-
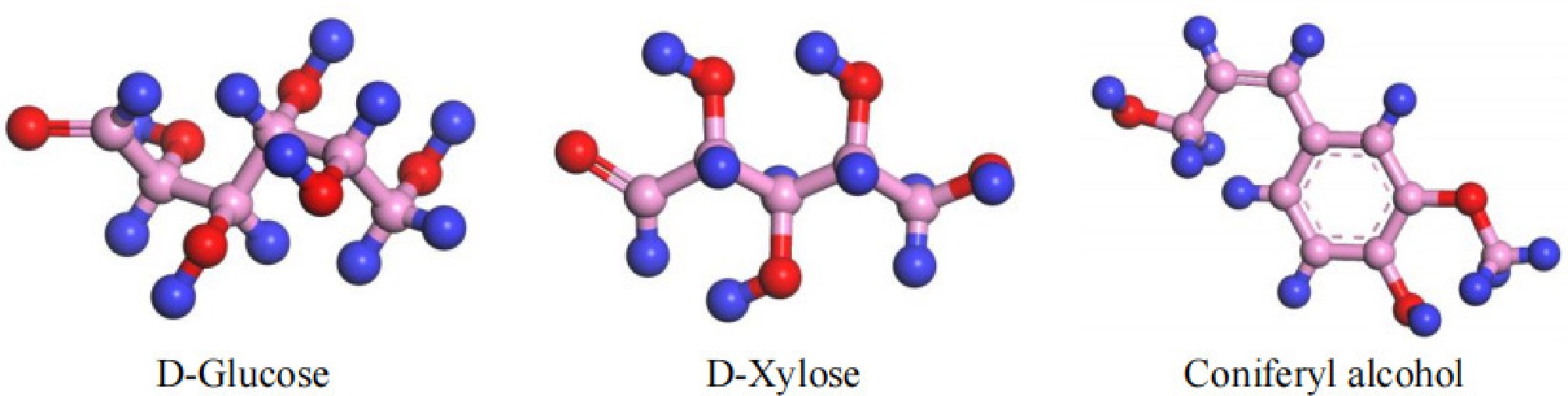
Figure 1.
Three modular models.
-
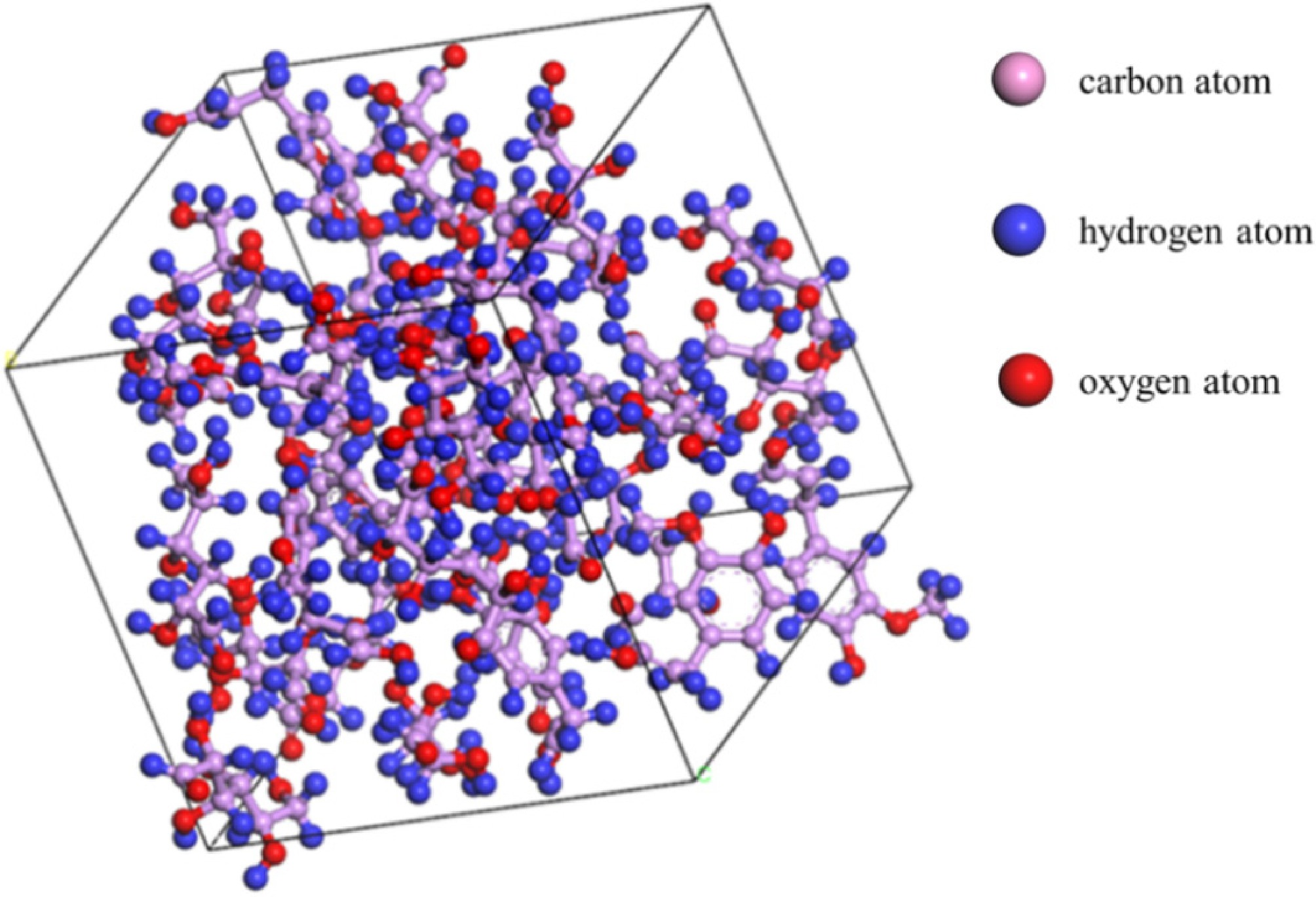
Figure 2.
