-
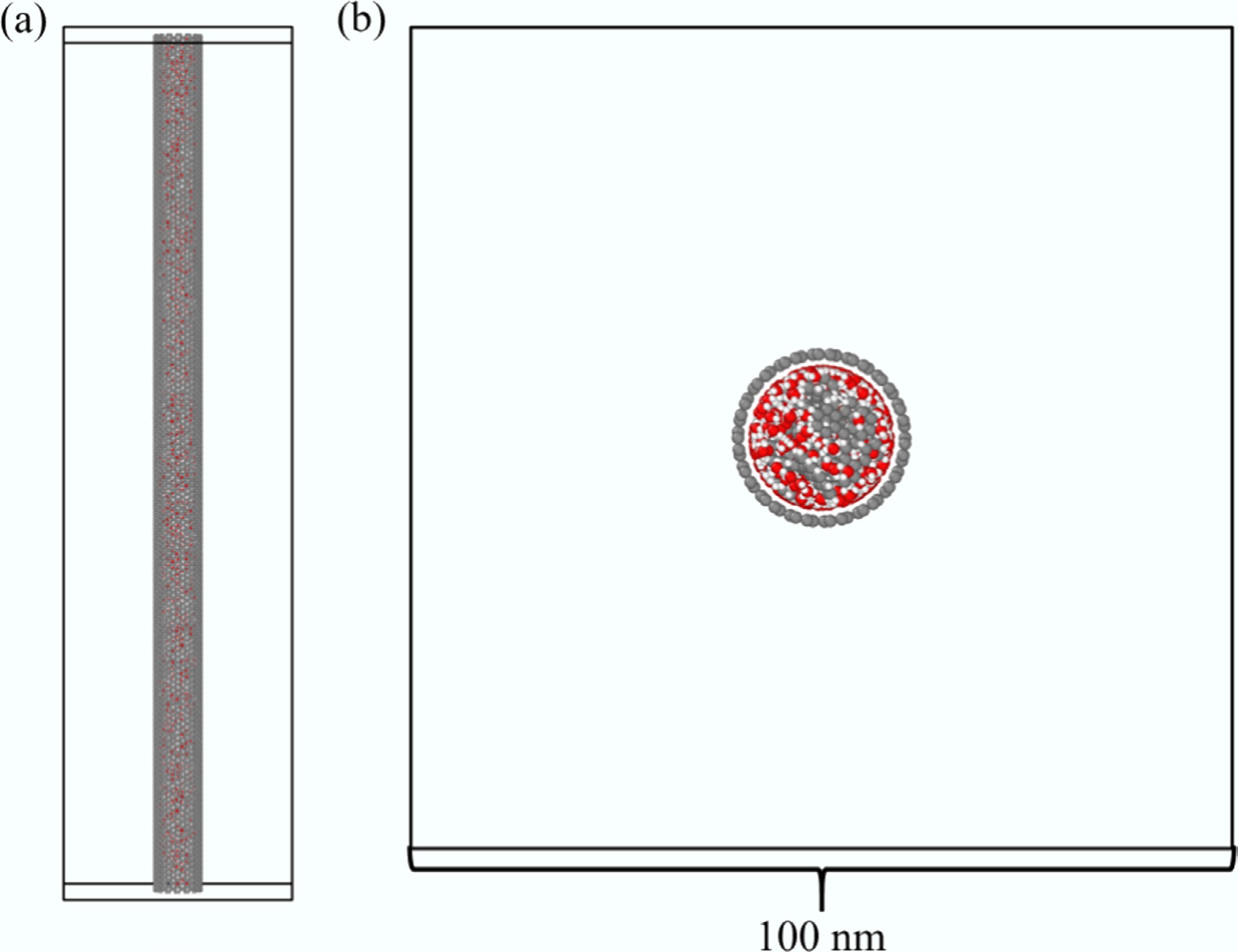
Figure 1.
Initial configuration of the simulated systems. (a) Side view; (b) Cross-sectional view.
