-
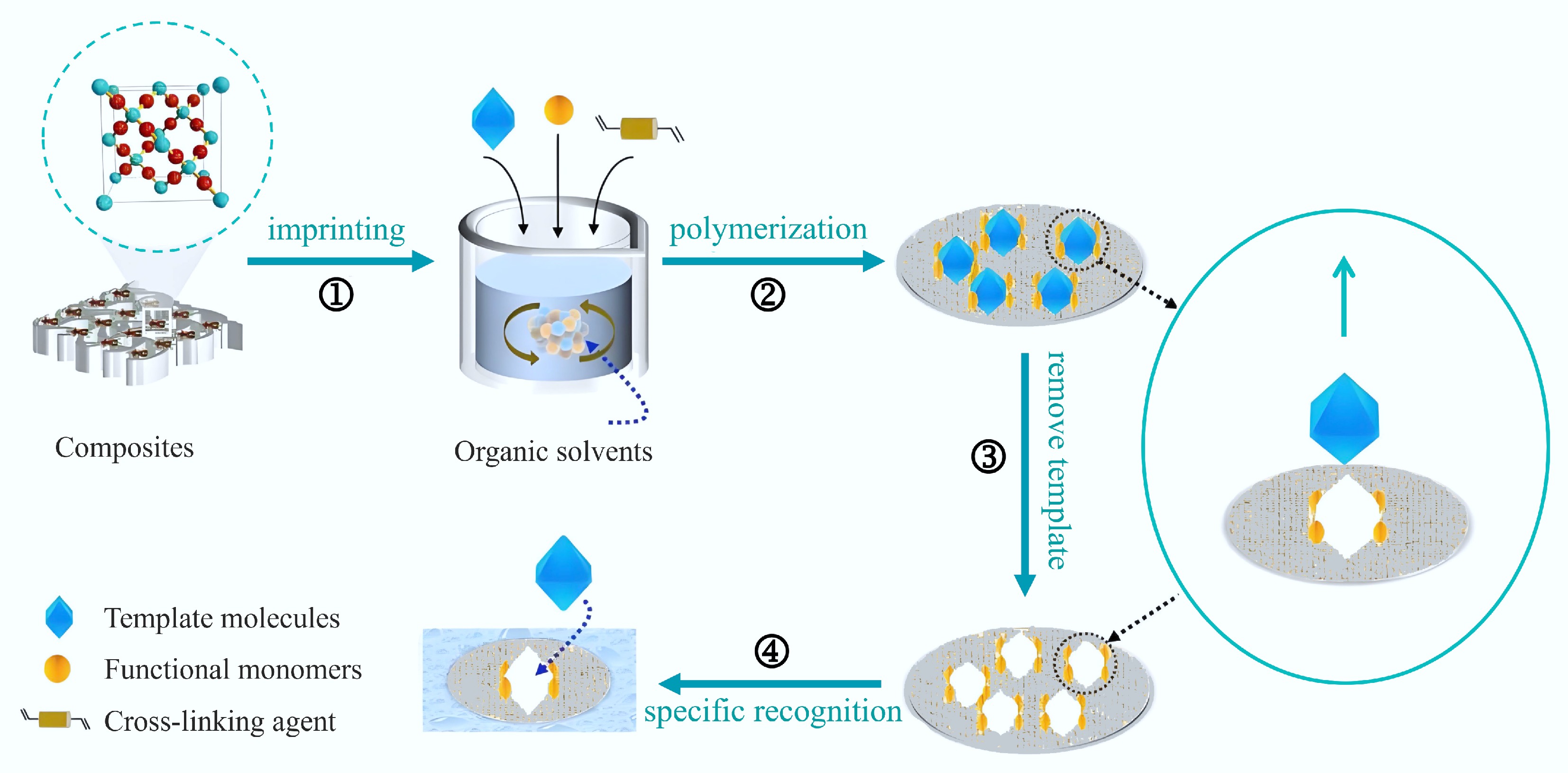
Figure 1.
Preparation of MIPs[4].
