-
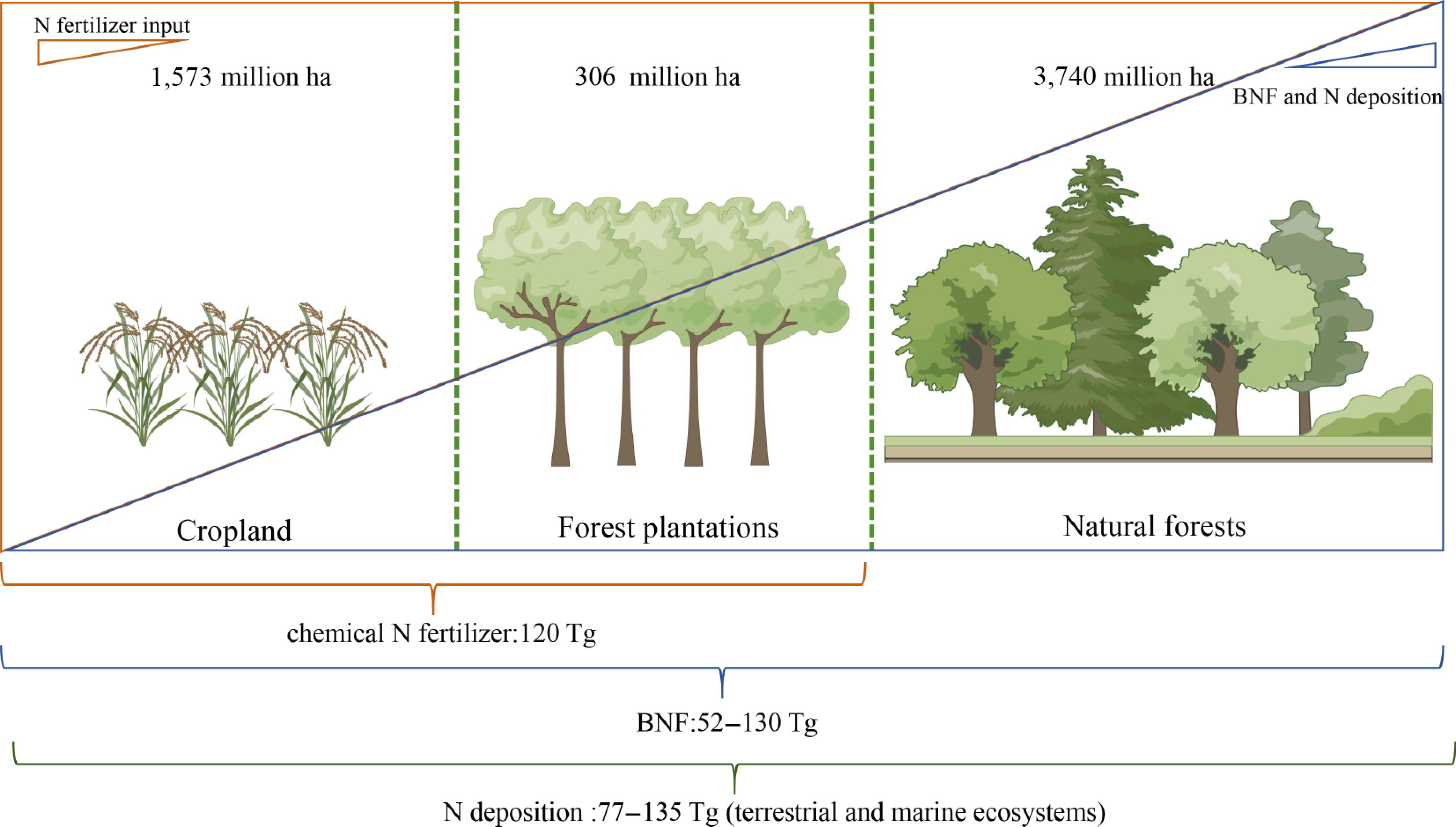
Figure 1.
Nitrogen acquisition in agricultural and forestry ecosystems. The cropland area, planted forest area, and natural forest area can reach 1,573 million ha, 306 million ha, and 3,740 million ha, respectively. The primary modes of nitrogen acquisition in agricultural and forest ecosystems include chemical fertilizers, biological nitrogen fixation, and nitrogen deposition, which amount to approximately 120, 52–130, and 77–135 Tg (terrestrial and marine ecosystems) annually.