-
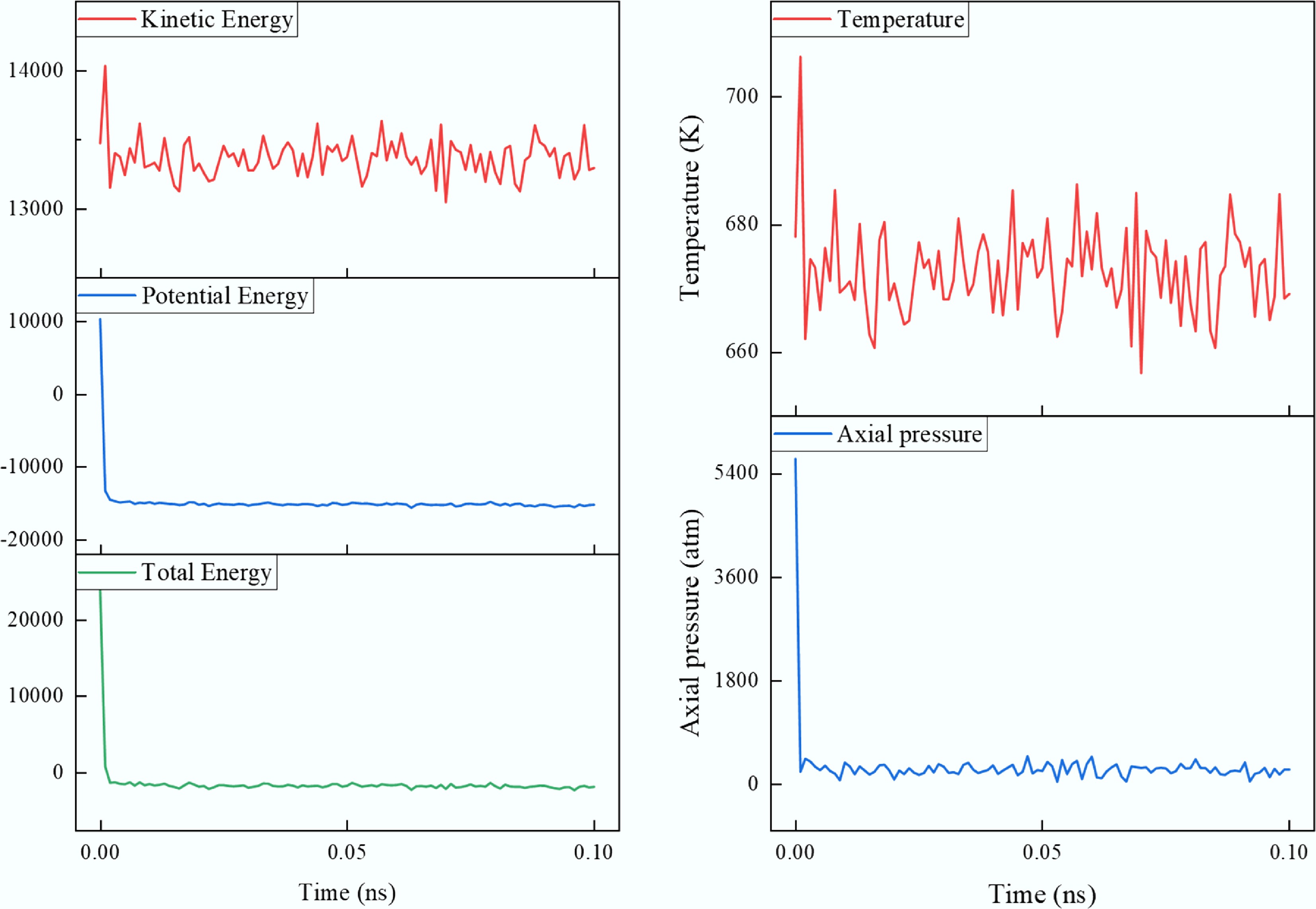
Figure 2.
Fluctuations of energy, pressure, and temperature during the pre-equilibration stage.
