-
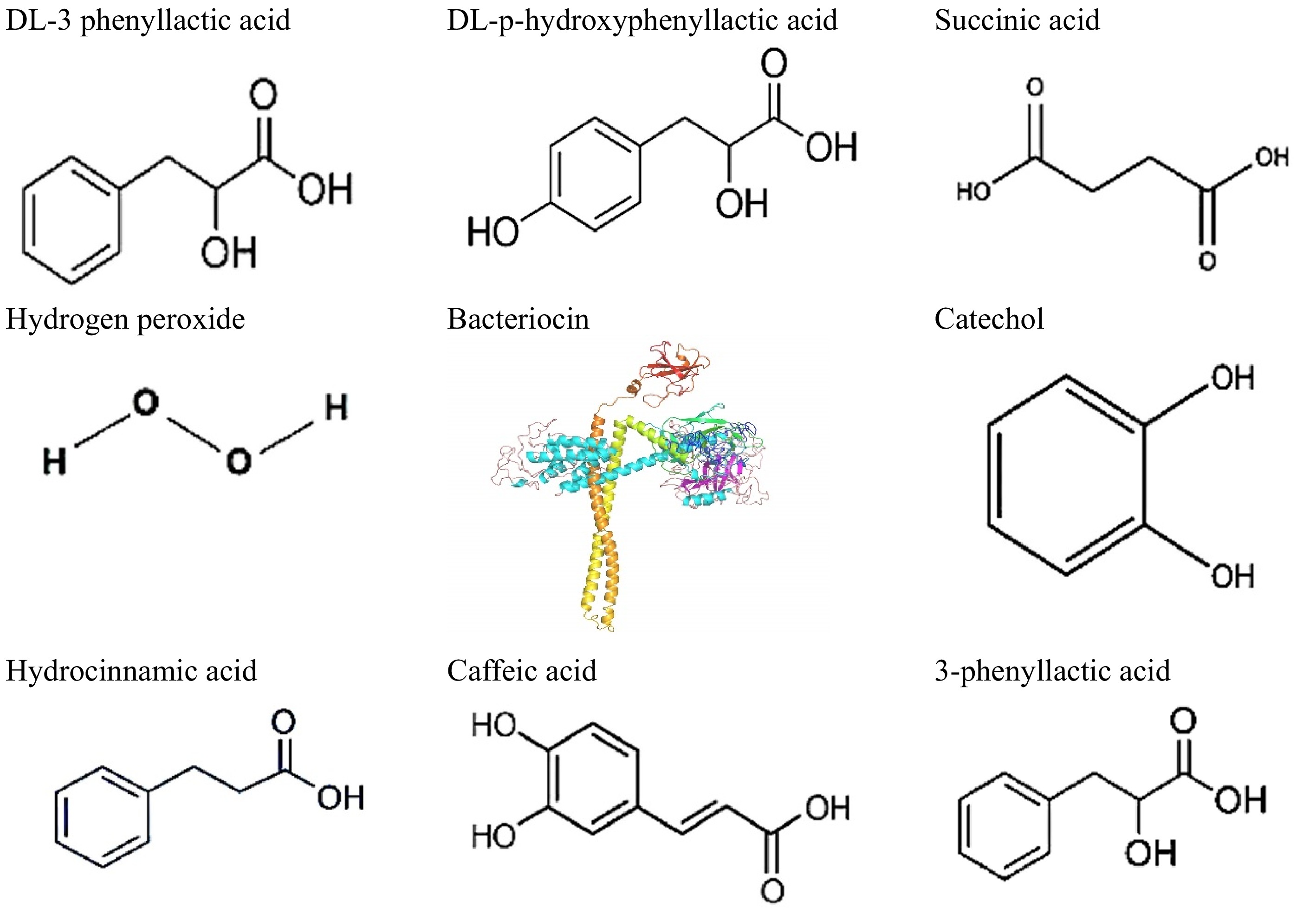
Figure 1.
Representative bioactive compounds produced by LAB species.