Bagasse model (Model A).
-
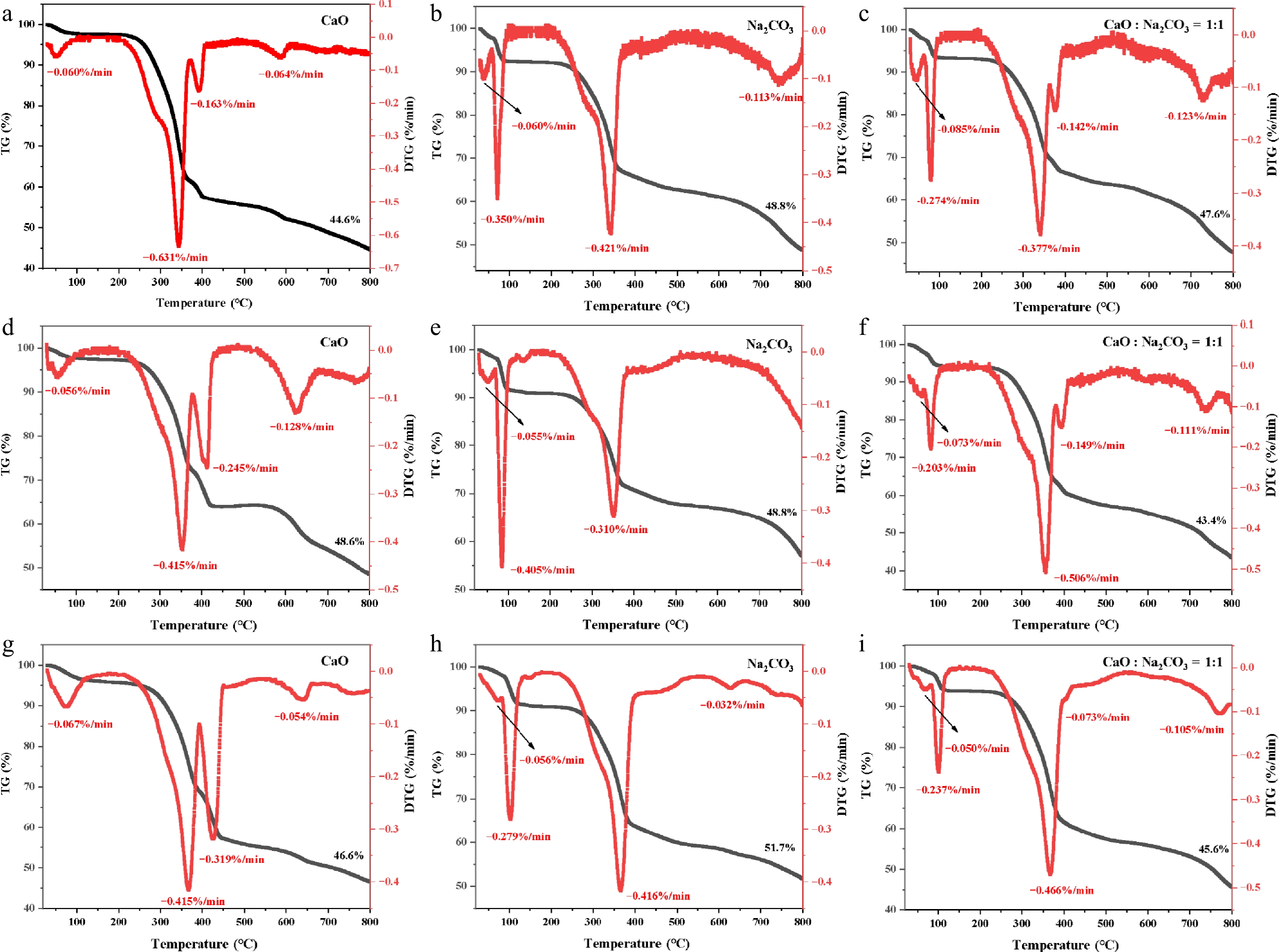
Figure 3.
TG and DTG curves of bagasse and catalyst: (a) CaO, 5 °C/min; (b) Na2CO3, 5 °C/min; (c) CaO : Na2CO3 = 1:1, 5 °C/min; (d) CaO, 10 °C/min; (e) Na2CO3, 10 °C/min; (f) CaO : Na2CO3 = 1:1, 10 °C/min; (g) CaO, 20 °C/min; (h) Na2CO3, 20 °C/min; (i) CaO : Na2CO3 = 1:1, 20 °C/min.