-
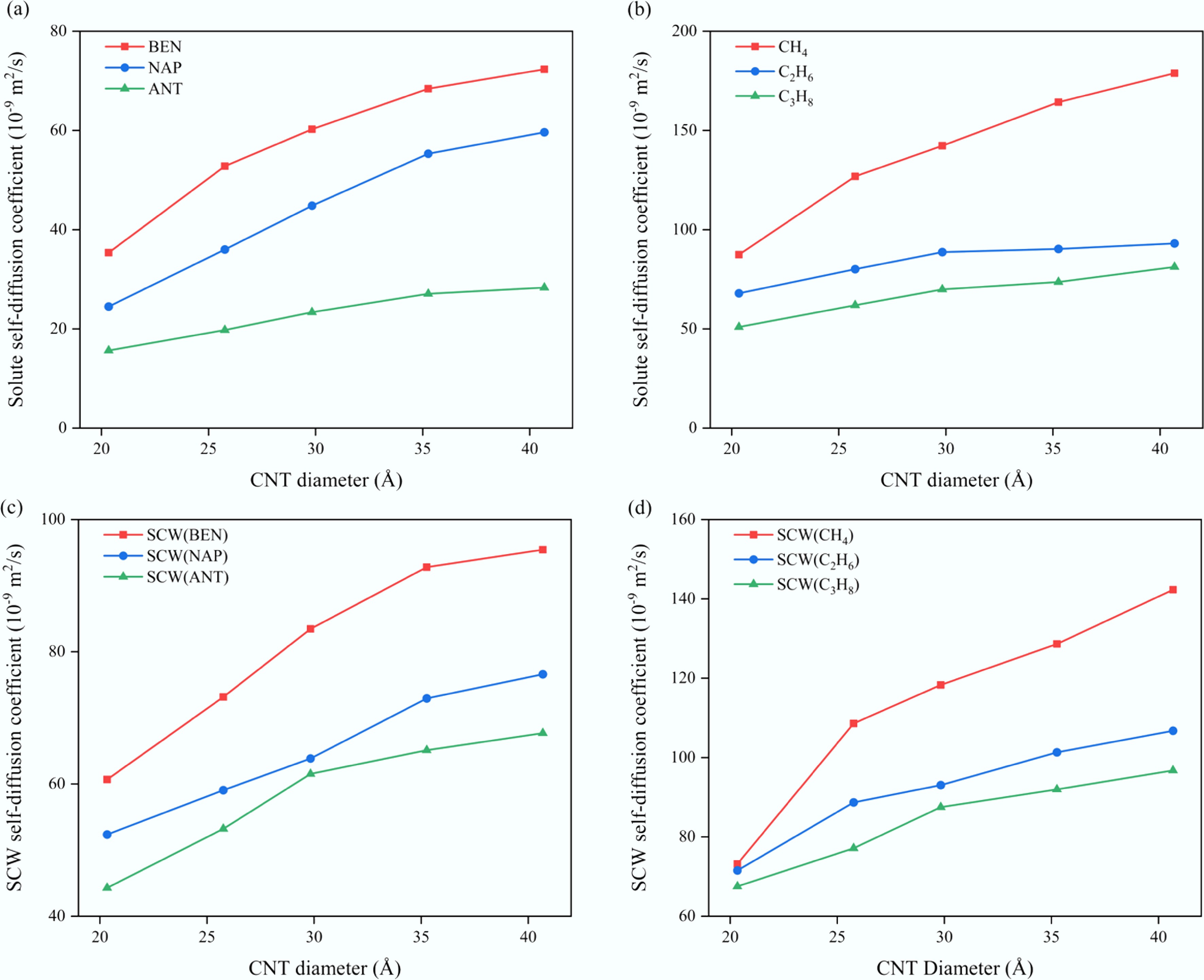
Figure 3.
Variation of self-diffusion coefficients with CNT diameter for (a) aromatic solutes, (b) aliphatic solutes, (c) SCW in aromatic systems, and (d) SCW in aliphatic systems.
-
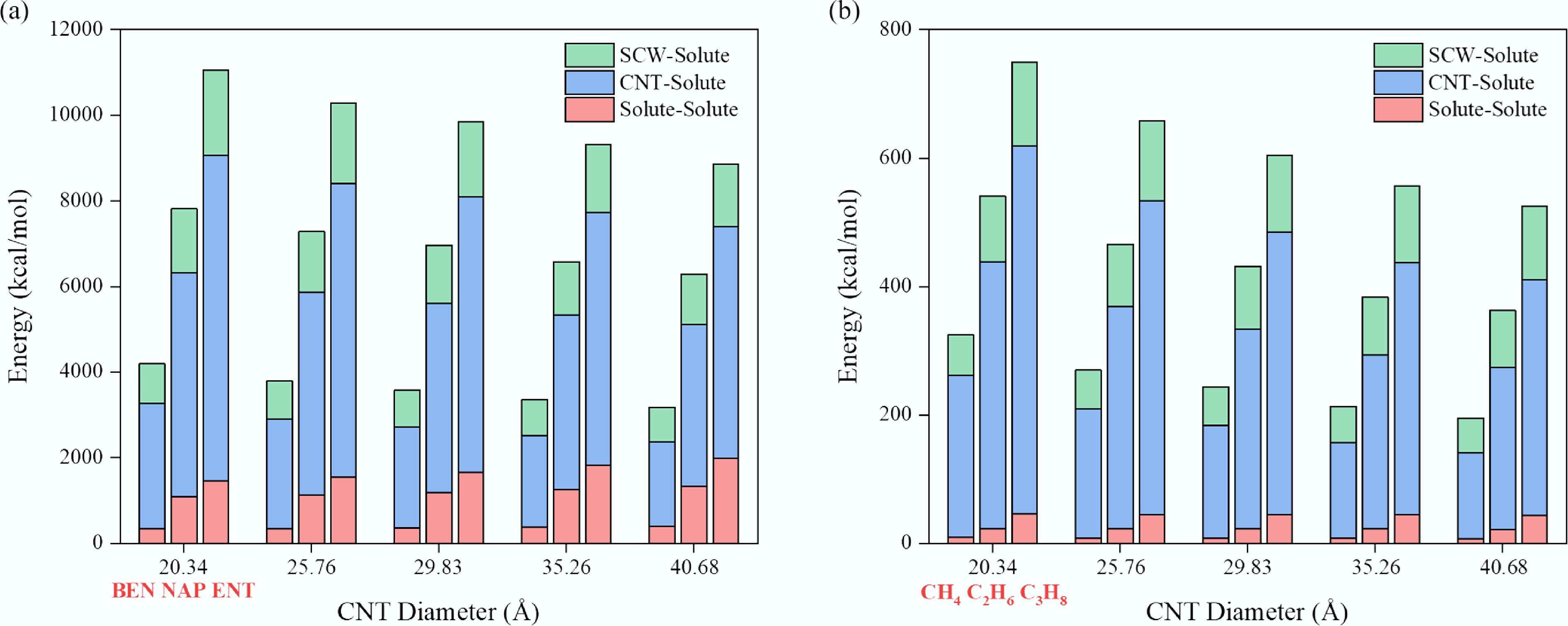
Figure 4.
