-
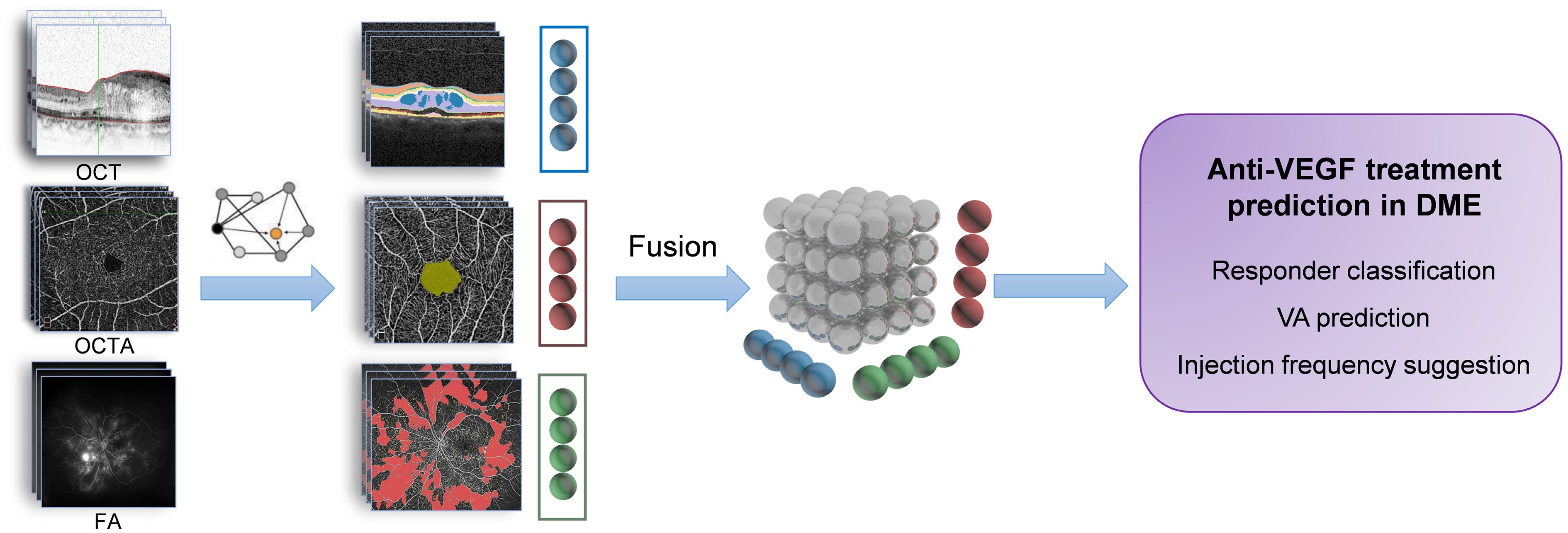
Figure 1.
Multimodal imaging fusion model to predict anti-VEGF treatment outcome in DME.
-
Year Authors Objective Imaging modality used Datasets AI model Outcome predicted Performance 2024 Baek et al.[4] Generate post-treatment OCT images, long-term anatomical prediction OCT + fundus 327 DME eyes from RCT KINGFISHER, one year follow-up GANs (CycleGAN, Pix2PixHD) Anatomical PPV, sensitivity, specificity, and kappa for residual fluid ranging from 0.500 to 0.889, 0.455 to 1.000, 0.357 to 0.857, and 0.537 to 0.929. PPV, sensitivity, specificity, and kappa for hard exudate were ranging from 0.500 to 1.000, 0.545 to 0.900, 0.600 to 1.000, and 0.642 to 0.894. 2022 Alryalat et al.[5] Predict anti-VEGF anatomical response (e.g., CST reduction) OCT 101 DME patients, three month follow-up U-Net, EfficientNet Anatomical The classification accuracy of classifying patients' images into good and poor responders was 75%. 2024 Meng et al.[6] Predict persistent DME after anti-VEGF via OCT-omics OCT 113 eyes from 82 patients with DME RF + Radiomics Anatomical The logistic classifier achieved a sensitivity of 0.904, specificity of 0.741, F1 score of 0.887, and AUC of 0.910. The SVM classifier showed a sensitivity of 0.923, specificity of 0.667, F1 score of 0.881, and AUC of 0.897. The BPNN classifier exhibited a sensitivity of 0.962, specificity of 0.926, F1 score of 0.962, and AUC of 0.982. OCT-omics scores were positively correlated with the rate of decline in CST after treatment (Pearson's R = 0.44). 2020 Rasti et al.[7] Predict anti-VEGF anatomical response (post-treatment retinal thickness) OCT 127 subjects treated for DME with three consecutive injections of anti-VEGF agents deep CNN Anatomical An average AUC of 0.866 in discriminating responsive from non-responsive patients, with an average precision, sensitivity, and specificity of 85.5%, 80.1%, and 85.0%. 2020 Cao et al.[8] Predict anti-VEGF anatomical response (good responders vs bad responder) OCT 712 DME eyes, treated with three monthly consecutive intravitreal conbercept injections OCT feature extractioin-CNN, responder prediction-RF/SVM Anatomical The sensitivity, specificity and AUC of responder prediction task was 0.900, 0.851, and 0.923. 2022 Xu et al.[9] Generate post-treatment OCT images, short-term anatomical prediction OCT 632 pairs of pre-therapeutic and post-therapeutic OCT images of patients with DME GAN(pix2pixHD) Anatomical The MAE of the CMT between the synthetic OCT images and the actual images was 24.51 ± 18.56 μm. 2022 Xie et al[10] Predict BCVA, CST reduction using OCT OCT n = 254, multi-nation cohort, six months SVM, RF Functional and anatomical The ACC and AUC of structural predictions of retinal pigment epithelial detachment were close to 1.000. The MAE and MSE of visual acuity predictions were nearly 0.3 to 0.4 logMAR. The ACC of treatment plan regarding continuous injection was approaching 70% 2020 Liu et al.