-
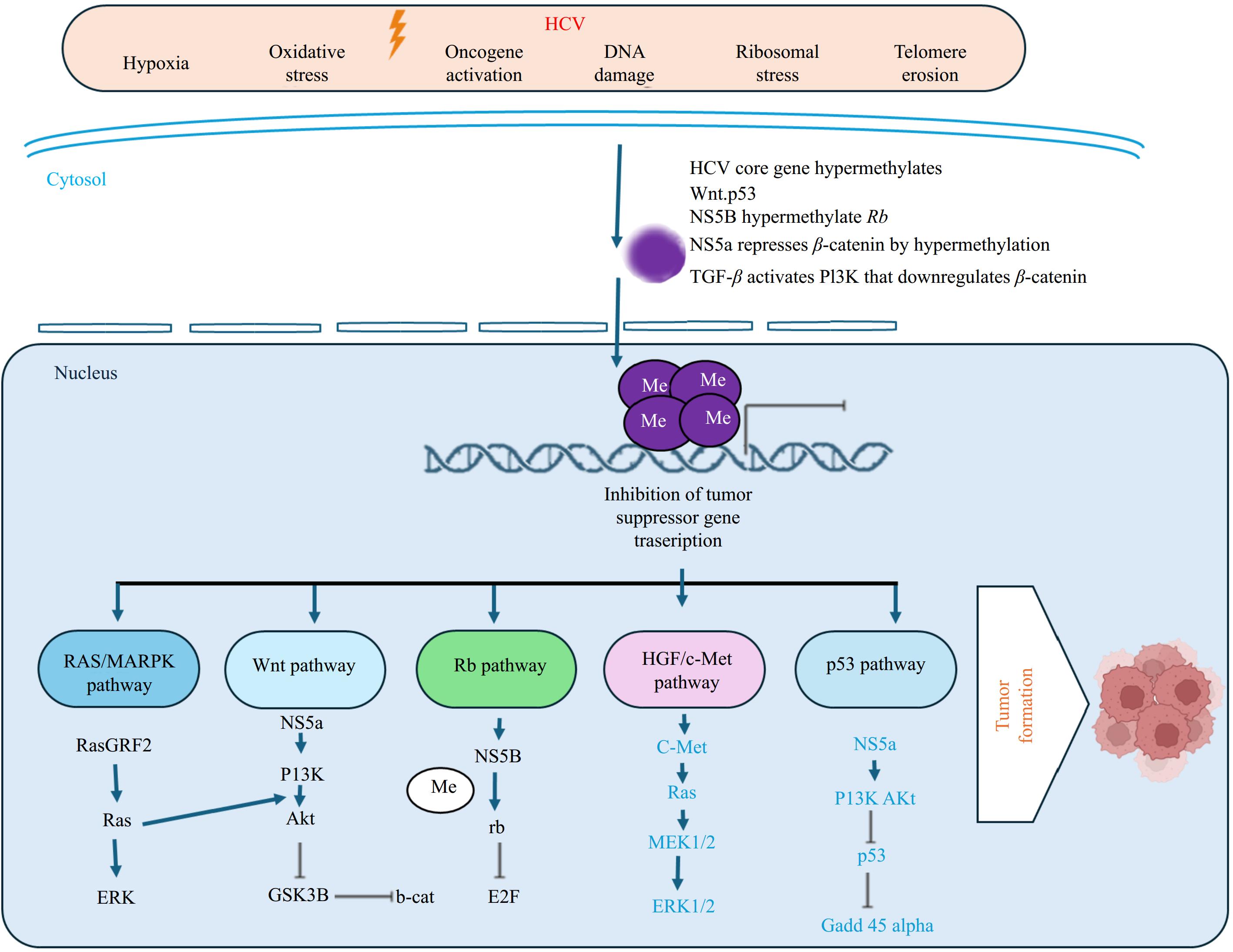
Figure 1.
