-
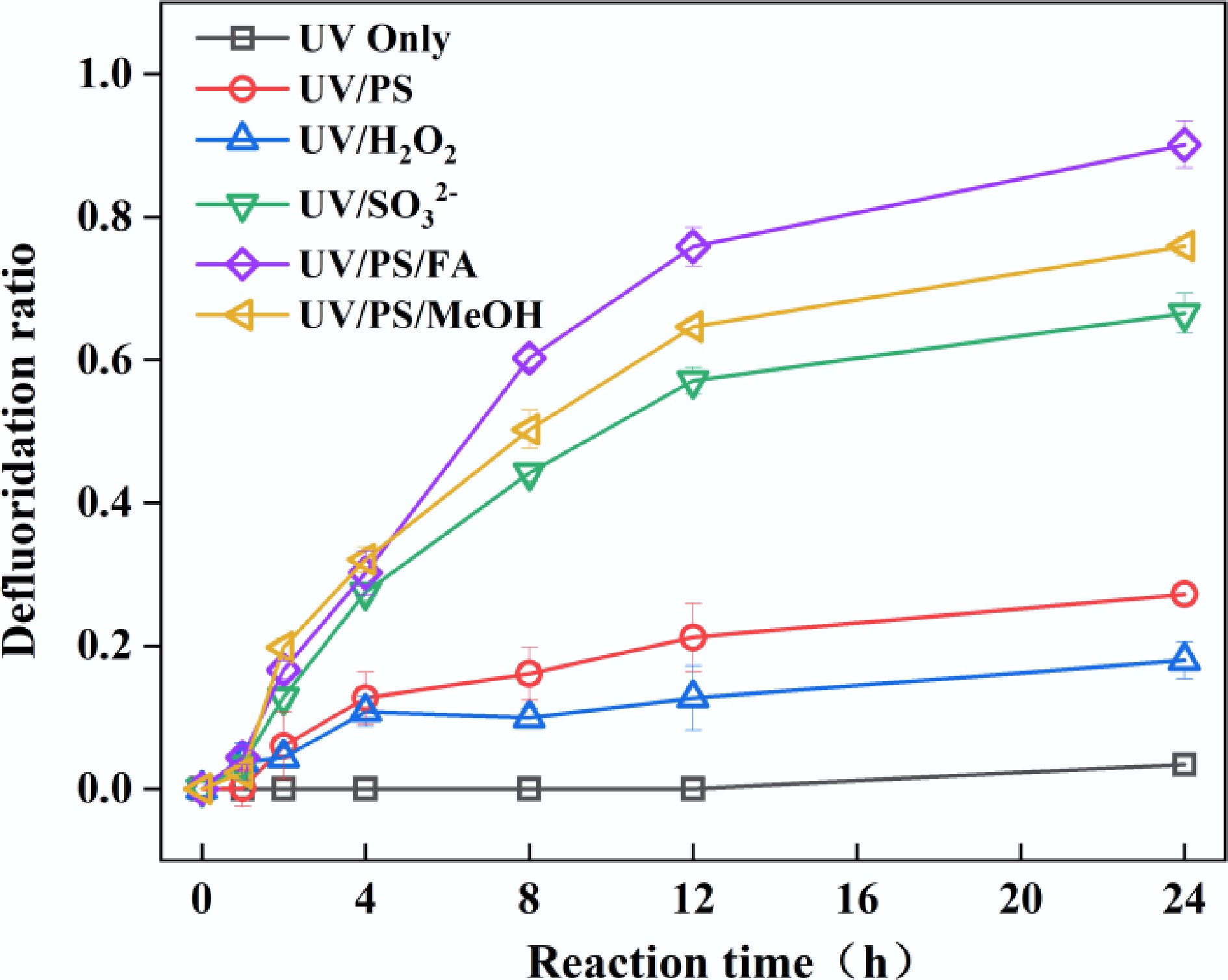
Figure 1.
Defluorination of PFOA in UV, UV/PS, UV/ H2O2, UV/SO32−, UV/PS/FA, and UV/PS/MeOH systems. Reaction conditions: PFOA = 20 µM, PS = 5 mM, H2O2 = 5 mM, SO32− = 10 mM, FA = 2 mM, MeOH = 2 mM, initial pH of 2.5, anaerobic environment for UV/SO32−, UV/PS/FA and UV/PS/MeOH systems, aerobic environment for the rest of the systems.