Variation of intermolecular interaction energies among system components as a function of CNT diameter. (a) Aromatic systems (benzene, naphthalene, and anthracene at each diameter). (b) Aliphatic systems (methane, ethane, and propane at each diameter).
-
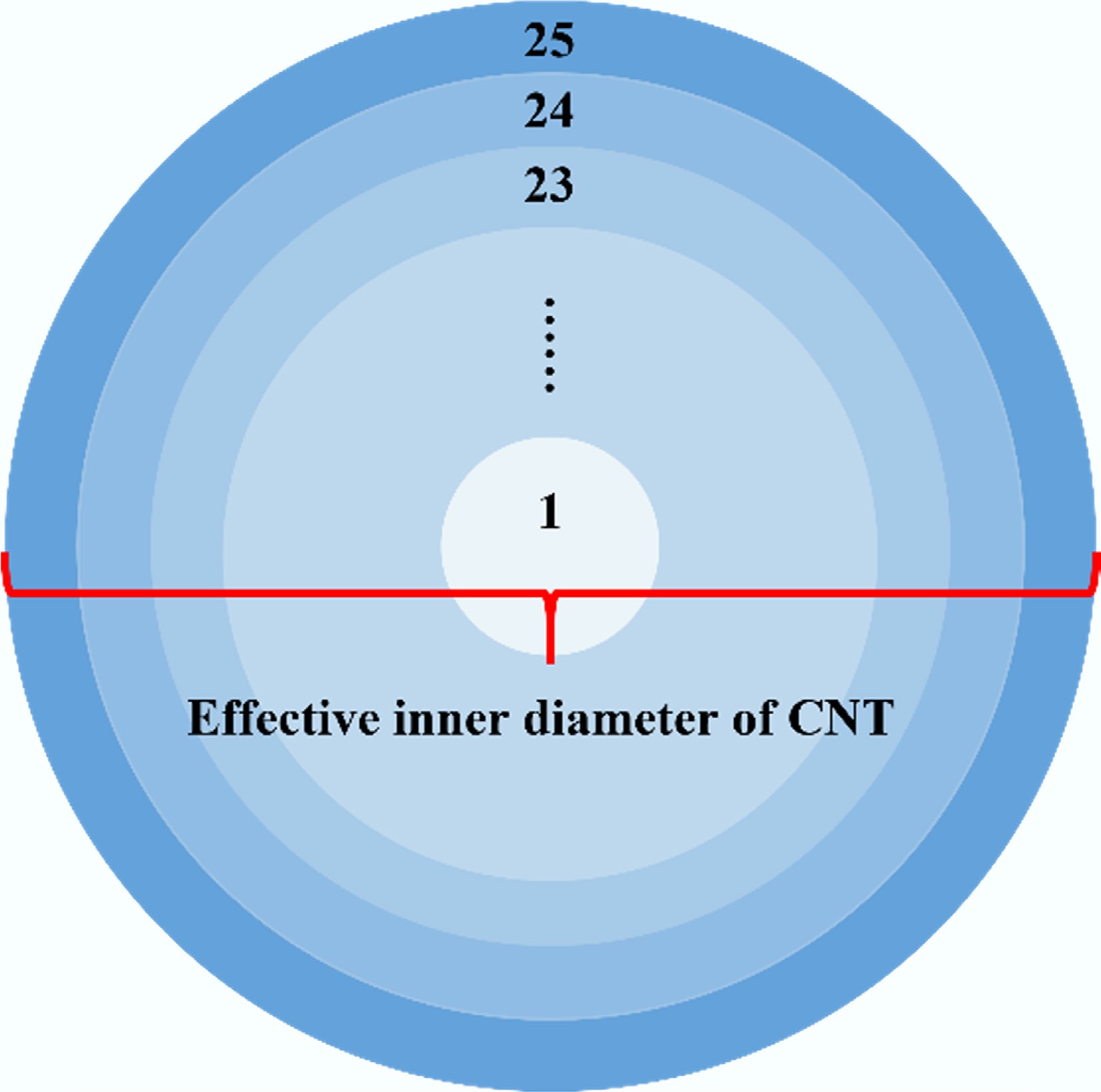
Figure 5.