-
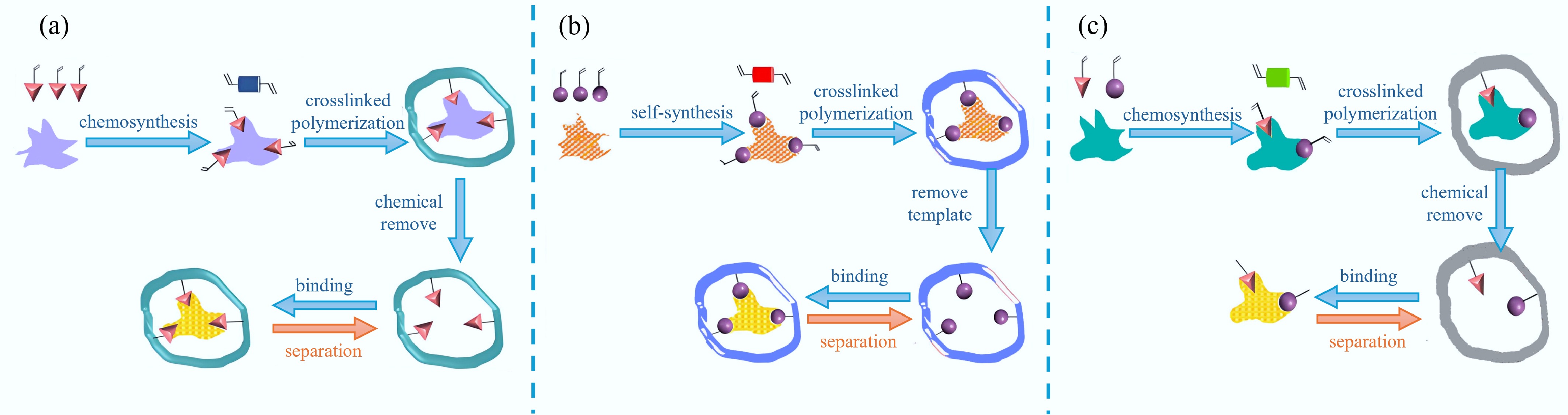
Figure 2.
Preparation of MIPs by (a) covalent, (b) non-covalent, and (c) semi-covalent[23].
-

Figure 3.
Simplified schematic of MIP electropolymerization on the electrode surface[50].
-
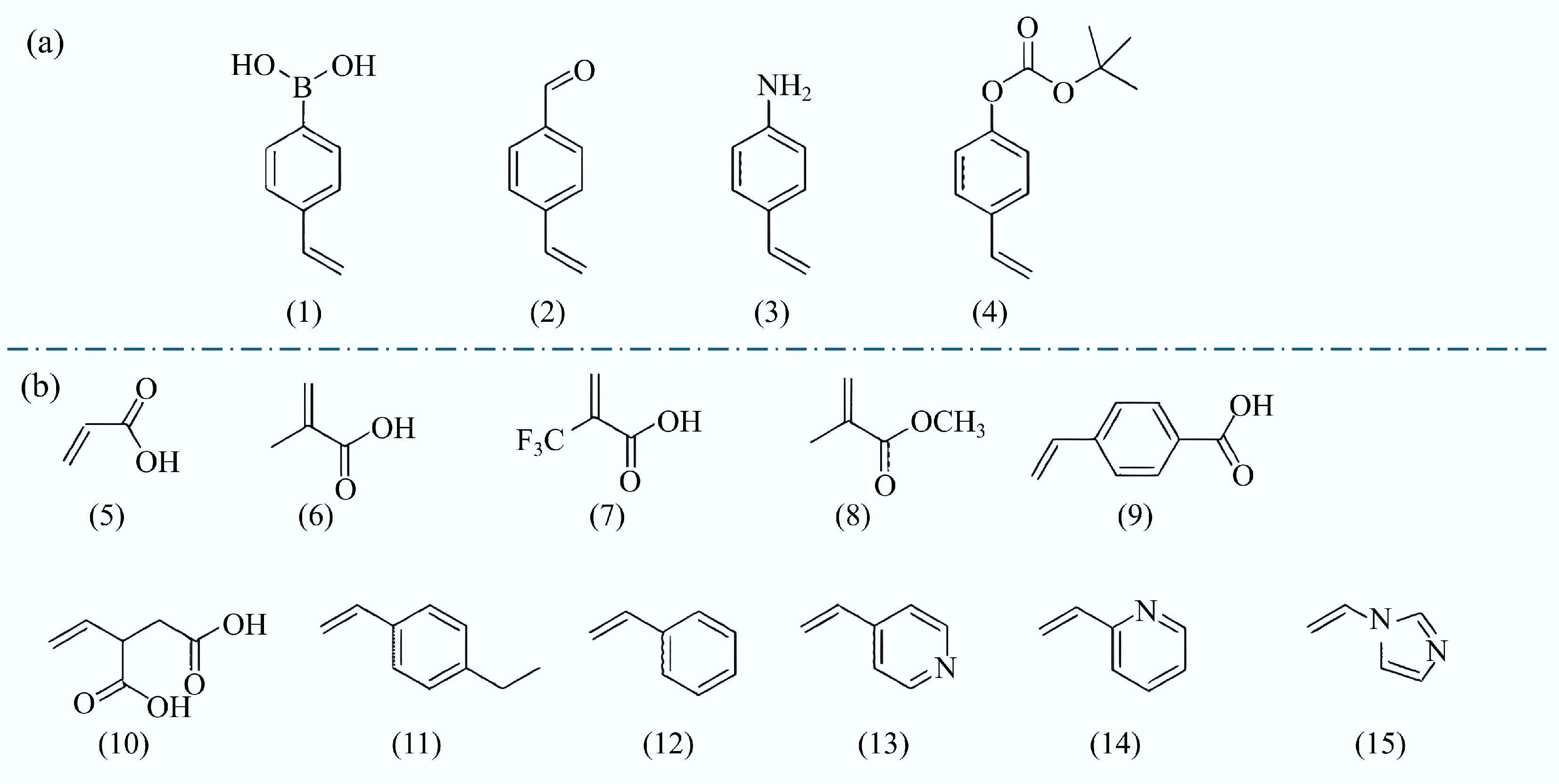
Figure 4.