-
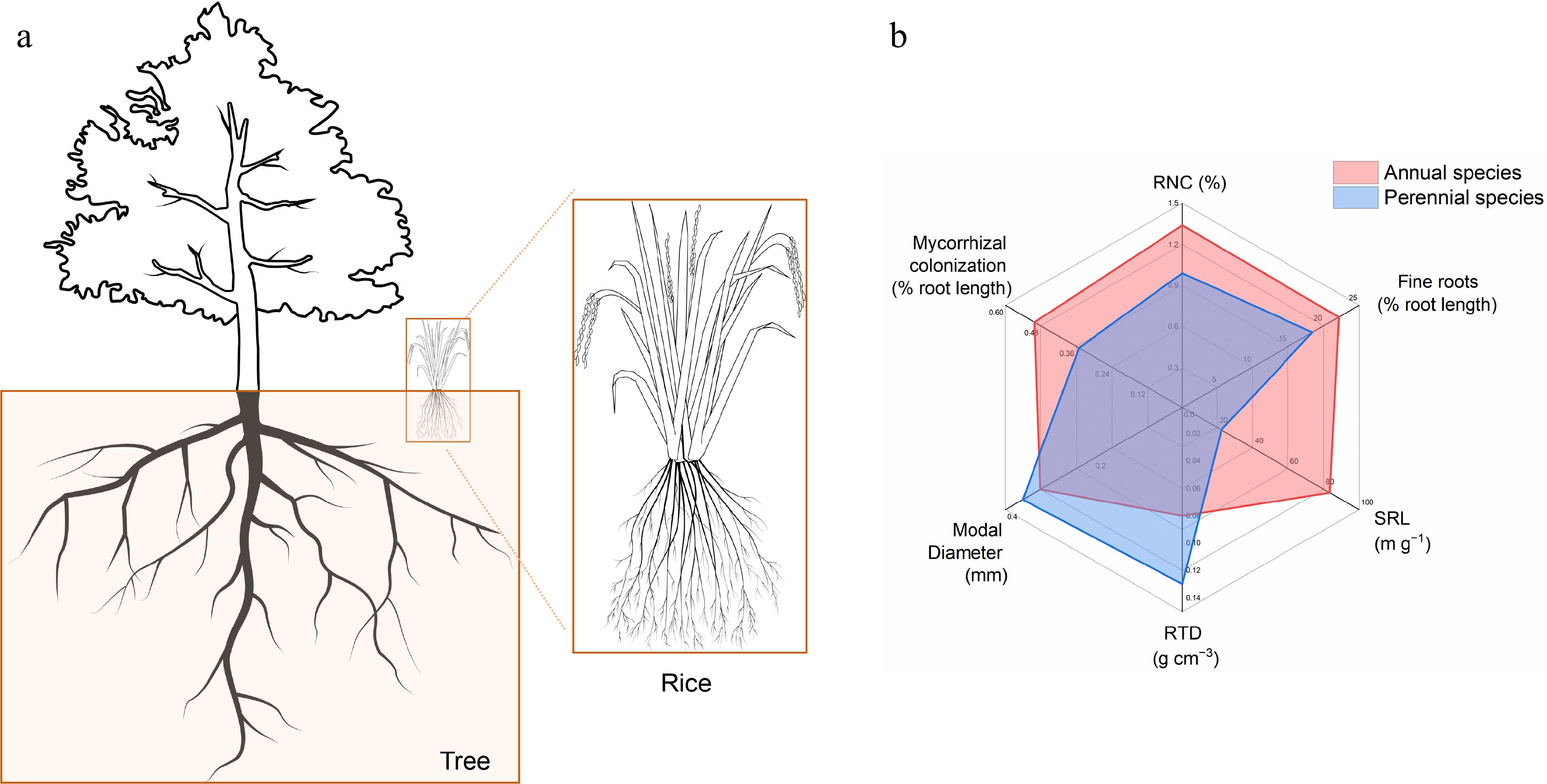
Figure 2.