Schematic illustration of the radial partitioning of the CNT cross-section.
-
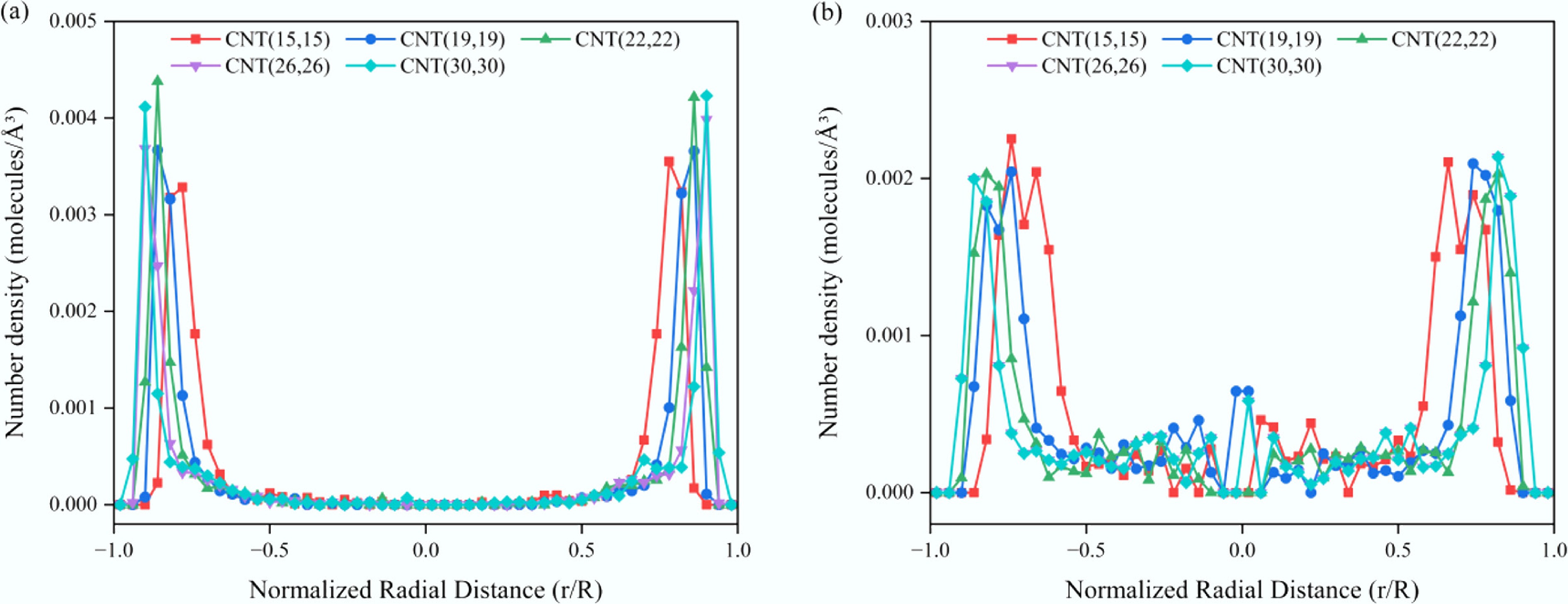
Figure 6.
Radial number density distributions of solute molecules within CNTs of different diameters. (a) SCW–anthracene system. (b) SCW–propane system.