Common functional monomers used in the molecular imprinting process. (a) Covalent. (1) 4-vinyl phenylboric acid, (2) 4-vinyl benzaldehyde, (3) 4-Vinyl aniline, and (4) Tert-butyl p-phenyl carbonate. (b) Non-covalent. (5) Acrylic acid (AA), (6) Methacrylic acid (MAA), (7) Trifluoromethylacrylic acid (TFMAA), (8) Methyl methacrylate (MMA), (9) P-vinylbenzoic acid, (10) Itaconic acid, (11) 4-ethylstyrene, (12) Styrene, (13) 4-vinylpyridine (4-VP), (14) 2-vinylpyridine (2-VP), and (15) 1-vinyl imidazole[58].
-
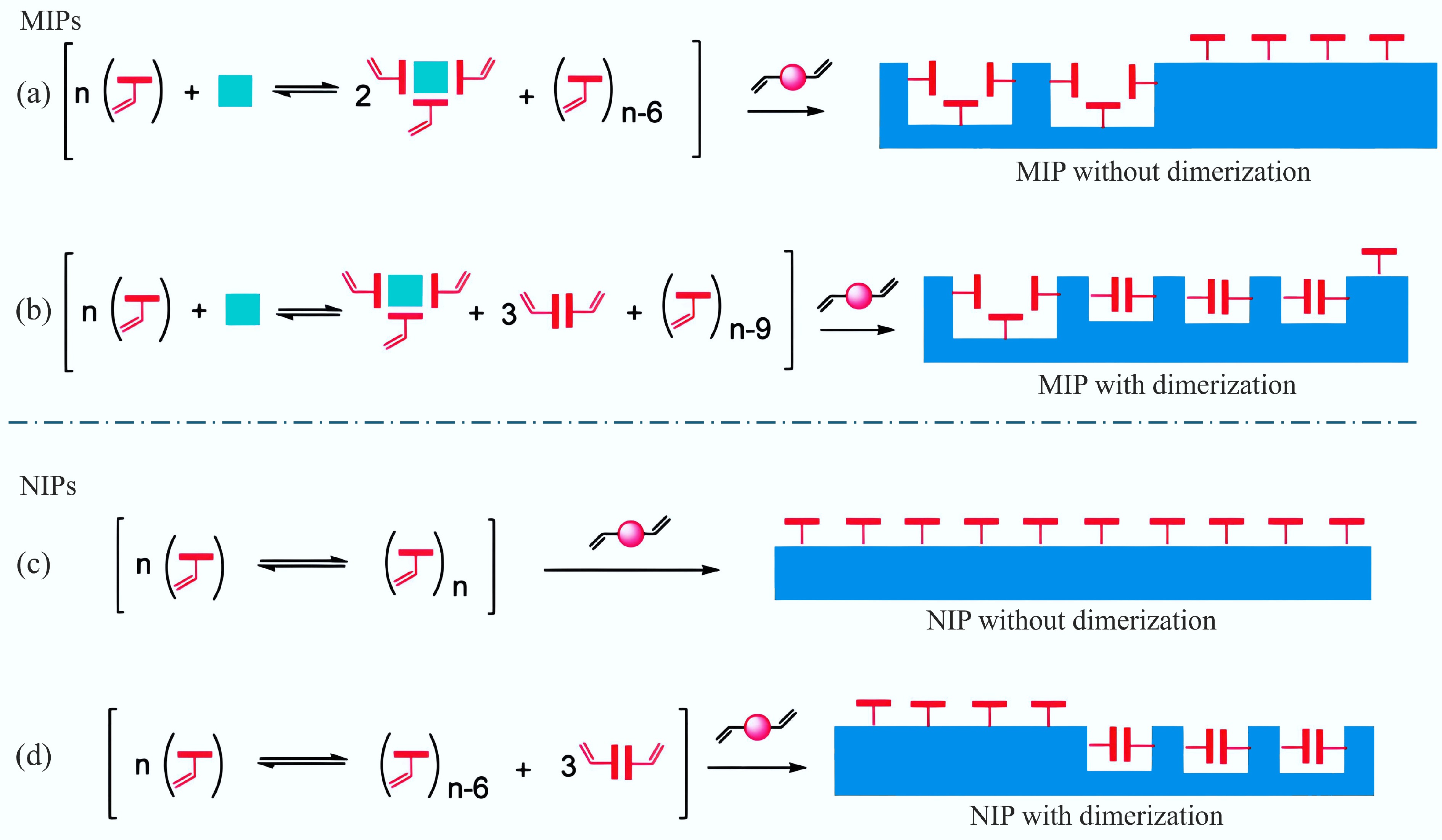
Figure 5.