-
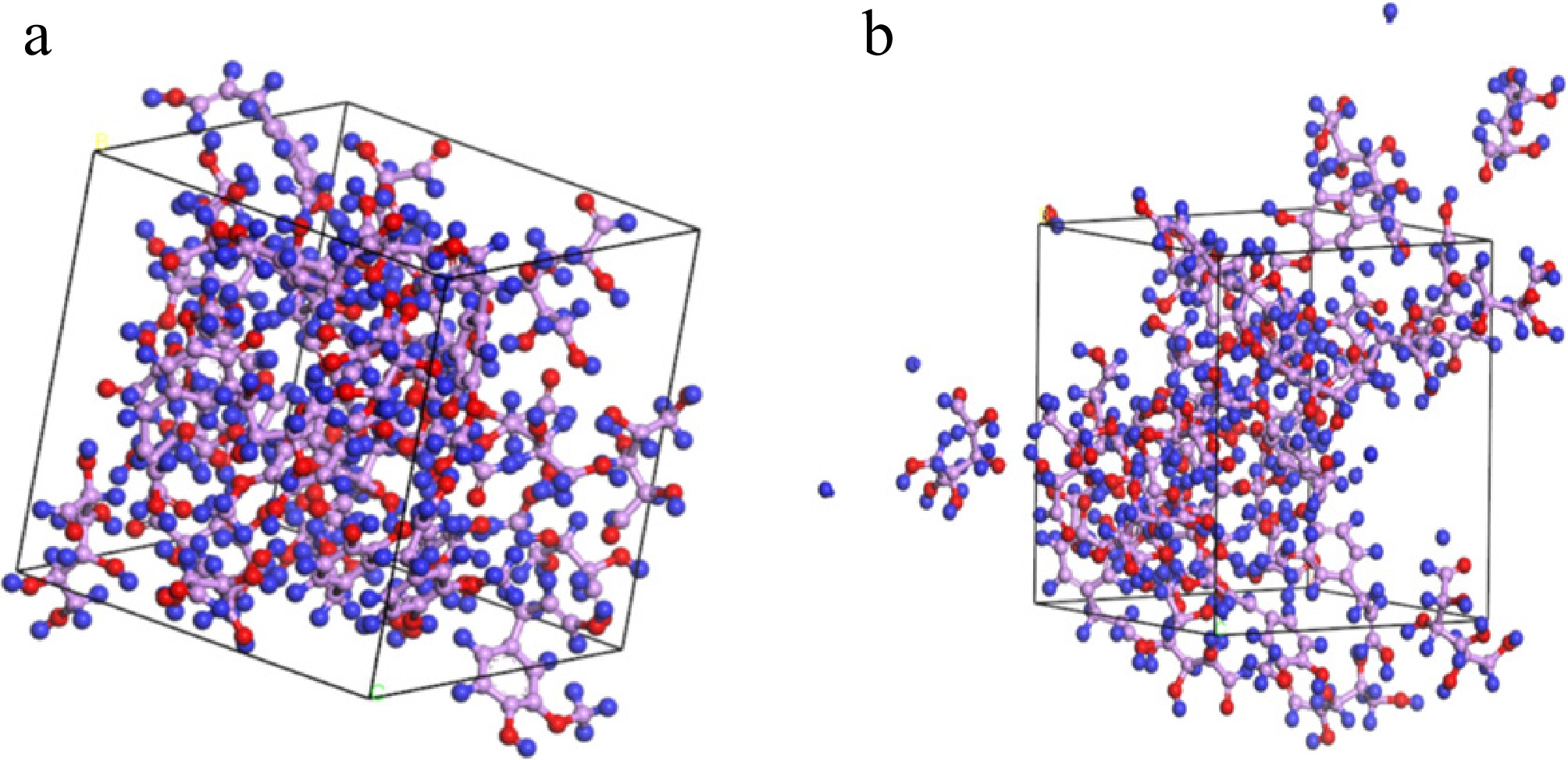
Figure 4.
Model A at (a) 1, 000 K, and (b)1,300 K.
-
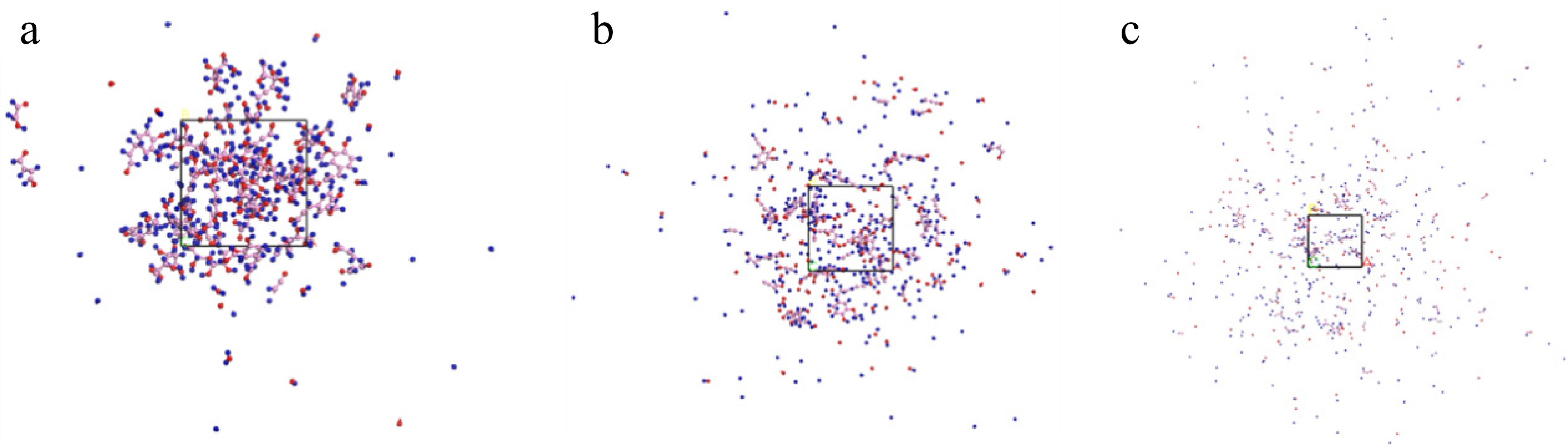
Figure 5.