-
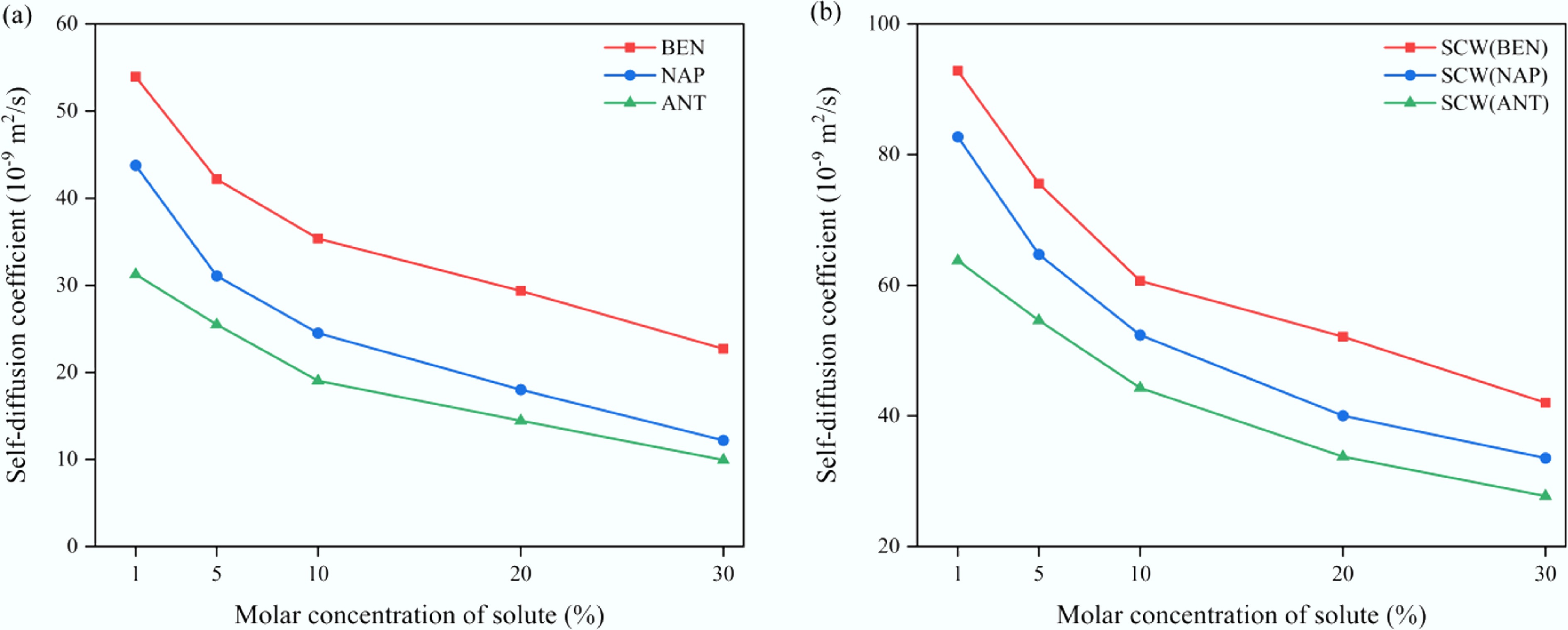
Figure 7.
Variation of self-diffusion coefficients in confined SCW–aromatic hydrocarbon systems as a function of solute molar concentration. (a) Solutes. (b) SCW.
-
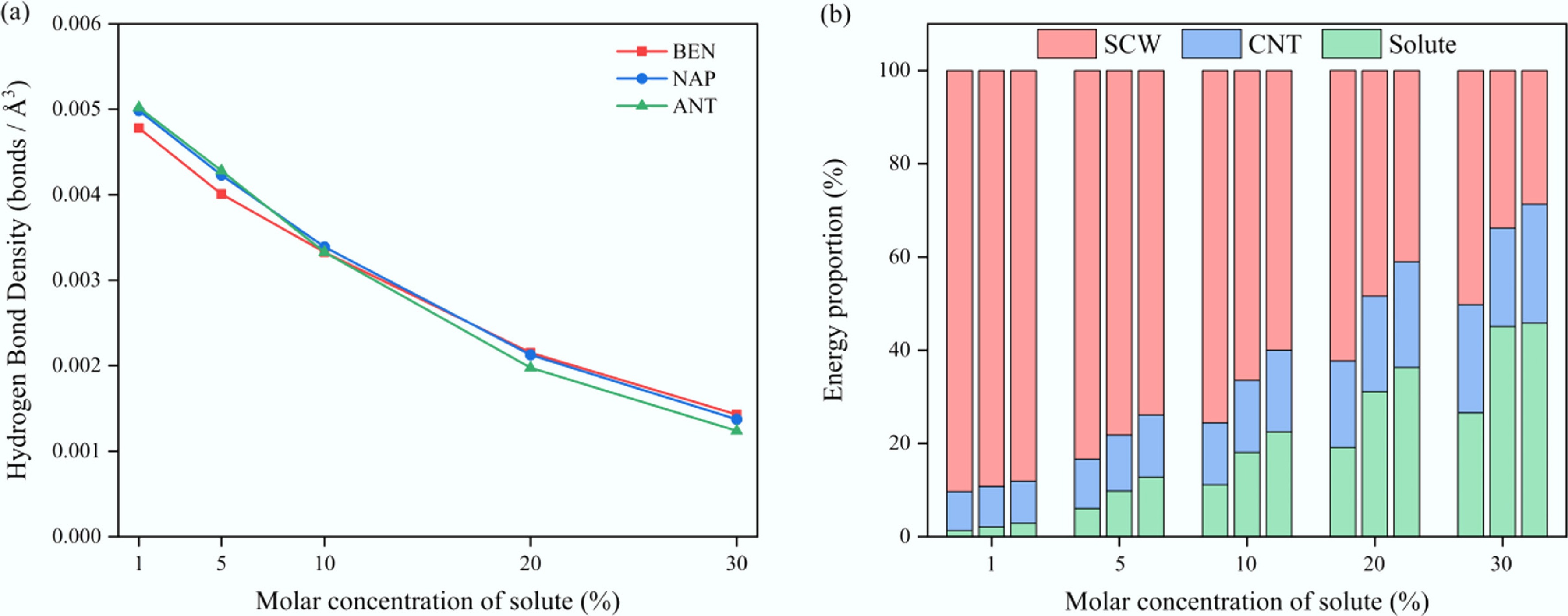
Figure 8.