(a), (b) Illustration of a comparison of imprinted polymers, and (c), (d) non-imprinted polymers formed from functional monomers with or without dimerization ability[58].
-
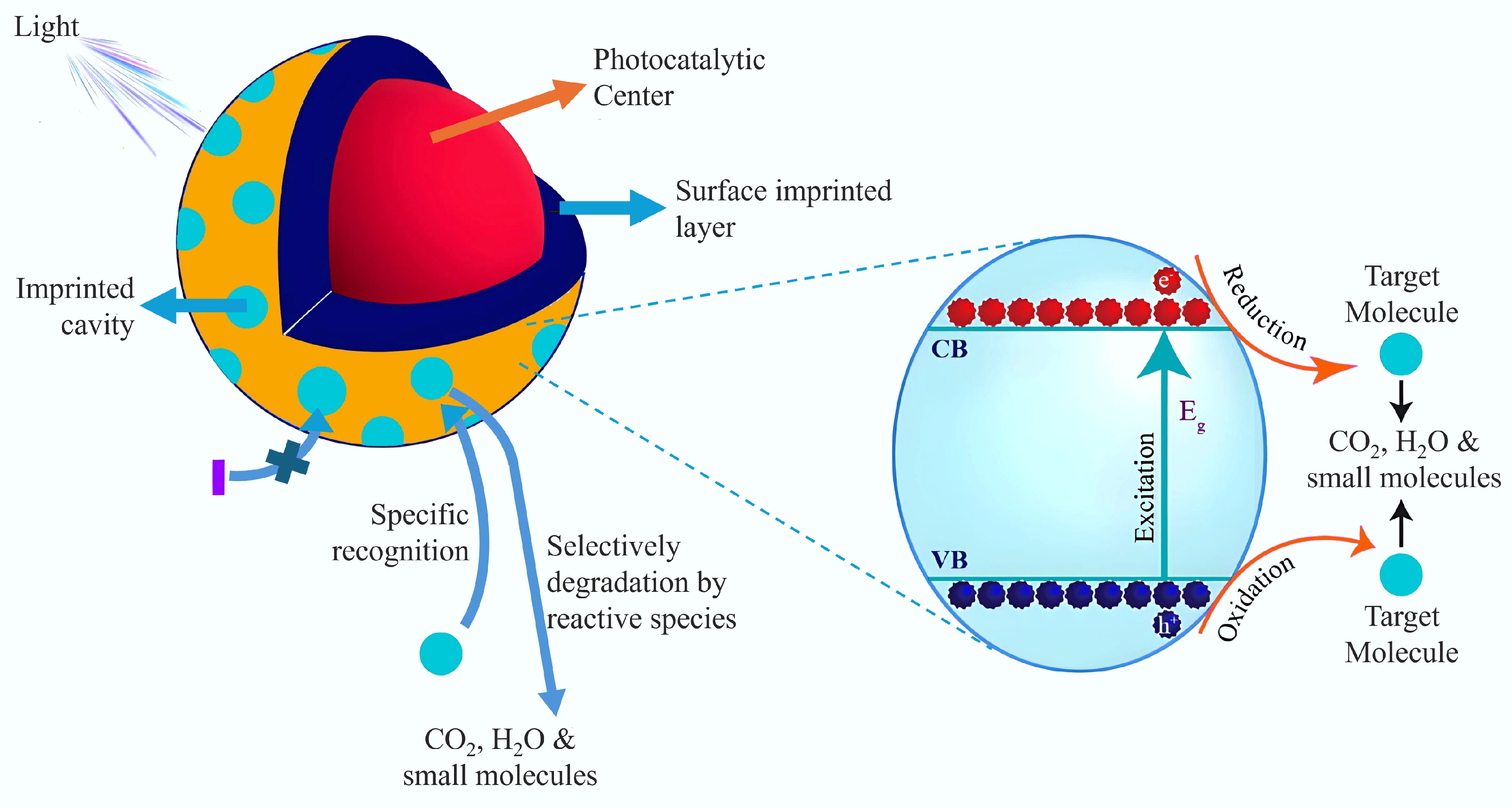
Figure 6.