-
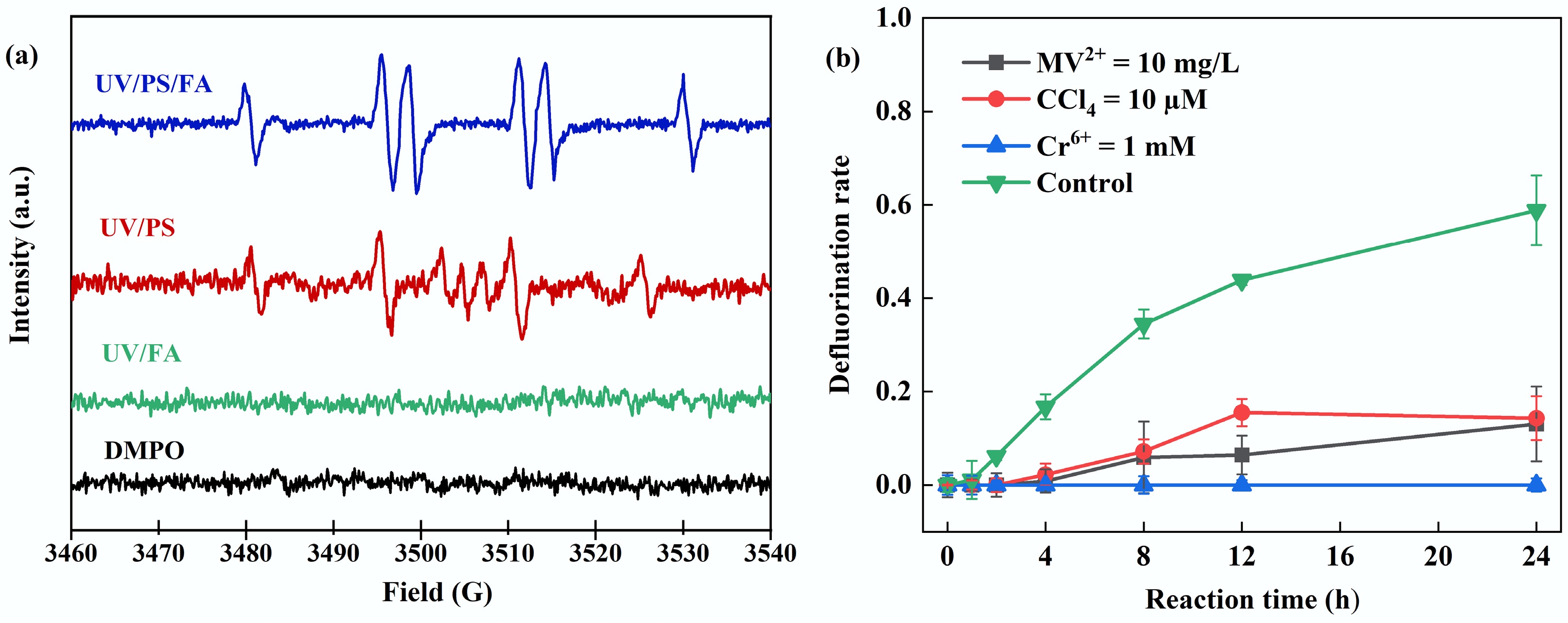
Figure 2.
(a) EPR analysis of UV/PS/FA, UV/PS, and UV/FA systems. (b) Free radical quenching experiments. Reaction conditions: PFOA = 20 µM, initial pH of 2.5, anaerobic environment. (a) PS = 2 mM, FA = 15 mM, DMPO = 100 mM, reaction time = 5 min, anaerobic environment. (b) PS = 4 mM, FA = 2 mM, MV2+ = 10 mg/L, CCl4 = 10 µM, Cr(VI) = 1 mM.