HCV infection hypermethylates promoter CpG island of Tumor Suppressor Gene and silences its activity. Different HCV genes control p53, rb, TGF-β, HGF/c-Met, RAS/MAPK, and Wnt pathways and disrupt the functionality of tumor suppressor genes Dysfunctional TSGs allow defective DNA to sneak out cell cycle checkpoints. Consequently, leads to the proliferation of cells with damaged DNA and develops tumorigenesis. Created with BioRender.com.
-
Cell cycle phases Tumor suppressor genes Effect of hypermethylation Function Refs G1-S PTEN[71], p15[72], p16[73], p18[74], p19[75], p27[76], SOCS1[77], CDKN2A[78], CDKN2B[78], RASSF1A[79], Rb[71] Loss of cell cycle control, uncontrolled proliferationCyclin D-CDK4/6 activation, bypass of G1 checkpoint HCV NS5b is associated with Rb gene of the host cell and down-regulates its expression. p27, a cyclin-dependent kinase inhibitor.SOCS-1 down-regulates cytokines of JAK/STAT3 pathway CDKN2A degrades p53 CDKN2B controls G1phase progression. RASSF1A is a potent, highly sensitive, and specific marker to differentiate between HCC and non-HCC samples. [58,64,80,81] S p53[82], p21[83] Impaired DNA repair, apoptosis, and cell cycle arrest NS5a forms a complex with the p53 gene and, therefore, down-regulates p21 expression. [84] G2-M PTEN[85] Inactivation of tumor-suppressive phosphatase activity PTEN encodes a phosphatase that prevents tumor formation. [48] M p53, p21[86], APC[87] Impaired DNA repair, apoptosis, and cell cycle arrest APC gene of the Wnt pathway was found to be hypermethylated in almost 80% of HCV-induced HCC cases [88] Table 1.
Tumor suppressor genes regulating cell cycle phases.
-
HCV genes Tumor suppressor genes Function Refs Core P53[99], rb[99], p16[100], PTEN[55] By means of methylation, the aberration core affects the cell cycle. It hypermethylates the p16 promoter and mitigates its expression. P16 is suggested as a negative regulator of the G1 checkpoint that inactivates during HCC pathogenesis. [92] Core-mediated Rb phosphorylation that releases E2F1 and activates DNMT-1 which is mandatory for HCV infection. [101,102] HCV Core diminishes the activity of ATRA to upregulate p53 and apoptosis. [103] The core gene down-regulates the PTEN gene. [104] NS3 P21[105], p53[99] NS3 down-regulates p53 and p21. [94] NS5a PTEN[106], p53[99], p21[107] Down-regulated p53 expression was observed when NS3 and NS5a were transfected in hepatoma cell line. Experiments conducted in HepG2 and Saos-2 cells showed that NS5a forms a complex with the p53 gene and, therefore, down-regulates p21 expression. [94,108] NS5a down-regulates PTEN and inhibits the PI3K-Akt pathway. [106] NS5b Rb[106] HCV NS5b associates with Rb gene of the host cell and down-regulates its expression [80] Table 2.
HCV genes down-regulate tumor suppressor genes' expression in HCC.
-
Epigenetic modifiers Description Inhibitors of modifiers Ref. Epidermal growth factor receptor (EGFR) HCV infection activates EGFR that epigenetically modify cytoskeleton remodeling Erlotinib [107] Unfolded Protein Response (UPR) HCV genome induces UPR. UPR inhibitor has been reported to partially recover the dysregulated gene expression. UPR inhibitor [107] Lysophosphatidic acid (LPA) pathway LPA initiates the HCC-inducing pathways. The efficacy of the inhibitors was observed in the diethylnitrosamine (DEN) rat model. These inhibitors suppress Rho and ERK pathways. ATX (AM063), LPAR1 (AM095) [107] Table 3.
HCV-induced epigenetic changes can be inhibited by epigenetic modifiers inhibitors. These inhibitors and their modifiers are given in the table.
Figures
(1)
Tables
(3)