Principle of MIP selective photocatalytic degradation[87].
-
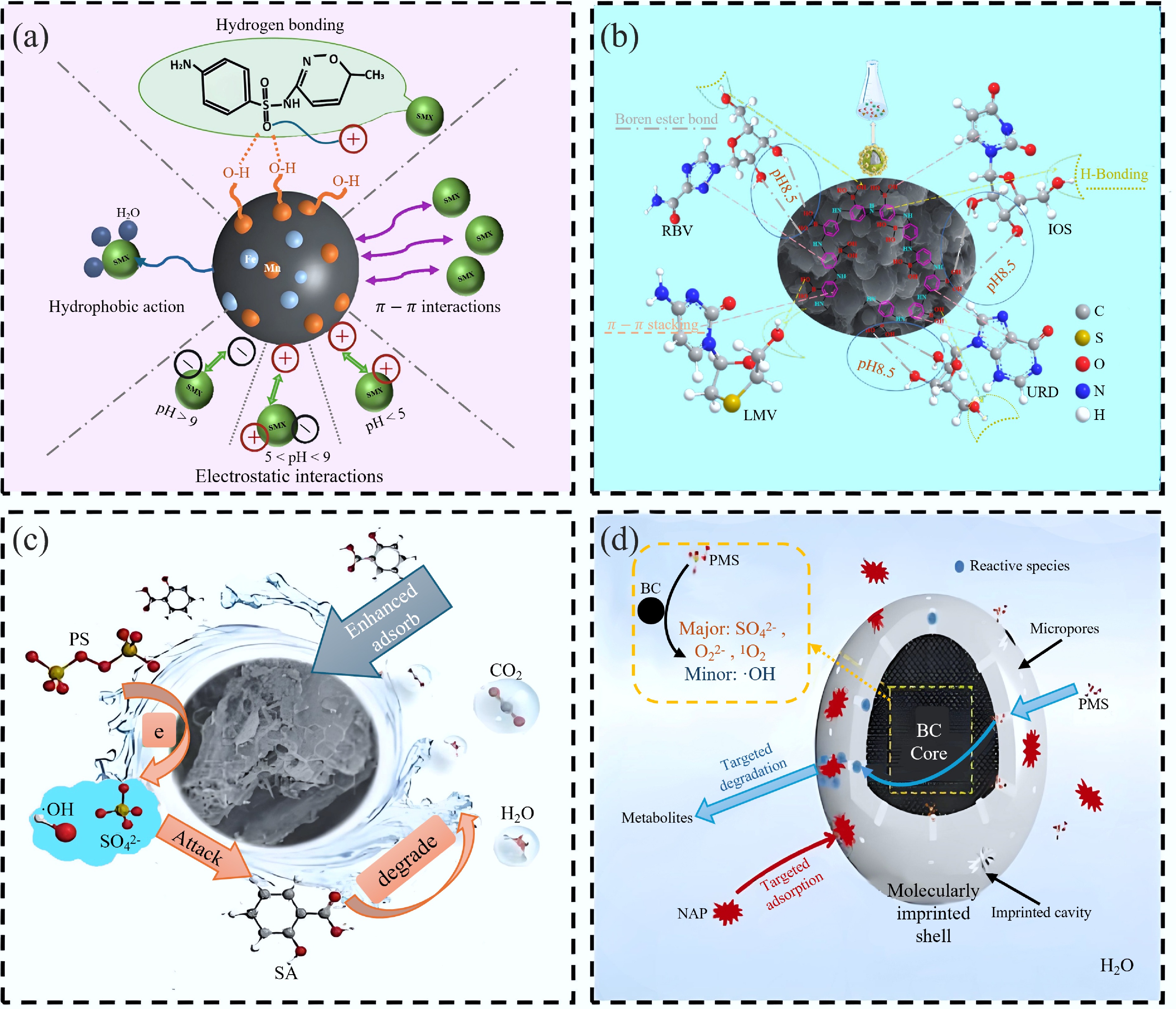
Figure 7.