Bagasse model at (a) 1,500 K, (b) 2,000 K, and (c) 2,500 K.
-
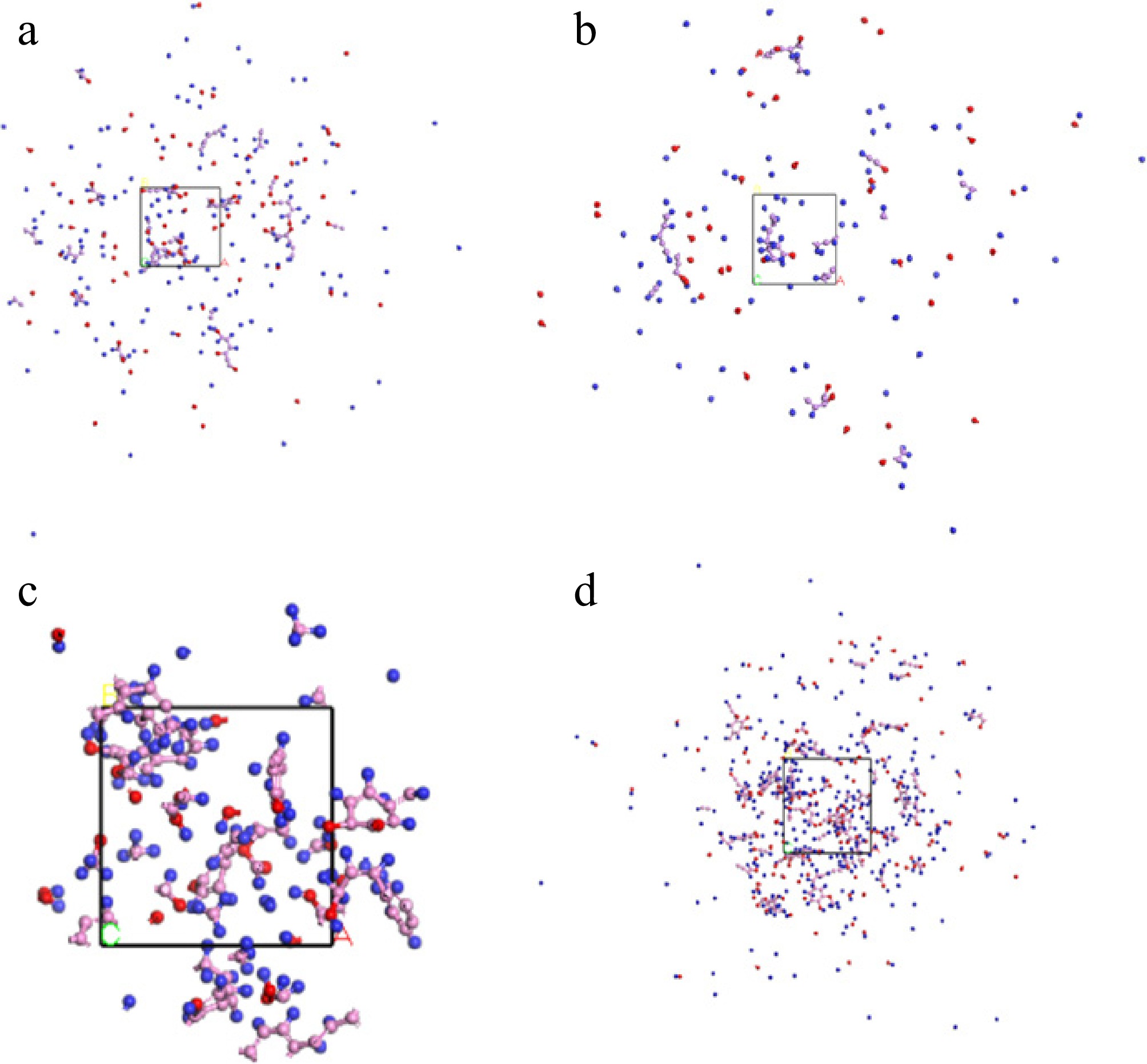
Figure 6.
(a) Glucose model; (b) Xylose model; (c) SA model; (d) Model A.
-

Figure 7.
Pyrolysis gas component concentration of bagasse under catalyst conditions (different ratios of CaO and Na2CO3).