-
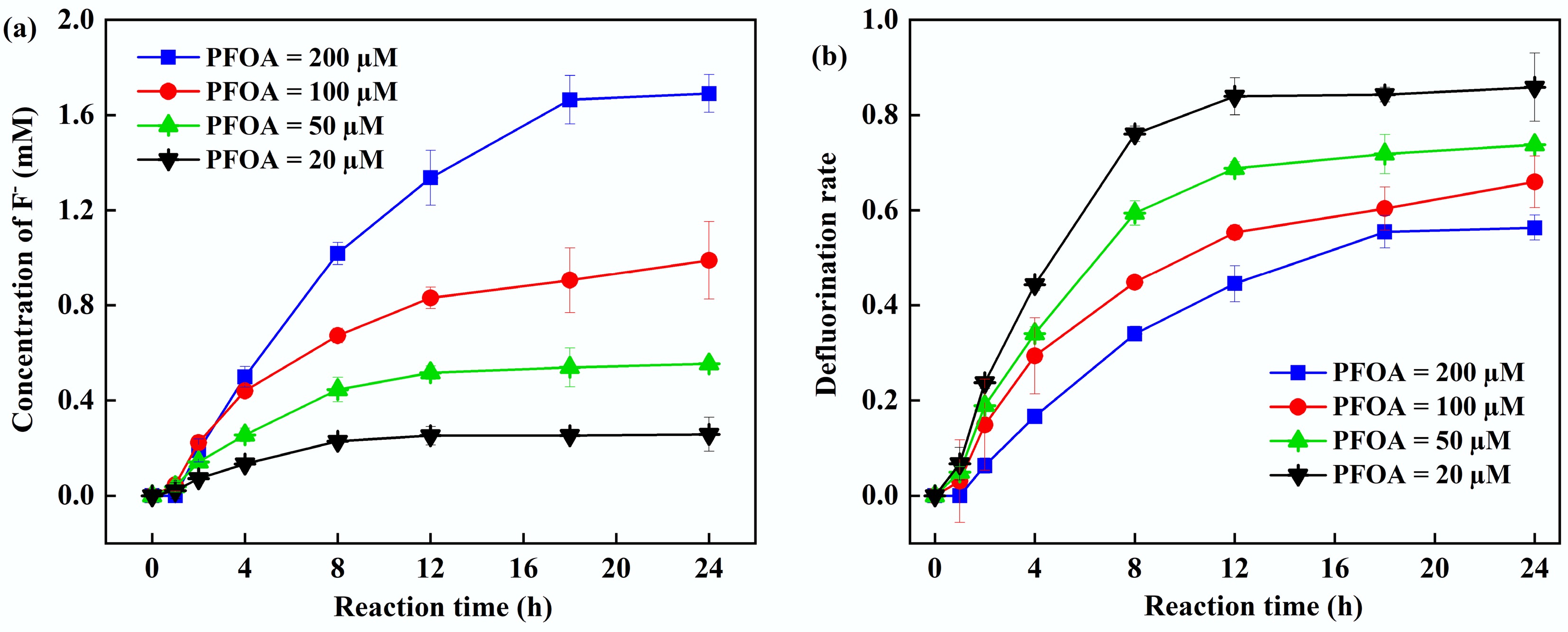
Figure 3.
(a) Fluoride ion concentration. (b) Defluorination rate at different initial PFOA concentrations. Reaction conditions: PFOA = 20–200 µM, PS = 5 mM, FA = 2 mM, initial pH of 2.5, anaerobic environment.
-
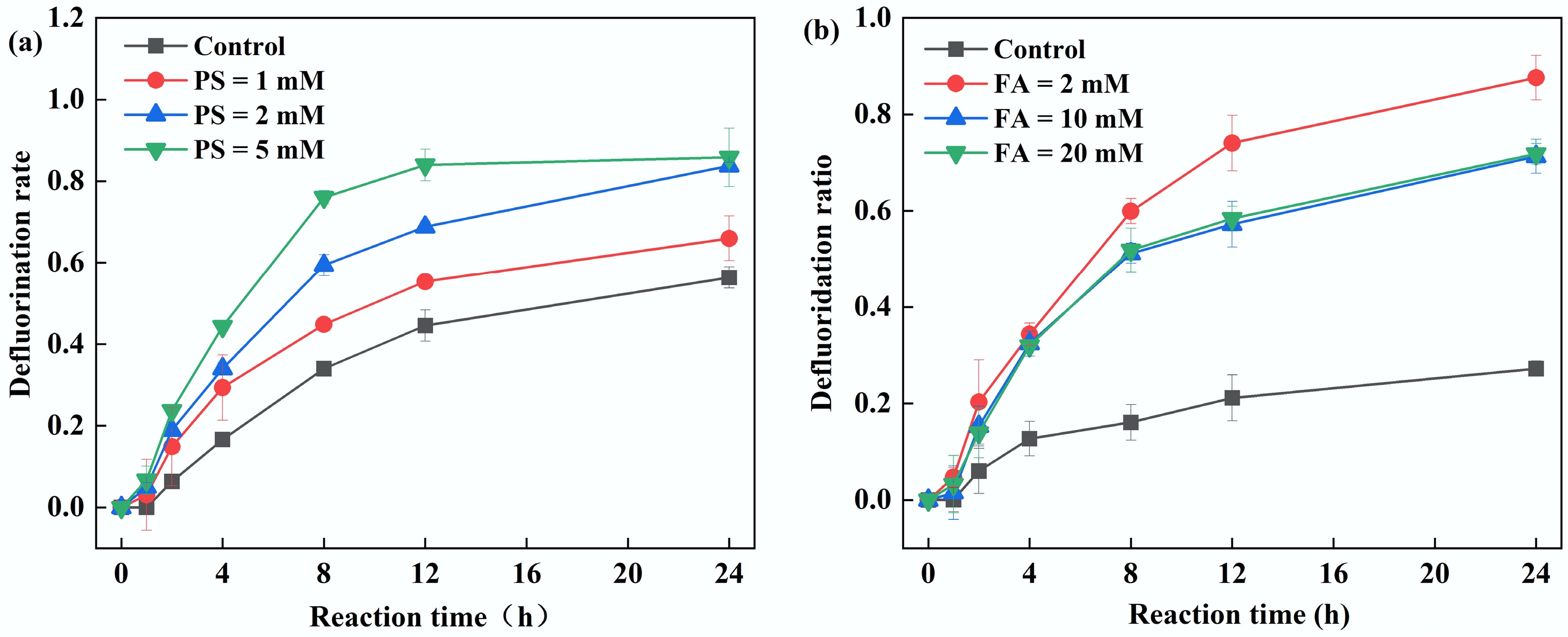