Root system architecture in perennial trees and annual crops. (a) Schematic diagram of RSA in trees and rice. (b) Root traits related to nitrogen absorption in annual species and perennial species according to the data of 18 field-grown species[25]. RNC: root nitrogen concentration; Fine roots: proportion of root length with diameter < 0.2 mm; SRL: specific root length; RTD: root tissue density; modal diameter, the size of the diameter that occurs most.
-
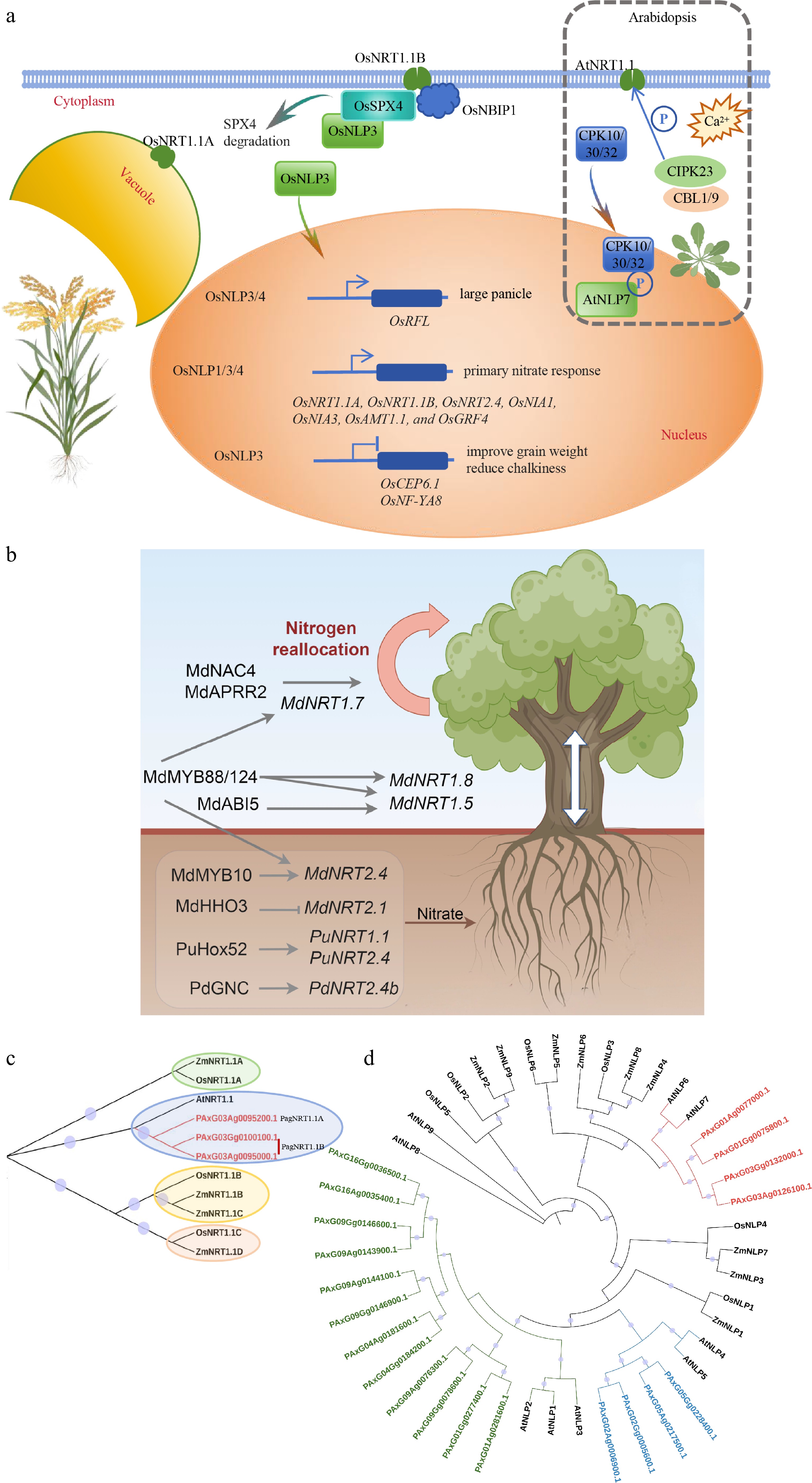
Figure 3.