(a) Main and other mechanisms of SMX adsorption onto MIP-BC[68]. (b) Adsorption mechanism of C@H@B-MIPs on RBV, LMV, URD, and IOS[59]. (c) Performance and specific recognition mechanism of Fe3O4/BC as an efficient persulfate activator for removing salicylic acid from wastewater[36]. (d) Mechanisms controlling NAP-targeted degradation in the MIP@BC/PMS system[38].
-
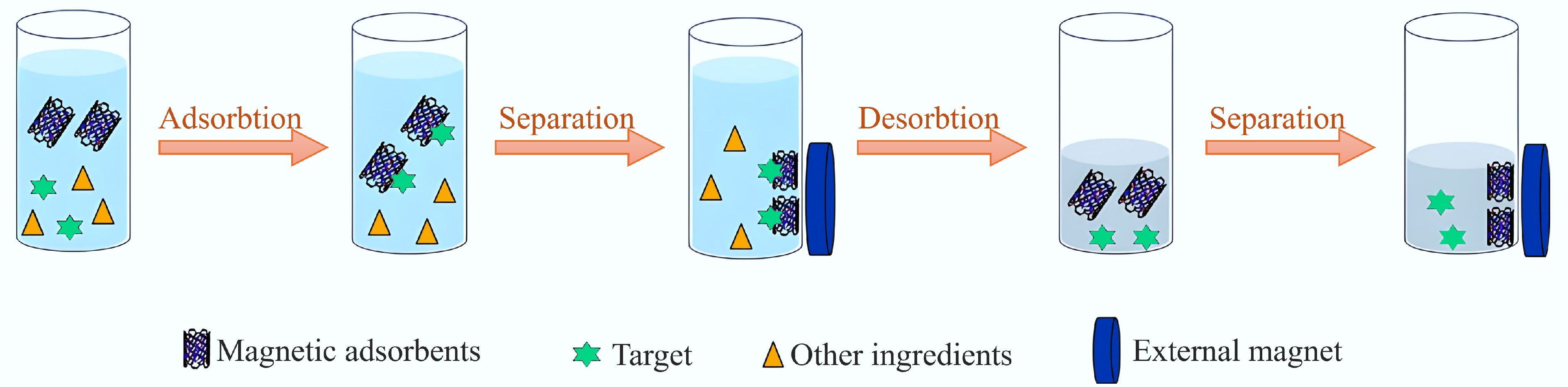
Figure 8.
Schematic of the MSPE program[125].
-
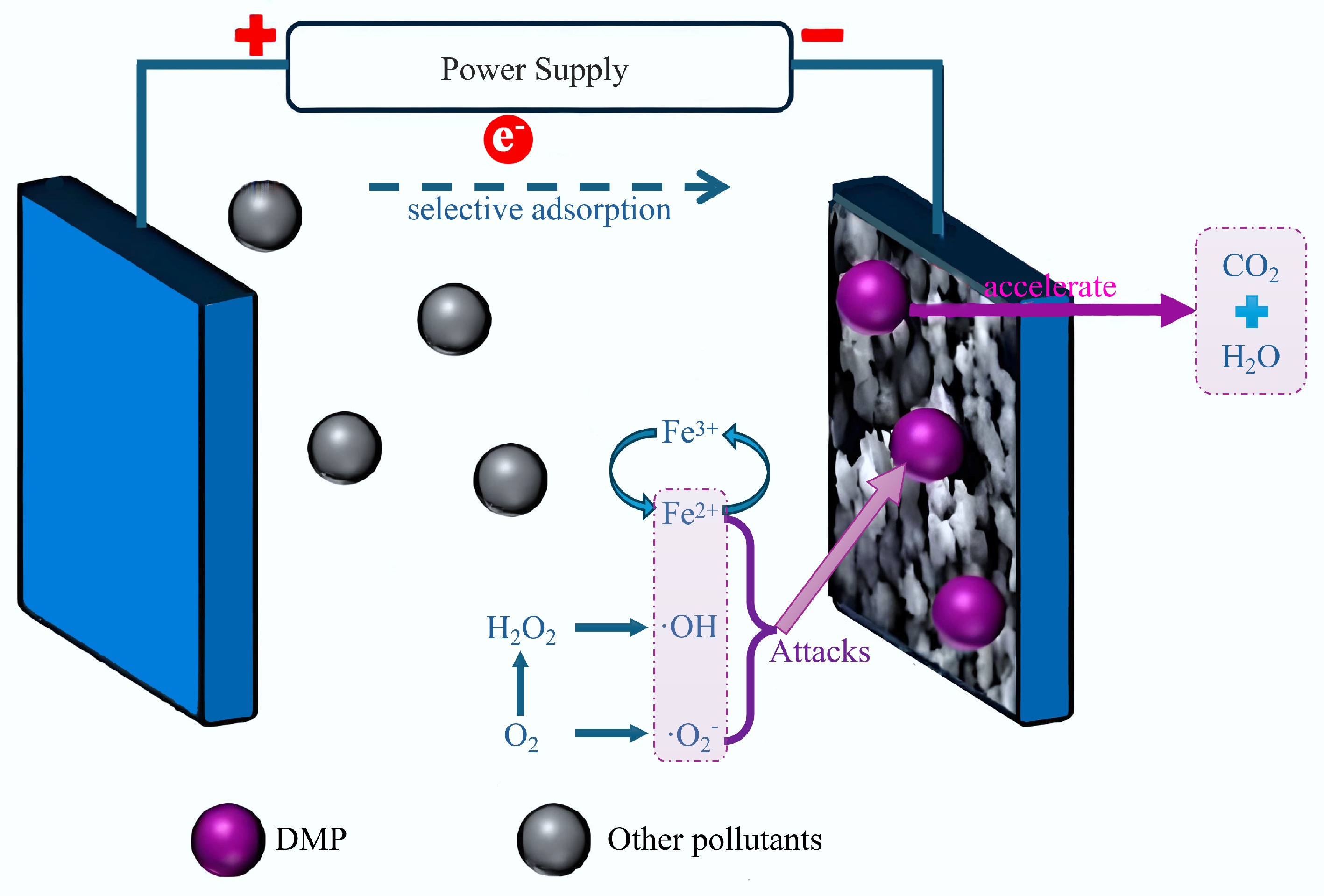
Figure 9.
Reaction mechanism for the degradation of DMP using an electrofenton system[18].
-
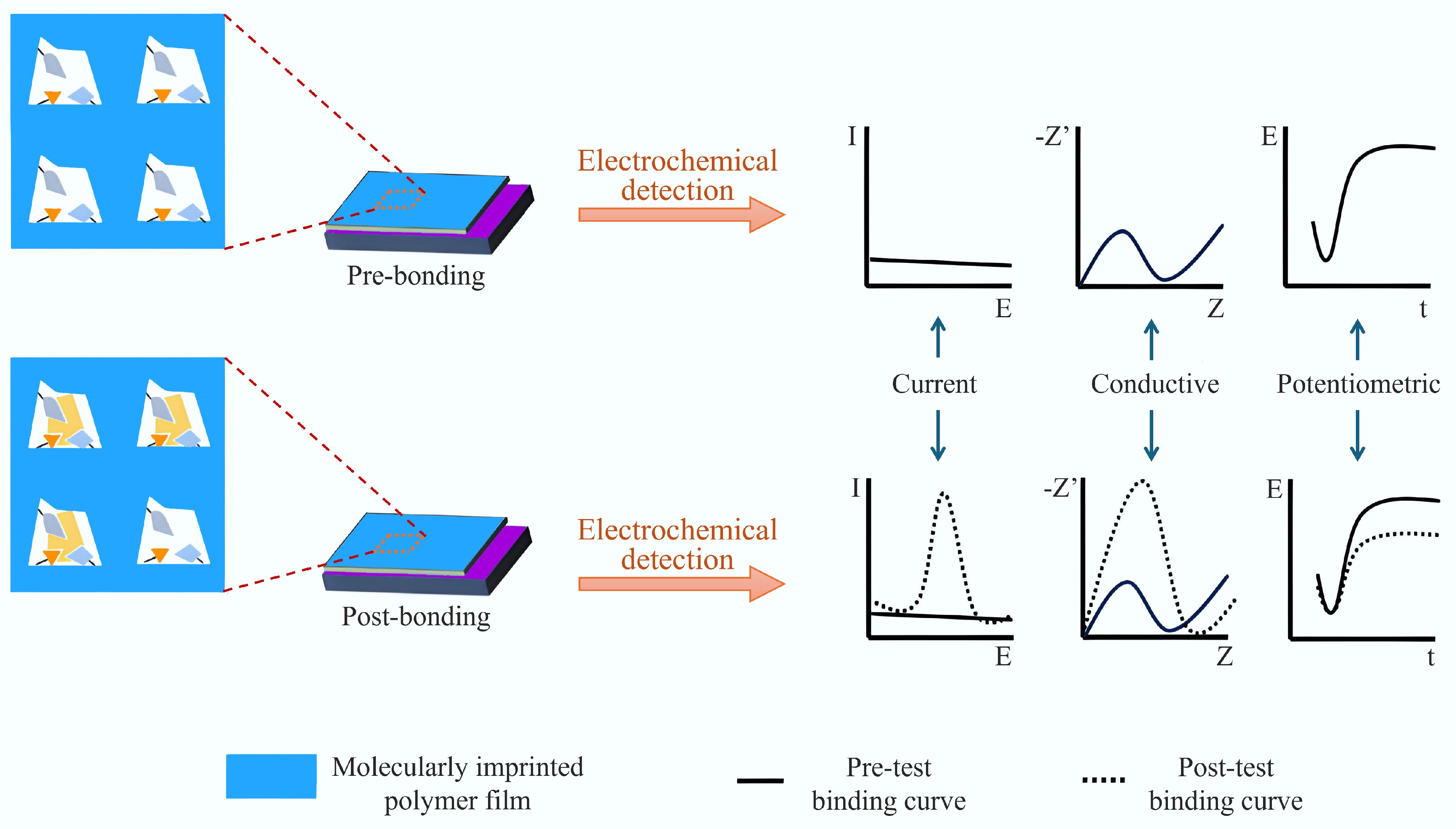
Figure 10.
