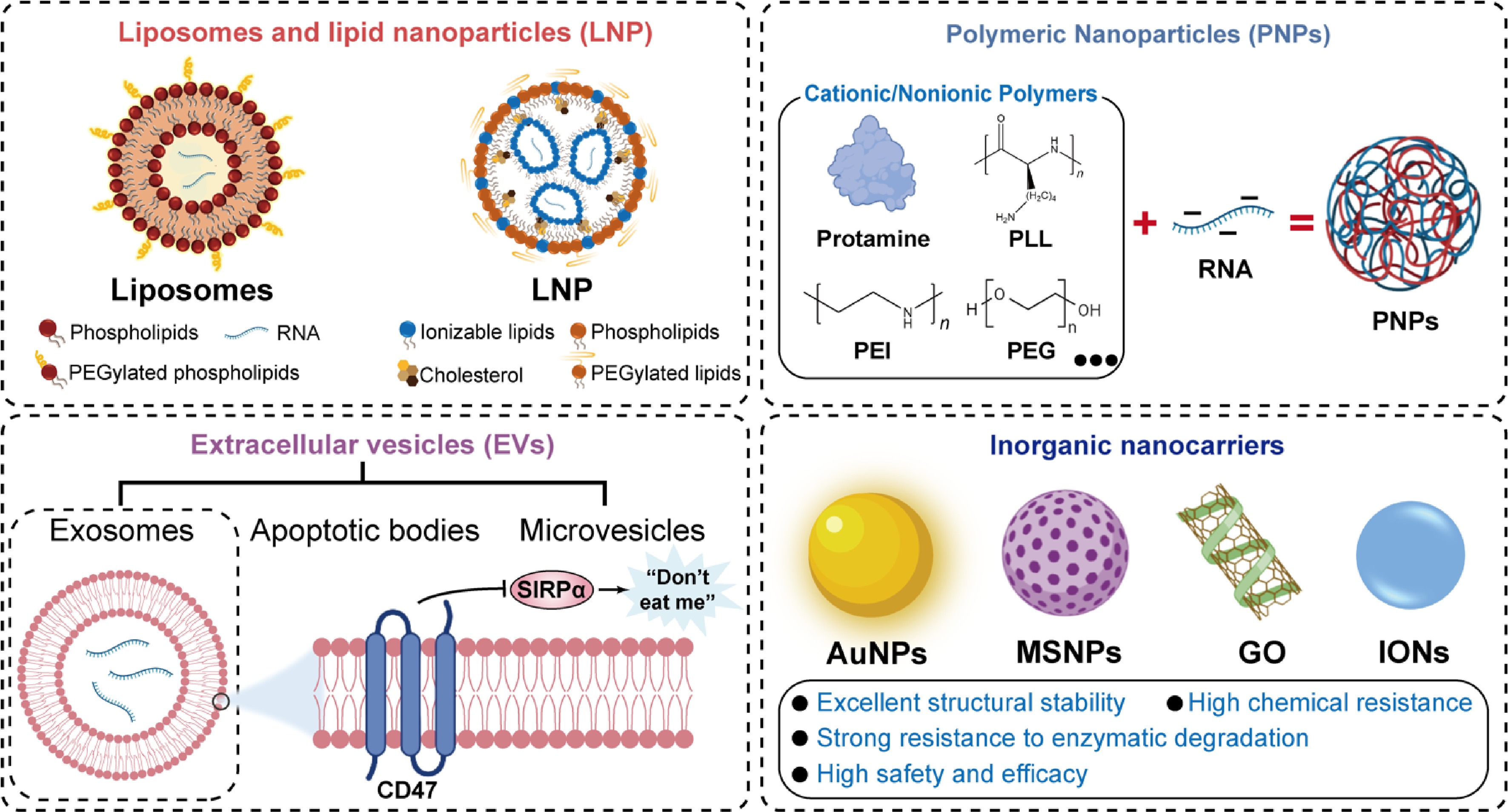
Figure 4.
Emerging drug carriers for delivering RNAs.