Nitrate sensors function in nutrient uptake and signaling. (a) The role of AtNRT1.1, AtNLP7, and their homologs in rice in nitrate absorption and signal transduction mechanisms. (b) Nitrate transporters and their modulation in poplar and apple trees. (c) Homologous sequence alignment of NRT1.1 in Arabidopsis, maize, rice, and poplar 84K. (d) Homologous sequence alignment of NLPs in Arabidopsis, maize, rice, and poplar 84K. The phylogenetic tree was constructed using maximum likelihood methods with 5,000 bootstraps in the Tbtools program.
-
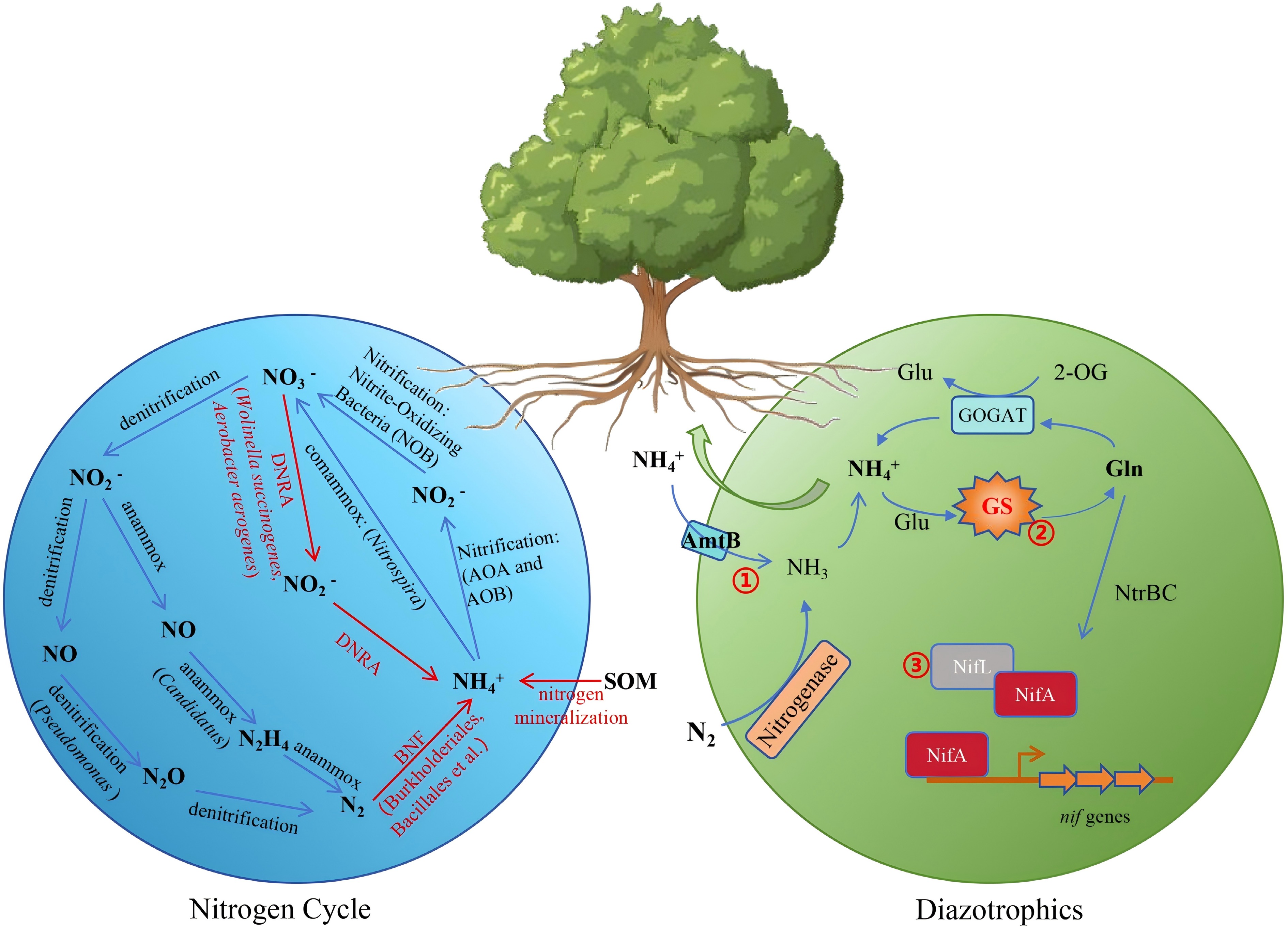
Figure 4.