-
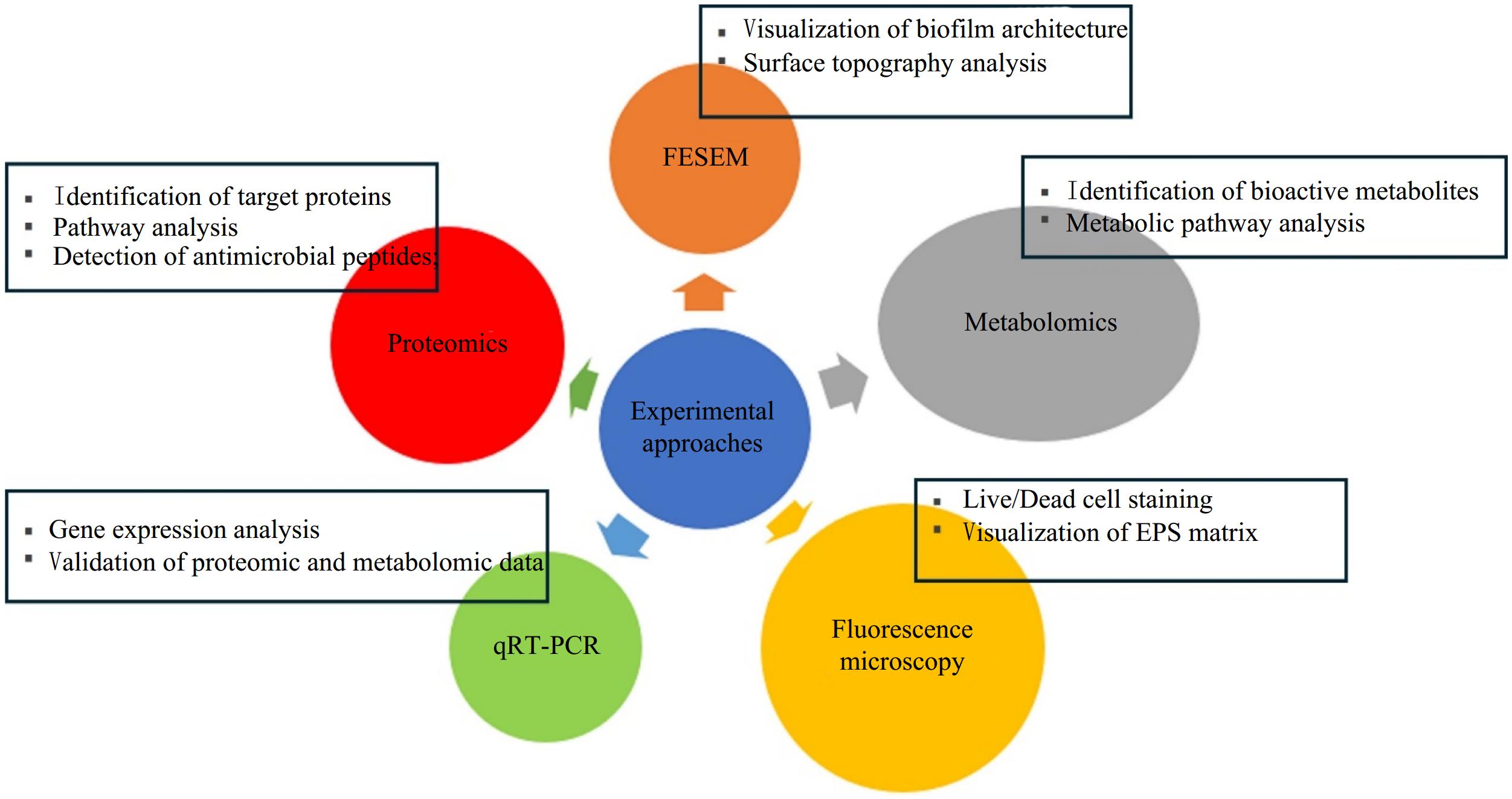
Figure 2.