Variation of (a) hydrogen bond density, and (b) energy distribution as a function of solute molar concentration in SCW–aromatic hydrocarbon systems confined within CNT(15,15) at 673 K.
-
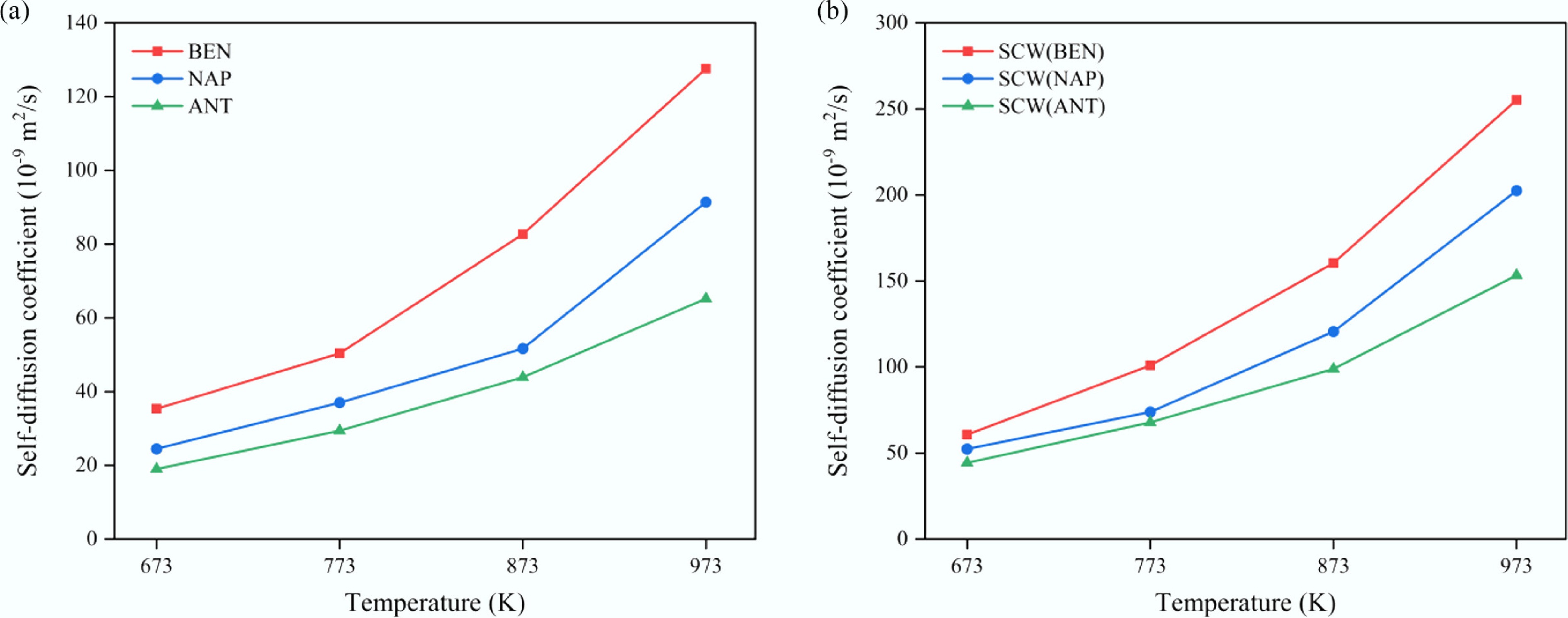
Figure 9.