Figure 4.
Effect of (a) PS, and (b) FA concentration on PFOA defluorination by the UV/PS/FA system. Reaction conditions: PFOA = 20 µM, initial pH of 2.5, anaerobic environment. (a) PS = 1–5 mM, FA = 2 mM. (b) PS = 5 mM, FA = 2–20 mM.
-
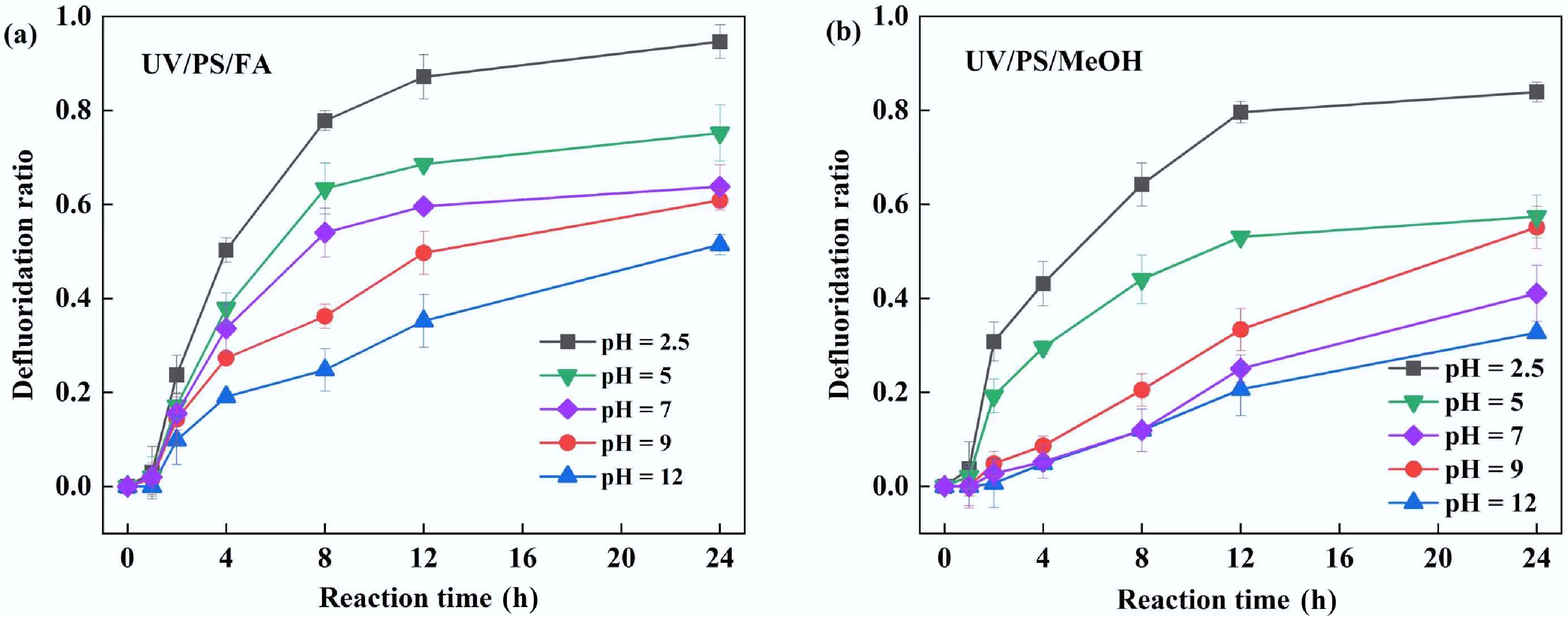
Figure 5.