Experimental approaches commonly used to investigate antibiofilm mode of action of CFS of LAB.
-
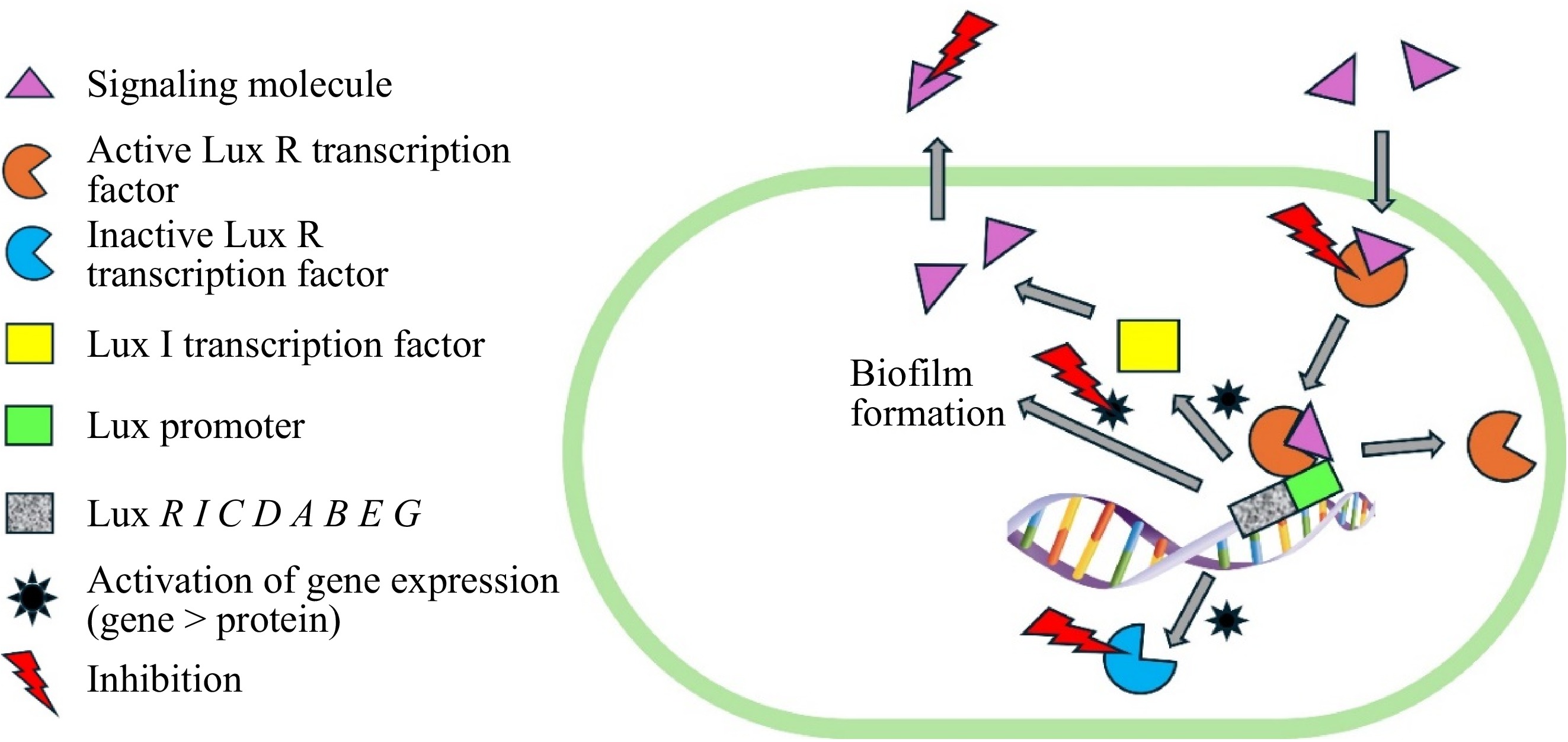
Figure 3.
Inhibition of biofilm formation in pathogenic bacteria via interference of quorum sensing mechanisms.