-
Disease classification Classification Development code Indications Target/mechanism of action RNA technology platform Delivery system Route of administration Launch year Ref. Infectious diseases Prophylactic mRNA vaccine mRNA-1345 Respiratory syncytial virus Respiratory syncytial virus stabilized prefusion F glycoprotein mRNA LNPs Intramuscular injection 2024 [145] Infectious diseases Prophylactic mRNA vaccine mRNA-1273 SARS-CoV-2/
COVID-19 virusSARS-CoV-2 spike protein mRNA LNPs Intramuscular injection 2020 [177] Infectious diseases Prophylactic mRNA vaccine mRNA-1283 SARS-CoV-2/
COVID-19 virusSARS-CoV-2/
COVID-19 virusmRNA LNPs Intramuscular injection 2025 [178] Infectious diseases Prophylactic mRNA vaccine BNT162b2 SARS-CoV-2/
COVID-19 virusSARS-CoV-2 spike protein mRNA LNPs Intramuscular injection 2020 [179] Infectious diseases Prophylactic mRNA vaccine ARCT-154 SARS-CoV-2/
COVID-19 virusSARS-CoV-2 spike protein mRNA LNPs Intramuscular injection 2025 [180] Infectious diseases Prophylactic mRNA vaccine SYS6006 SARS-CoV-2/
COVID-19 virusSARS-CoV-2 spike protein mRNA LNPs Intramuscular injection 2023 [181] Liver and metabolic diseases RNA interference (RNAi) therapy KJX839 Familial hypercholesterolemia (FH) and cardiovascular risk reduction PCSK9 mRNA siRNA GalNAc Subcutaneous injection 2020 [182] Hepatic and metabolic diseases RNA aptamer therapy NX1838 Neovascular (Wet)
age-related macular degenerationSpecifically targets
the VEGF165
isoformRNA aptamer Not required Intravitreal injection 2004 [183] Genetic diseases Antisense oligonucleotide (ASO) therapy Nusinersen Spinal muscular atrophy (SMA) Modulation of SMN2 pre-mRNA splicing ASO Ligand-conjugated active delivery Intrathecal injection 2016 [184] Genetic diseases Antisense oligonucleotide (ASO) therapy AVI-4658 Duchenne muscular dystrophy (DMD) DMD gene pre-mRNA ASO No specialized delivery system required Intravenous infusion 2016 [185] Genetic diseases Antisense oligonucleotide (ASO) therapy IONIS-TTRRx Hereditary transthyretin-mediated amyloidosis (hATTR) mRNA silencing of
the TTR geneASO Ligand-conjugated active delivery Subcutaneous injection 2018 [186] Genetic diseases Antisense oligonucleotide (ASO) therapy BIIB067 Amyotrophic lateral sclerosis (ALS) Targeted degradation of SOD1 mRNA ASO Ligand-conjugated active delivery Intrathecal injection 2023 [187] Genetic diseases CRISPR gene editing therapy CTX001 β-thalassemia,
sickle cell diseaseEdits the BCL11A gene in autologous hematopoietic stem cells to reactivate
fetal hemoglobin productionCRISPR-Cas9 Electroporation Intravenous infusion 2023 [188] Genetic diseases RNA interference (RNAi) therapy ALN-TTR02 Hereditary transthyretin-mediated amyloidosis (hATTR) mRNA silencing of
the TTR genesiRNA LNPs Intravenous infusion 2018 [189] Genetic diseases RNA interference (RNAi) therapy ALN-AS1 Acute hepatic porphyria (AHP) Targeted silencing
of ALAS1 mRNAsiRNA GalNAc Subcutaneous injection 2019 [190] Table 1.
RNA therapeutics on market for disease treatment.
-
Disease classification Classification Development code Indications Target/mechanism of action RNA technology platform Delivery system Route of administration Status Ref. Cancer Personalized neoantigen vaccine mRNA-4157 Melanoma Personalized neoantigen mRNA LNPs Intramuscular injection Phase II [135] Cancer Personalized neoantigen vaccine BNT122 Melanoma, colorectal cancer, non-small cell
lung cancerPersonalized neoantigen mRNA LNPs Intravenous
infusionPhase II [191] Cancer Shared-antigen vaccine BNT111 Melanoma Shared-antigen mRNA LNPs Intravenous
infusionPhase II [137] Cancer Shared-antigen vaccine CV9104 Prostate cancer Shared-antigen mRNA Protamine complex Intradermal injection and subcutaneous injection Phase II [138] Cancer RNA interference (RNAi) therapy MTL-CEBPA Hepatocellular carcinoma CEBPA gene activation siRNA LNPs Intravenous
infusionPhase II [132] Cancer RNA interference (RNAi) therapy siG12D-LODER Pancreatic cancer KRAS G12D silencing siRNA LODER™ (LOcal Drug EluteR) Endoscopic Intratumoral injection Phase II [192] Cancer mRNA-encoded cytokine/antibody mRNA-2752 Solid tumors, Lymphoma Cytokine encoding mRNA LNPs Intratumoral injection Phase I [193] Cancer microRNA therapy MRX34 Advanced or metastatic hepatocellular carcinoma (HCC), melanoma, lymphoma, and various other solid tumors miR-34a mimic Double-stranded
RNA mimicLNPs Intratumoral injection Phase I [194] Cancer microRNA therapy MesomiR-1 Malignant pleural mesothelioma, advanced non-small cell lung cancer miR-16 mimic Double-stranded