Microbial-mediated nitrogen cycling and BNF enhance NUE in forest trees. The blue circle illustrates various processes of the nitrogen cycle and representative bacterial genera, including denitrification, ammonia oxidation, anammox, and comammox. The green circle represents nitrogen metabolism in associative nitrogen-fixing bacteria and engineering targets for ammonium-excreting strains. (1) Disruption of ammonium transporter AmtB to prevent the ammonium transport back into the cell. (2) Modifying glutamine synthetase to obstruct ammonium assimilation. (3) Modulation of the NifLA system to regulate nitrogenase expression.
-
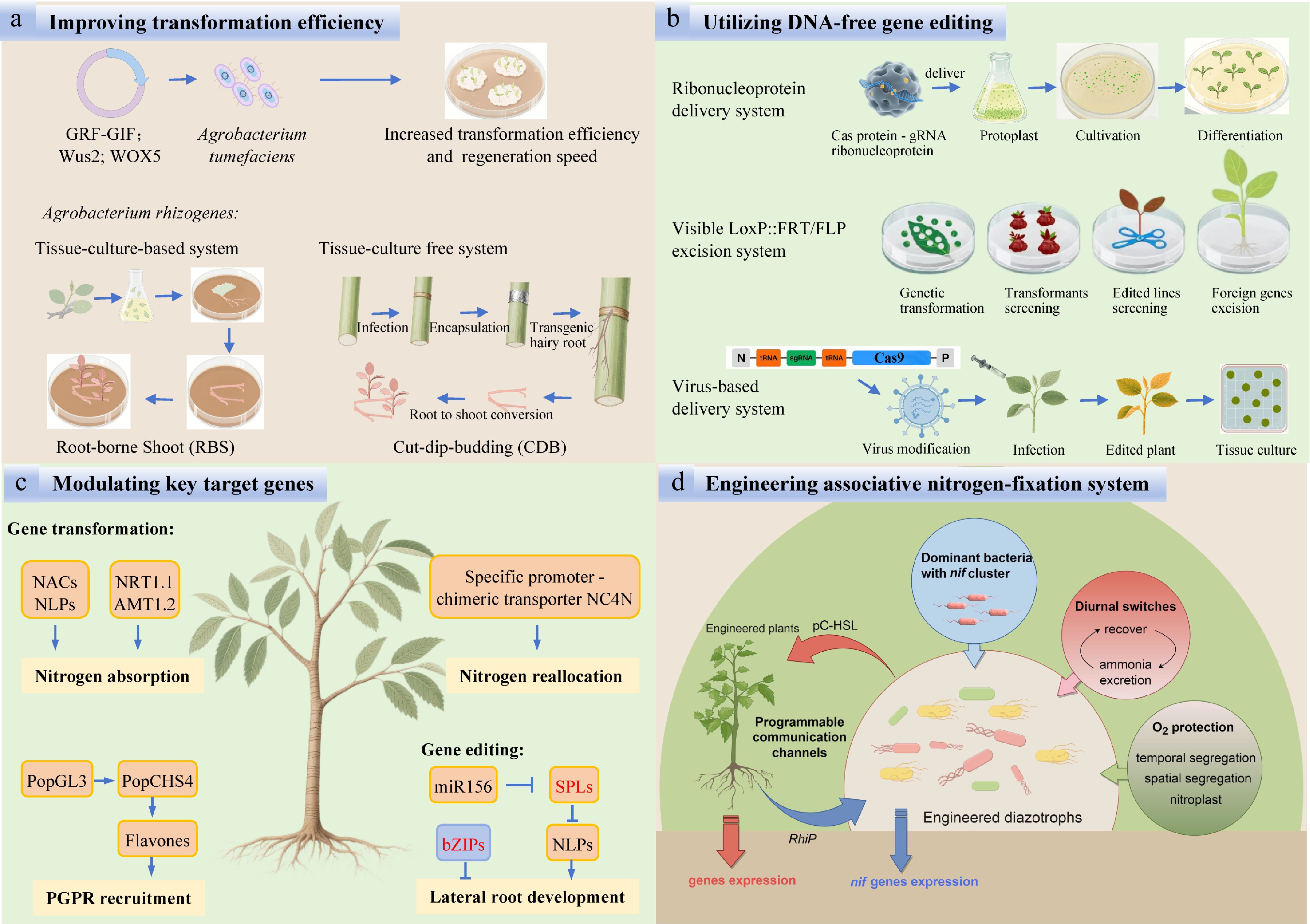
Figure 5.