Effect of initial pH on PFOA defluorination in (a) UV/PS/FA and (b) UV/PS/MeOH systems. Reaction conditions: PFOA = 20 µM, PS = 4 mM, initial pH = 2.5, 5, 7, 9, 12, anaerobic environment. (a) FA = 2 mM. (b) MeOH = 2 mM.
-
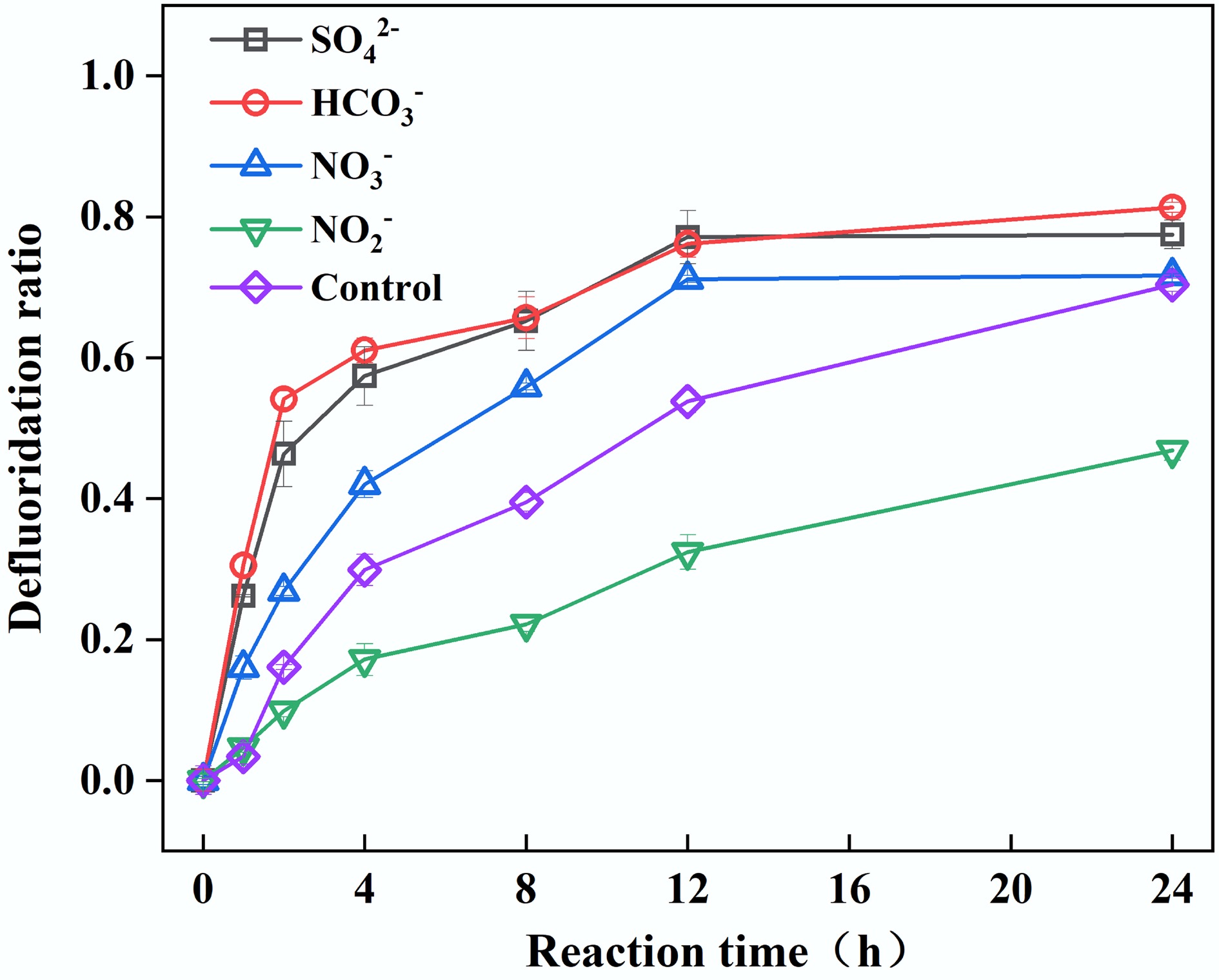
Figure 6.