-
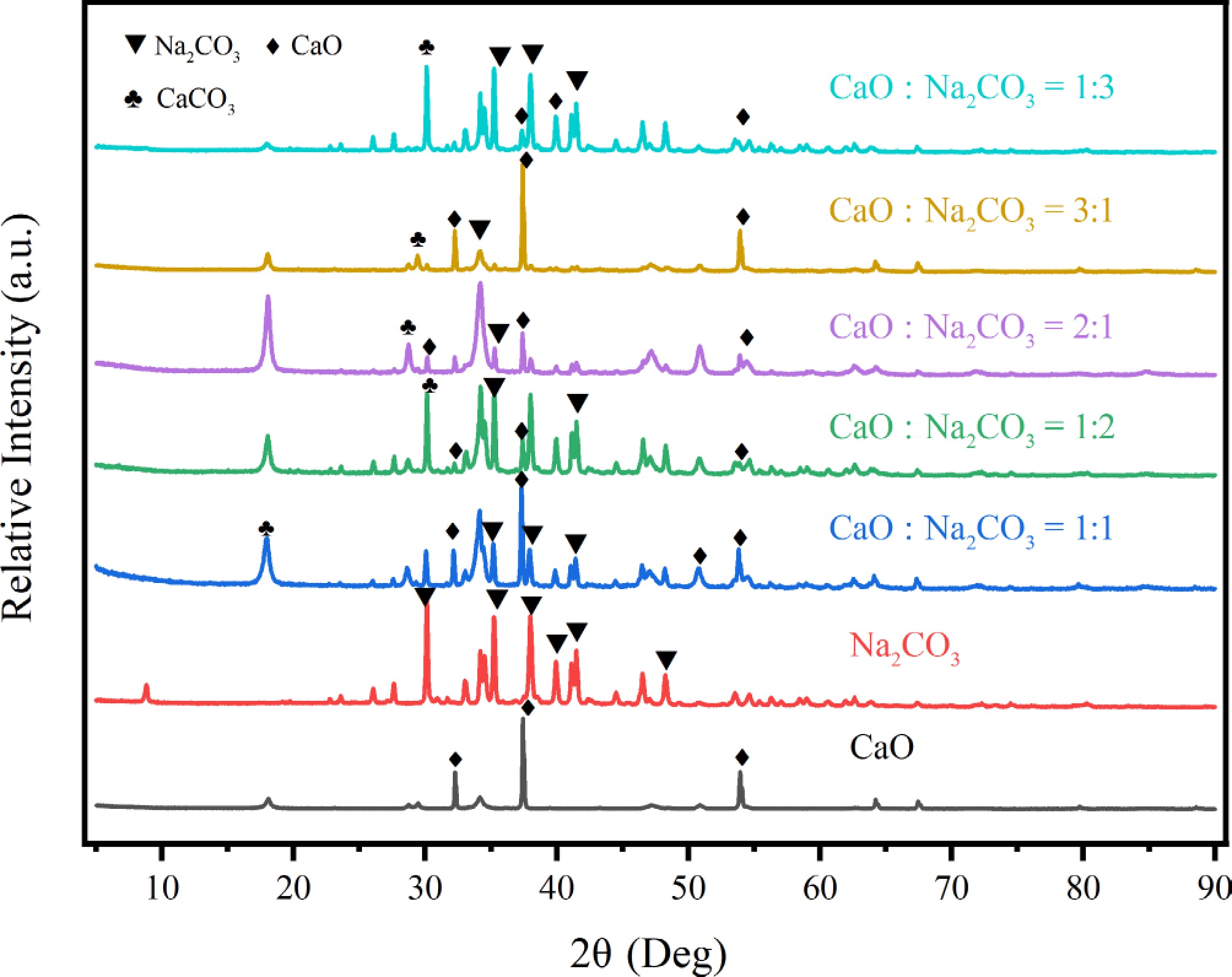
Figure 8.
XRD pattern of catalysts.
-
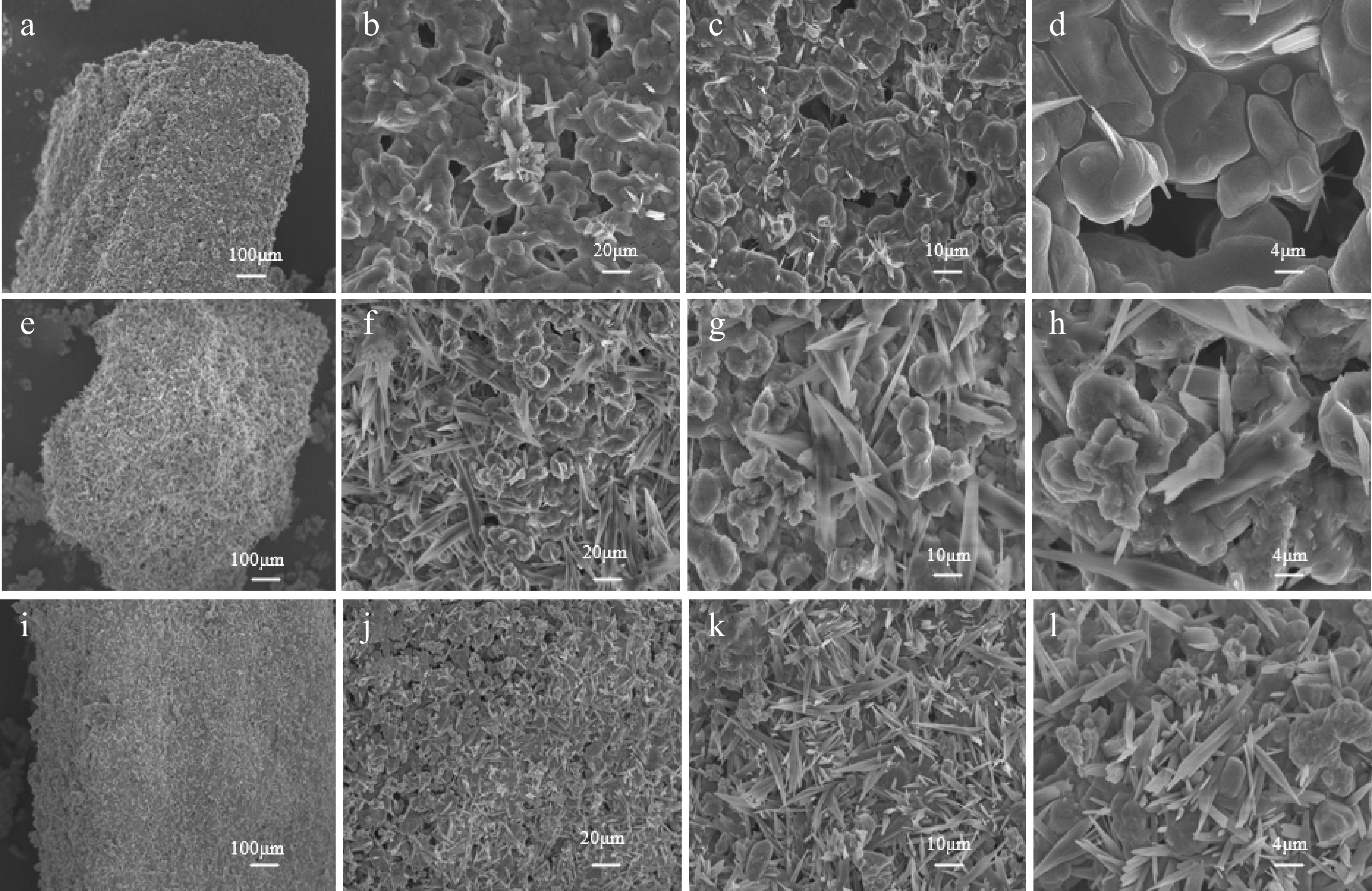
Figure 9.
SEM characterization of (a)–(d) Na2CO3 catalysts; (e)–(h) CaO : Na2CO3 = 1:2 catalysts; (i)–(l) CaO : Na2CO3 = 1:3 catalysts.
-
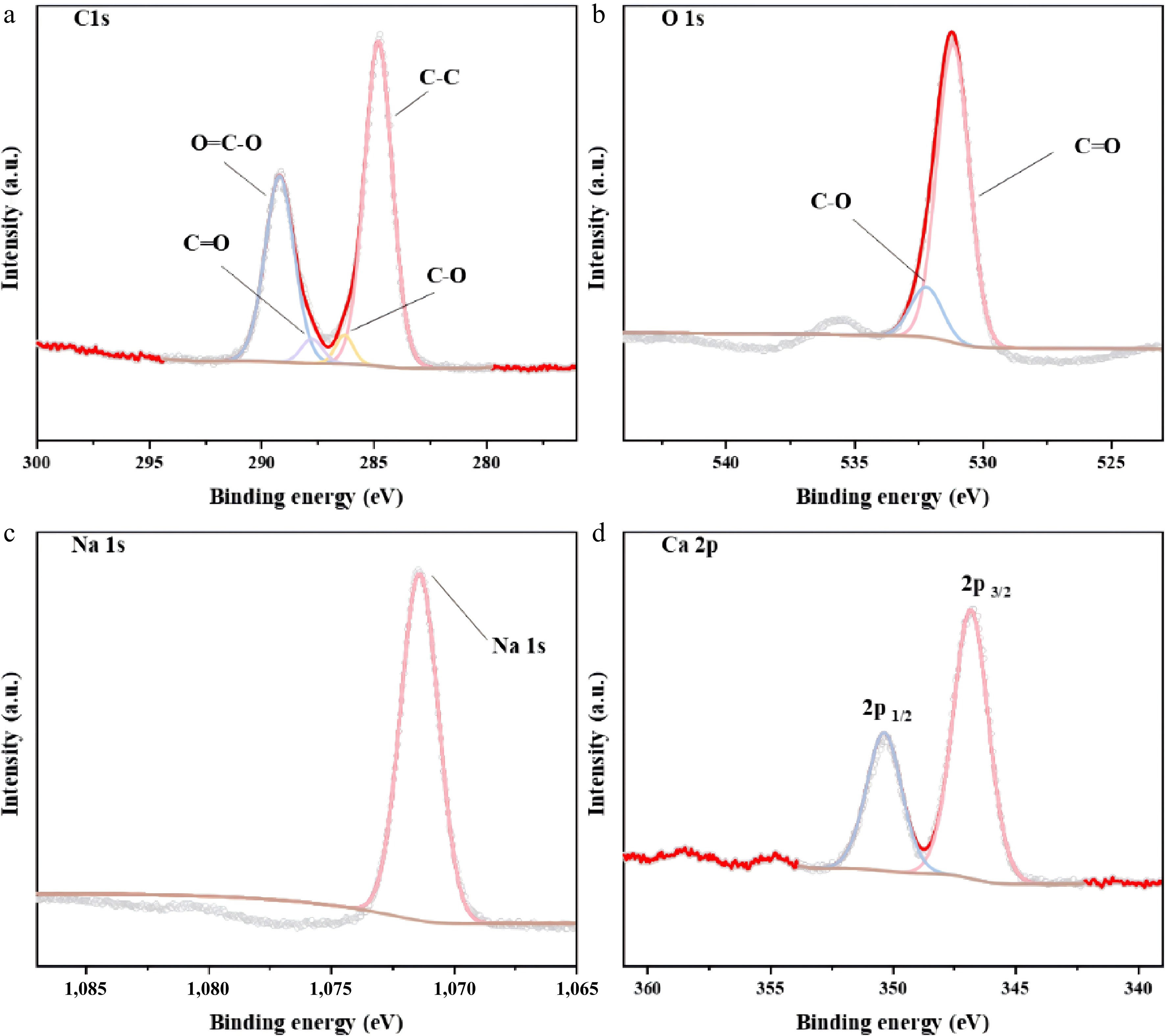
Figure 10.