[11] Predict CST and BCVA outcomes post three injections OCT Multi-center cohort, 363 OCT images and 7,587 clinical data records from 363 eyes Ensemble (RF + DL) Functional and anatomical For CFT prediction, MAE, RMSE, and R2 was 66.59, 93.73, and 0.71 in the training set, with an AUC of 0.90 for distinguishing the eyes with good anatomical response. For BCVA prediction, MAE, RMSE, and R2 was 0.19, 0.29, and 0.60, in the training set, with an AUC of 0.80 for distinguishing eyes with a good functional response. 2022 Zhang et al.[12] Predict VA one month after anti-VEGF therapy OCT (features extracted manually)
+ clinical data281 DME eyes linear regression + RF regression Functional For the prediction of VA variance at one month, the MAEs were 0.164–0.169 logMAR, and the MSEs were 0.056–0.059 logMAR. 2024 Wang et al.[13] Predict BCVA post-anti-VEGF therapy in telemedicine OCT + clinical/
demographic dataAPTOS 2021 Dataset, pre-treatment and post-treatment 2864 OCT images of 221 patients Semi-supervised CNN Functional Accuracy of VA prediction 38.18%, MAE 0.106, RMSE 0.141, R2 0.722 2022 Kar et al.[14] Predict anti-VEGF BCVA outcomes (responders vs non-responder) UWFA+OCT DME eyes from the PERMEATE study (29 eyes for UWFA study and 28 eyes for OCT study) ResNet50, ResNet101, Inception-v3 and DenseNet201 Functional The best performing DL model had a mean AUC of 0.507 ± 0.042 on UWFA images, and highest observed AUC of 0.503 for fluid-compartmentalized OCT images. 2021 Prasanna et al.[15] Predicting therapeutic durability of intravitreal aflibercept injection UWFA 13 eyes with DME and 14 eyes with RVO from the PERMEATE study Machine learning Functional The cross-validated AUC was 0.77 ± 0.14 using baseline leakage distribution features and 0.73 ± 0.10 for the UWFA baseline tortuosity measures. 2021 Gallardo et al.[16] Predict low and high treatment demand in patients with DME OCT+demographic data 333 eyes (285 patients) with RVO or DME RF Treatment interval Mean AUC of 0.76 and 0.78 for low and high demand in RVO and DME model. PPV, Positive Predictive Value; RCT, Randomized Controlled Trial; GAN, Generative Adversarial Network; CNN, Convolutional Neural Network; RF, Random Forest; SVM, Support Vector Machine; MSE, Mean Squared Error; MAE, Mean Absolute Error; RMSE, Root Mean Squared Error, DL: Deep Learning. Table 1.
Summary of research articles on applicability of AI in DME treatment outcome prediction.
Figures
(1)
Tables
(1)