Challenges and measures for improving NUE in forest plantations. (a) Improving transformation efficiency. (b) Utilizing DNA-free gene editing. (c) Modulating key target genes. (d) Engineering associative nitrogen-fixation system.
-
Tree species Gene Function Ref. Populus PsiSKP2B; PtabZIP1L; PeFUS3; PtrABR1-PtrYY1 module Promoting lateral root growth [26−28,39] MicroRNA319 Decreasing density of lateral roots [29] PagWOX11/12a Improving adventitious root development; promoting root elongation and biomass [31,40] MicroRNA390 Stimulating lateral root development [30] PuZFP1 Inhibiting lateral root emergence; promoting adventitious root elongation [36] Malus MdARF3 Promoting root elongation [32] MdSIZ1 Promoting lateral root formation [34] miR156/SPLs/NLP7 module Stimulating lateral root development [35] MhIDA Increasing primary root length and lateral root number [33] Citrus CcRR5 Promoting root length and lateral root number [37] CrWRKY57; CrABF3 Increasing primary root length and lateral root number [38] Table 1.
Key genes of root system architecture regulation in woody plants.
-
Tree species Bacteria Function Ref. Populus Pseudomonas Enhanced growth, nitrogen acquisition, and secondary root development [50] Burkholderia vietnamiensis Nitrogen fixation [51] Olea europaea Azospirillum, Bacillus Nitrogen fixation, phosphate and potassium solubilization [52] Abies nordmanniana Bacillus, Paenibacillus Enhanced seed germination, increased secondary root formation [53] Camellia sinensis Klebsiella, Serratia, Sporosarcina, Brevibacillius Nitrogen fixation,
promote growth,
chelate Fe ion[54] Eucalyptus Acidobacteria, Verrucomicrobia, Chloroflexi Nitrogen fixation, Nitrogen cycle [55] Table 2.
Microbes enhancing plant growth and nitrogen use efficiency in forest trees.
-
Plantation conditions Major limiting factors Key intervention/target Expected effect Cobble-and-sand riparian zone Extremely low available nitrogen Engineer ammonium-excreting diazotrophic like strains Burkholderia vietnamiensis Enhance BNF-derived N supply Nitrogen-poor marginal land Low concentration of nitrogen and shallow root Mixed forest with N2-fixing tree species; overexpress PsiSKP2B, PagWOX11/12a and CcRR5 genes Enhance the input of available nitrogen;
improve root elongation and lateral-root developmentFrequent rotation Nitrogen depletion Overexpress PuHox52, PtaNAC1, PdGNC, OsNLPs and OsNRTs Boost lateral-root development and N-uptake efficiency Organic-rich zone Low organic-N uptake efficiency; N emission in nitrogen cycle Enhance fungal colonization; enhance DNRA to retain nitrogen as ammonium Enhance nitrogen acquisition via fungal pathways; improve soil ammonium content Nitrogen-rich soil Restricted lateral-root development;
limited nitrogen assimilation rateOverexpress PsiSKP2B, PagWOX11/12a, CcRR5 and GS genes Increase soil-contact area to facilitate nitrogen acquisition; increase nitrogen assimilation rate Saline-alkaline soil Suppressed nitrification Overexpress PtAMT1;2 or AtAMT1;1 Increase ammonium uptake and salt tolerance Arid region Decreased soluble nitrogen; decreased NRTs gene expression Overexpress OsNRT1.1B Increase nitrate uptake and drought tolerance Table 3.
Strategies for improving NUE in forest plantations across contrasting cultivation environments.
Figures
(5)
Tables
(3)