XPS spectra of mixed catalysts (CaO : Na2CO3=1:3): (a) C1s; (b) O1s; (c) Na1s; (d) Ca2p.
-
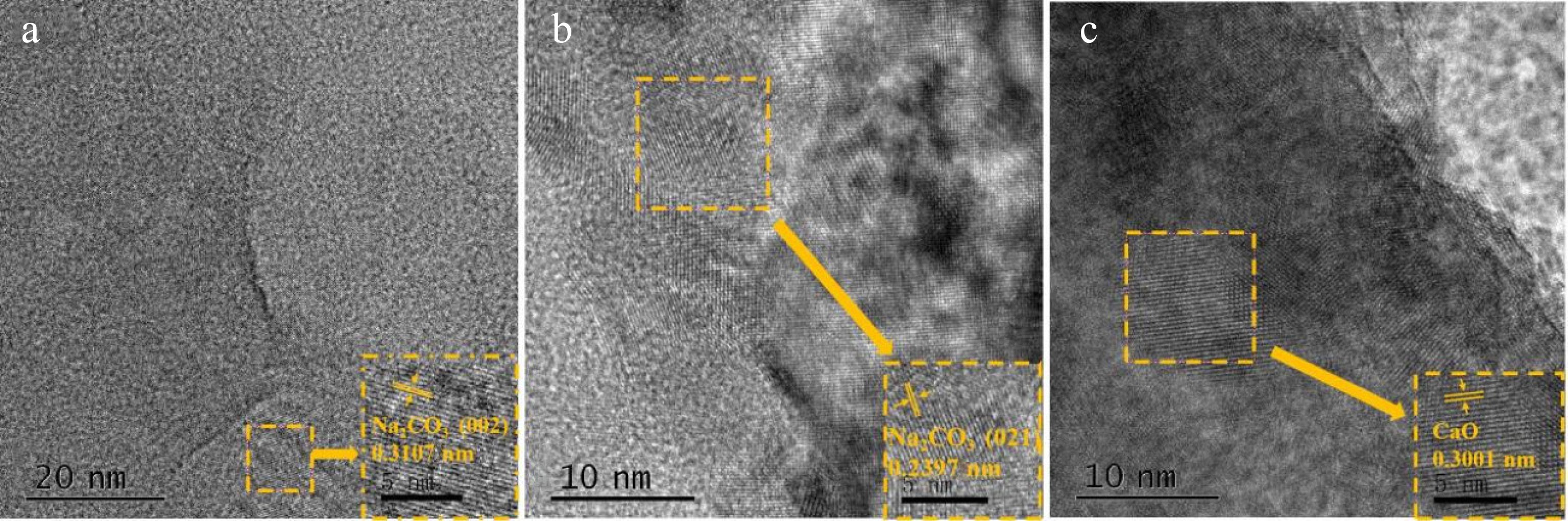
Figure 11.
TEM patterns of (a) Na2CO3 catalyst, and (b) and (c) mixed catalyst (CaO : Na2CO3=1:3).
-
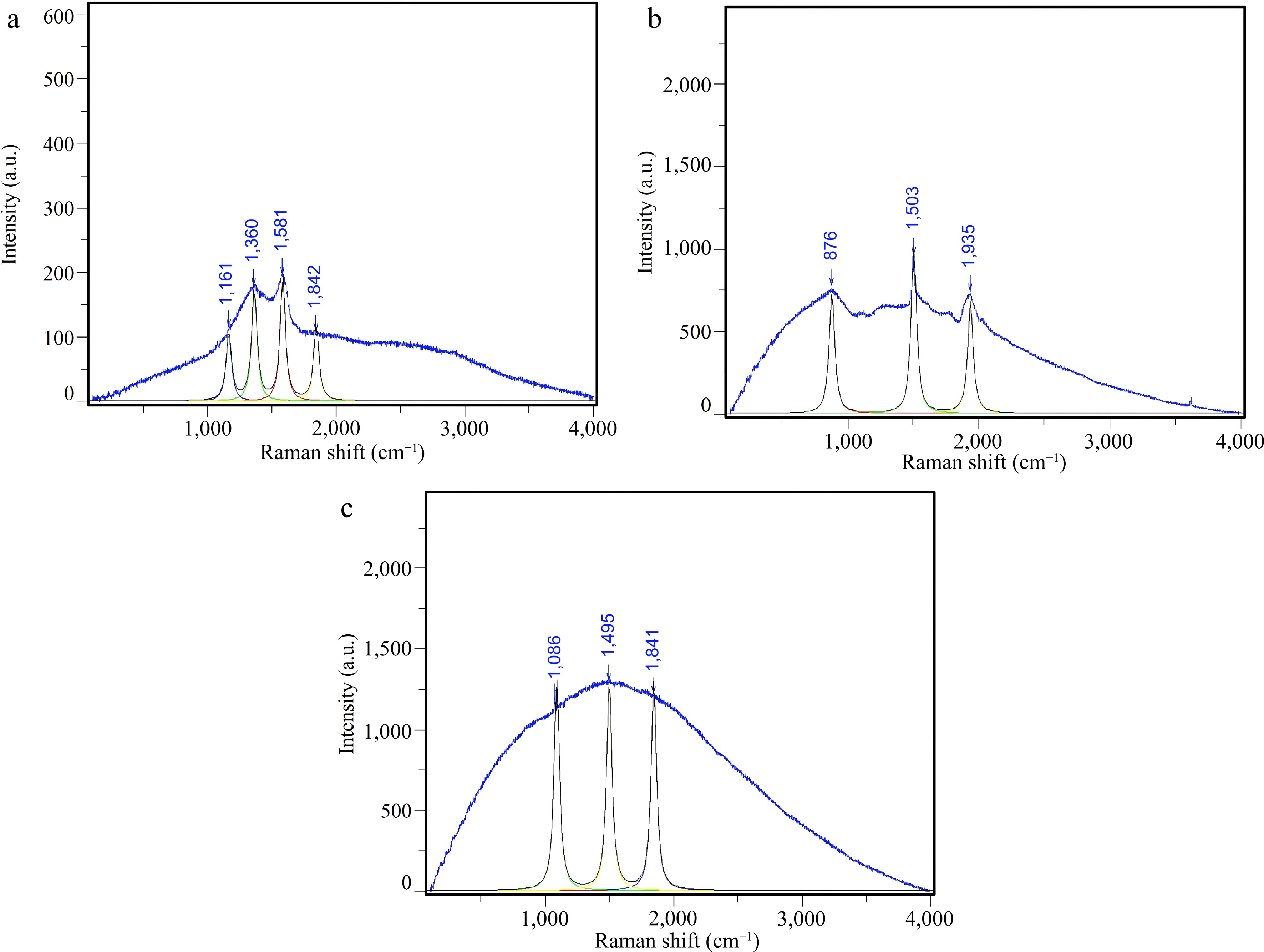
Figure 12.
Raman profiles of (a) Na2CO3 catalyst; (b) CaO : Na2CO3 = 1:2 catalyst; (c) CaO : Na2CO3 = 1:3 catalyst.
-
Serial number Feedstock Catalyst Feedstock catalyst ratio 1 Bagasse CaO Feedstock : catalyst = 3:1 2 Bagasse Na2CO3 3 Bagasse CaO : Na2CO3 = 1:1 4 Bagasse CaO : Na2CO3 = 1:2 5 Bagasse CaO : Na2CO3 = 2:1 6 Bagasse CaO : Na2CO3 = 3:1 7 Bagasse CaO : Na2CO3 = 1:3 Table 1.
Experimental feedstock and catalyst ratios.
-
Number of molecules H· O· OH· H2O CO2 C1 C2 C2 C4 C5 C6 C7 C8 C9 SRS 1,000 K 0 0 0 0 0 0 0 0 0 9 14 0 0 0 9 1,500 K 46 5 15 6 0 2 4 6 2 8 9 0 0 0 9 2,000 K 143 65 24 0 1 8 15 12 5 4 4 0 0 1 8 2,500 K 251 117 12 1 0 15 26 13 4 1 3 0 0 1 7 Table 2.
Product statistics at four pyrolysis temperatures for model A.
-
Number of molecules H· O· OH· H2O CO CO2 C1 C2 C3 C4 C5 C6 C7 C8 C9 SRS Glucose 113 51 6 0 1 0 1 5 4 3 0 8 0 0 0 0 Xylose 62 36 2 0 0 0 1 2 4 0 4 0 0 0 0 0 SA 26 7 5 0 0 0 8 3 1 3 1 2 1 0 0 5 Total 201 94 13 0 1 0 10 10 9 6 5 10 1 0 0 5 Model A 143 65 24 0 0 1 8 15 12 5 4 4 0 0 1 8 Table 3.