Effect of NO3−, NO2−, SO42−, and HCO3− on PFOA defluorination. Reaction conditions: PFOA = 20 µM, PS = 4 mM, FA = 2 mM, NO3−, NO2−, SO42−, HCO3− = 10 mM, unadjusted pH, anaerobic environment.
-
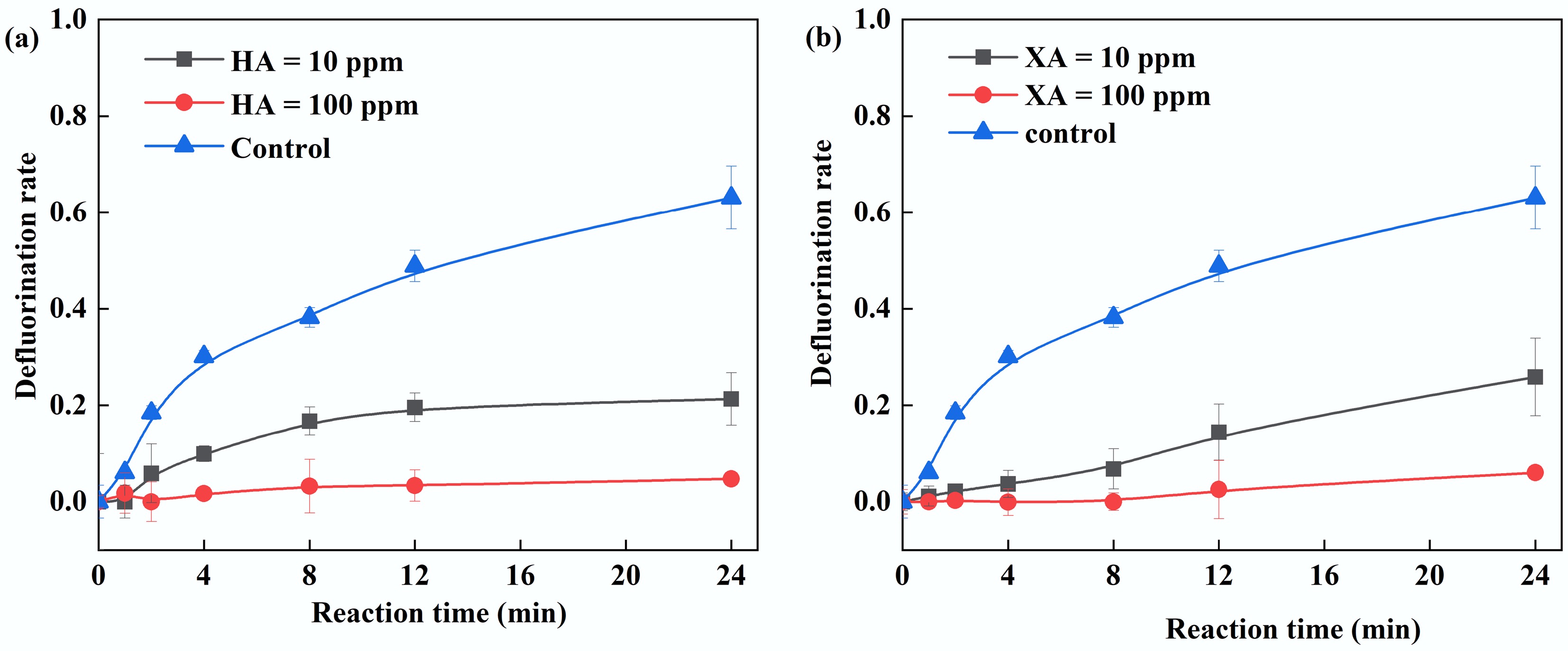
Figure 7.
(a) Effect of humic acid on PFOA defluorination. (b) Effect of xanthohumic acid on PFOA degradation. Reaction conditions: PFOA = 20 µM, PS = 4 mM, FA = 2 mM, initial pH of 2.5, anaerobic environment. (a) HA = 10–100 ppm, (b) XA = 10–100 ppm.
Figures
(7)
Tables
(0)