Temperature dependence of the self-diffusion coefficients of (a) aromatic solutes, and (b) SCW in confined SCW–aromatic hydrocarbon systems.
-
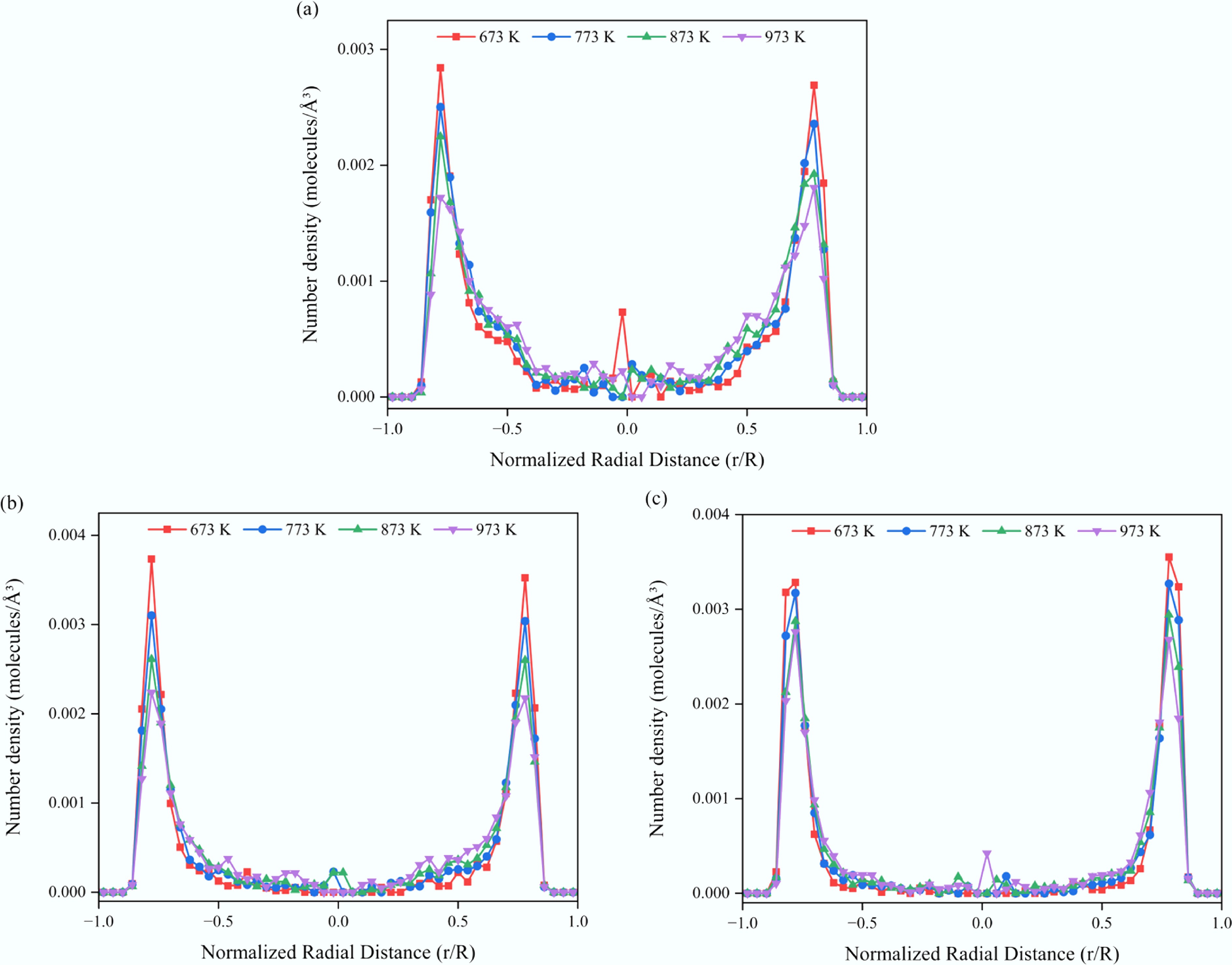
Figure 10.
Radial number density profiles of aromatic solutes in confined SCW–CNT(15,15) systems at different temperatures. (a) Benzene. (b) Naphthalene. (c) Anthracene.
-
CNT d (Å) deff (Å) (15,15) 20.34 16.94 (19,19) 25.76 22.36 (22,22) 29.83 26.43 (26,26) 35.26 31.86 (30,30) 40.68 37.28 Table 1.
Structural parameters of the simulated CNTs, including chirality, geometric diameter (d), and effective inner diameter (deff)
-
Table 2.
Comparison between simulated and experimental self-diffusion coefficients of bulk water and representative solute molecules
Figures
(10)
Tables
(2)