Pyrolysis results of different models at 2,000 K.
-
Product H2 CO2 CO CmHn Content (%) 12.09 25.05 37.51 25.35 Table 4.
Gas product of separate pyrolysis of bagasse.
-
Raw material Catalysts H2 content Ref. Bagasse and microalgal poultry manure residue Fe biochar 27.89 vol.% [37] Bagasse and microalgal poultry manure residue HZSM-5 31.0 vol.% [37] Bagasse 10 wt.% Ni-dolomite 24.7 vol.% [38] Bagasse Ni/Al2O3 24.3 vol.% [42] Bagasse CaO 14.57 vol.% This work Bagasse Na2CO3 16.89 vol.% This work Bagasse CaO : Na2CO3 = 1:3 17.26 vol.% This work Table 5.
The comparison of hydrogen evolution efficiency.
-
Number of cycles 1 2 3 4 5 H2 content (%) 17.3 16.8 15.6 11.9 11.5 Table 6.
Effect of cycle times on CaO and Na2CO3 catalyst systems.
-
Catalysts Surface area
(m2/g) BETPore volume (cm3/g) Pore
diameter
(nm)Mesoporous Microporous Total Na2CO3 21.525 0.104 0.051 0.155 3.06 CaO : Na2CO3 = 1:1 16.616 0.033 0.026 0.059 3.063 CaO : Na2CO3 = 1:3 17.228 0.035 0.028 0.063 3.063 Table 7.
BET results of catalysts.
Figures
(12)
Tables
(7)