Working principle and classification of molecularly imprinted electrochemical sensors[20].
-
Preparation method Composite material Functional monomer Crosslinker Initiator Target molecule Application Ref. Precipitation polymerization MI-FBC MAA EDGMA AIBN Salicylic acid (SA) Water pollution control [36] MBC@MIPs MAA EDGMA AIBN Oxytetracycline (OTC) Water pollution control [37] MIP@BC MAA EDGMA AIBN Naphthalene (NAP) Water pollution control [38] A-SBC@MIP MAA EDGMA AIBN Carbaryl Solid-phase extraction (SPE) [42] MIP@BC MAA EDGMA AIBN Dimethyl phthalate (DMP) Electrical Fenton system [18] A-SBC@MIP MAA EDGMA AIBN Metanaphos Solid-phase extraction (SPE) [40] BC/Cr2O3/Ag/MIP/GCE AM, MAA EDGMA AIBN Nitrofurazone (NFZ) Electrochemical sensor [41] Emulsion polymerization MMIPMs MAA DVB AIBN Tetracycline (TC) Magnetic solid-phase extraction (MSPE) [43] MIPMs MAA DVB AIBN Tetracycline (TC) Solid-phase extraction (SPE) [44] Electropolymerization MIP/TBC/GCE o-PD, o-AP − − Norfloxacin (NOR) Electrochemical sensor [21] IIP-BBC/GCE
(Pb-IIP-BBC/GCE
Cd-IIP-BBC/GCE)L-Cys − − Pb2+, Cd2+ Electrochemical sensor [20] MIP-DBP-CTS/F-CC3/GCE CTS Glutaraldehyde − Dibutyl phthalate (DBP) Electrochemical sensor [45] Table 1.
Synthesis methods, materials, target molecules and applications of MIBs
-
Preparation method Key feature Advantage Limitation Ref. Precipitation polymerization Heterogeneous reaction, polymer precipitation on substrate surface Simple operation, controllable pore structure, low cost, high yield Imprint sites may be buried, high mass transfer resistance, high solvent consumption [28] Emulsion polymerization Biphasic system, surfactant-stabilized micelles as templates Enables preparation of nano-/micron-scale spherical materials with tunable particle size and morphology, featuring uniform biochar dispersion Requires surfactant removal, involving complex processes with poor reproducibility [43] Electropolymerization Electrochemical in-situ polymerization on conductive substrates Precise film thickness control, process monitoring capability, excellent reproducibility, and rapid response Limited to conductive substrates, restricted monomer selection, and film uniformity dependent on parameter optimization [49] Sol-gel method Silane precursors undergo hydrolysis and condensation
to form gelsMild conditions, aqueous phase compatibility, high thermal stability and mechanical strength, excellent film-forming properties Gel shrinkage may cause cavity deformation, slower mass transfer, and complex kinetic control [51] Table 2.
Advantages and disadvantages of precipitation polymerization, emulsion polymerization, electrochemical polymerization, and sol-gel methods
-
Material type Adsorption capacity Selectivity (imprinting factor) Recyclability Cost Primary application scenario Unmodified BC Medium-high Low Medium Low Non-selective adsorption water purification Standard MIPs Medium High High Medium-high Solid-phase extraction, sensor identification MIB composite material High High High-very high Medium Selective environmental remediation, sensing, advanced catalysis Metal-organic framework (MOF) Very high Medium-high Low-medium (poor water stability) High Gas storage, catalysis, specific adsorption Covalent Organic Frameworks (COFs) High Medium-high Medium High Chromatographic separation, catalysis, precision adsorption Table 3.
Performance comparison of MIB composite materials with other adsorption/recognition materials
Figures
(10)
Tables
(3)