RNA mimicTargeted Bacterial Minicells Intratumoral injection Phase I [195] Infectious diseases Prophylactic mRNA vaccine mRNA-1010 Influenza Hemagglutinin (HA) glycoproteins of four influenza virus strains mRNA LNPs Intramuscular injection Phase III [196] Infectious diseases Prophylactic mRNA vaccine PF-07252220 Influenza Hemagglutinin (HA) glycoproteins of four influenza virus strains mRNA LNPs Intramuscular injection Phase I [197] Infectious diseases Prophylactic mRNA vaccine mRNA-1647 Cytomegalovirus Cytomegalovirus mRNA LNPs Intramuscular injection Phase III [198] Infectious diseases Prophylactic mRNA vaccine mRNA-1443 Cytomegalovirus Hemagglutinin (HA) protein of influenza virus mRNA LNPs Intramuscular injection Phase I [199] Infectious diseases Prophylactic mRNA vaccine mRNA-1189 E–B virus Epstein–Barr virus envelope glycoproteins gH, gL, gp42, and gp220 mRNA LNPs Intramuscular injection Phase I/II [200] Infectious diseases Prophylactic mRNA vaccine mRNA-1215 Nipah virus Nipah virus prefusion-stabilized F protein and G protein monomer mRNA LNPs Intramuscular injection Phase I [201] Infectious diseases Prophylactic mRNA vaccine mRNA-1893 Zika virus Zika virus mRNA LNPs Intramuscular injection Phase II [202] Infectious diseases Prophylactic mRNA vaccine mRNA-1608 Herpes simplex virus Herpes simplex virus mRNA LNPs Intramuscular injection Phase I/II [203] Infectious diseases Prophylactic mRNA vaccine mRNA-1283 SARS-CoV-2/
COVID-19 virusSARS-CoV-2 spike protein mRNA LNPs Intramuscular injection Phase III [178] Infectious diseases RNA interference (RNAi) therapy ARO-HBV Hepatitis B virus Degradation of HBV mRNA siRNA Dynamic Polyconjugates Subcutaneous injection Phase I/II [204] Liver and metabolic diseases RNA interference (RNAi) therapy Fazirsiran α-1 antitrypsin
deficiency (A1ATD)Specific silencing of mutant SERPINA1 siRNA TRiM™ Subcutaneous injection Phase III [161] Liver and metabolic diseases Antisense oligonucleotide (ASO) therapy Vupanorsen Familial hypercholesterolemia (FH) and cardiovascular risk reduction Targeted silencing of ANGPTL3 ASO GalNAc Subcutaneous injection Phase II [205] Liver and metabolic diseases Messenger RNA (mRNA) therapy mRNA-3927 Propionic acidemia (PA) PCCA and PCCB mRNA LNPs Intravenous
infusionPhase I/II [164] Liver and metabolic diseases Messenger RNA (mRNA) therapy ARCT-810 Ornithine transcarbamylase deficiency (OTCD) Encoding a functional
OTC enzymemRNA LNPs Intravenous
infusionPhase II [206] Liver and metabolic diseases RNA interference (RNAi) therapy AB-729 Hepatitis B virus GalNAc-conjugated siRNA targeting all HBV RNA transcripts that produces
a marked and durable reduction in hepatitis B surface antigen (HBsAg) levelssiRNA GalNAc Subcutaneous injection Phase II [207] Hepatic and metabolic diseases Hybrid antisense oligonucleotide (ASO) therapy TPI ASM8 Asthma mRNA targeting the two signaling molecules CCR3 and the βc chain ASO No delivery system required Nebulized inhalation Phase II [208] Hepatic and metabolic diseases RNA aptamer therapy ARC1779 Thrombotic Microangiopathy, Acquired Hemophilia,
and other thrombotic disordersTargets the A1 domain of von Willebrand Factor (vWF) RNA aptamer Not required Intravitreal
injectionPhase III [209] Hepatic and metabolic diseases RNA aptamer therapy ARC1905 Age-related Macular Degeneration Complement C5 protein RNA aptamer Not required Intravitreal
injectionPhase III [210] Genetic diseases Antisense oligonucleotide (ASO) therapy RO7234292 Huntington's disease (HD) Targeted silencing of
mutant huntingtin mRNAASO Ligand-conjugated active delivery Intrathecal
injectionPhase III [211] Genetic diseases CRISPR gene editing therapy NTLA-2001 Hereditary Transthyretin Amyloidosis Knocks out the TTR gene in hepatocytes to reduce the production of disease-causing transthyretin protein CRISPR-Cas9 LNPs Intralesional injection Phase I/II [212] Genetic diseases CRISPR gene editing therapy NTLA-2002 Hereditary
angioedemaKnocks out the Kallikrein B1 gene in hepatocytes to reduce bradykinin and prevent swelling attacks CRISPR-Cas9 LNPs Intralesional injection Phase I/II [213] Genetic diseases CRISPR gene editing therapy EDIT-101 Leber Congenital Amaurosis Type 10 Use CRISPR to remove the pathogenic mutation in intron 26 of the CEP290 gene in retinal cells to restore normal protein function CRISPR-Cas12a Adeno-associated virus CRISPR-Cas12a Phase I/II [214] Table 2.
RNA therapeutics under investigation for disease treatment.
Figures
(4)
Tables
(2)