-
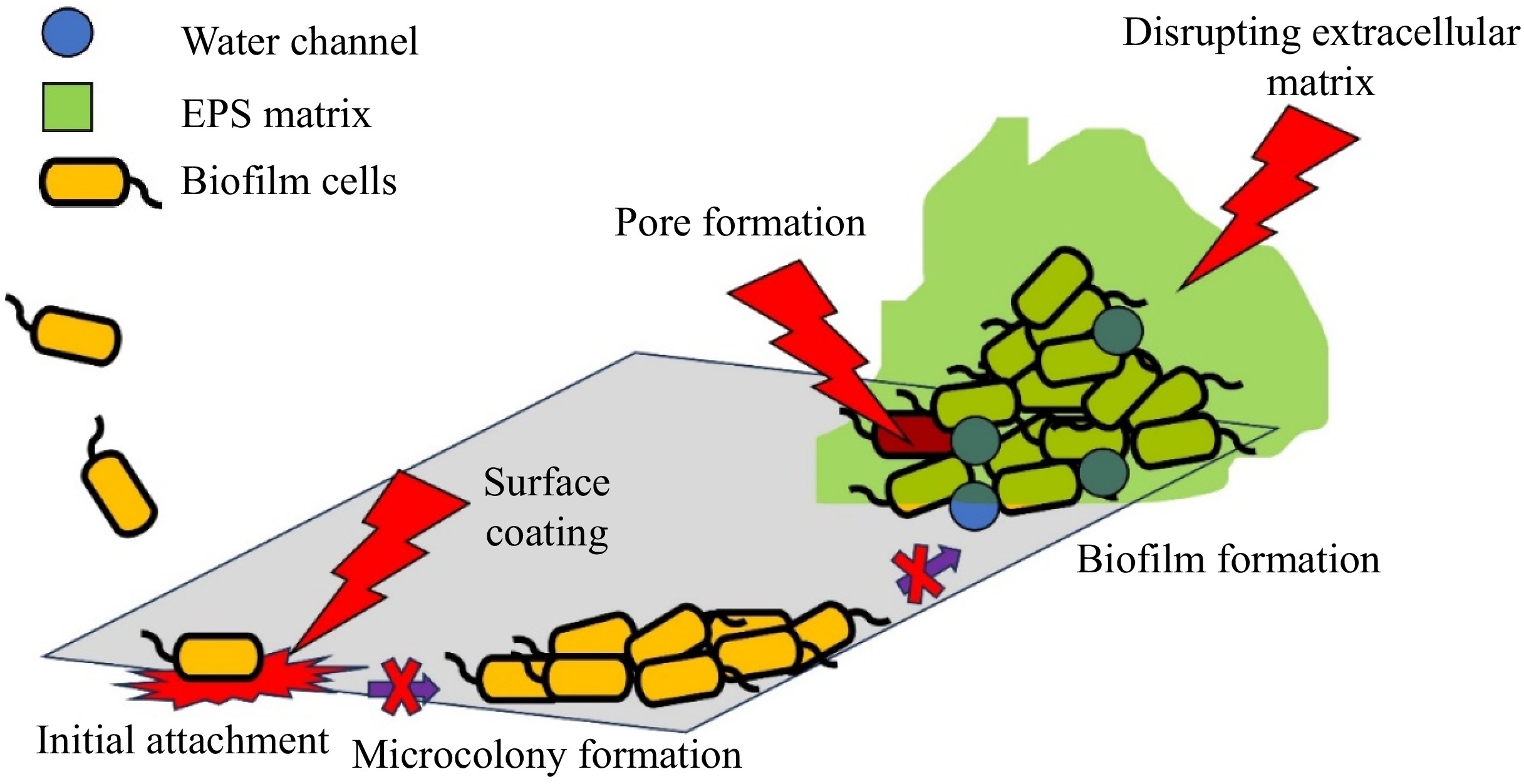
Figure 4.
Antibiofilm mode of action of bacteriocin.
-
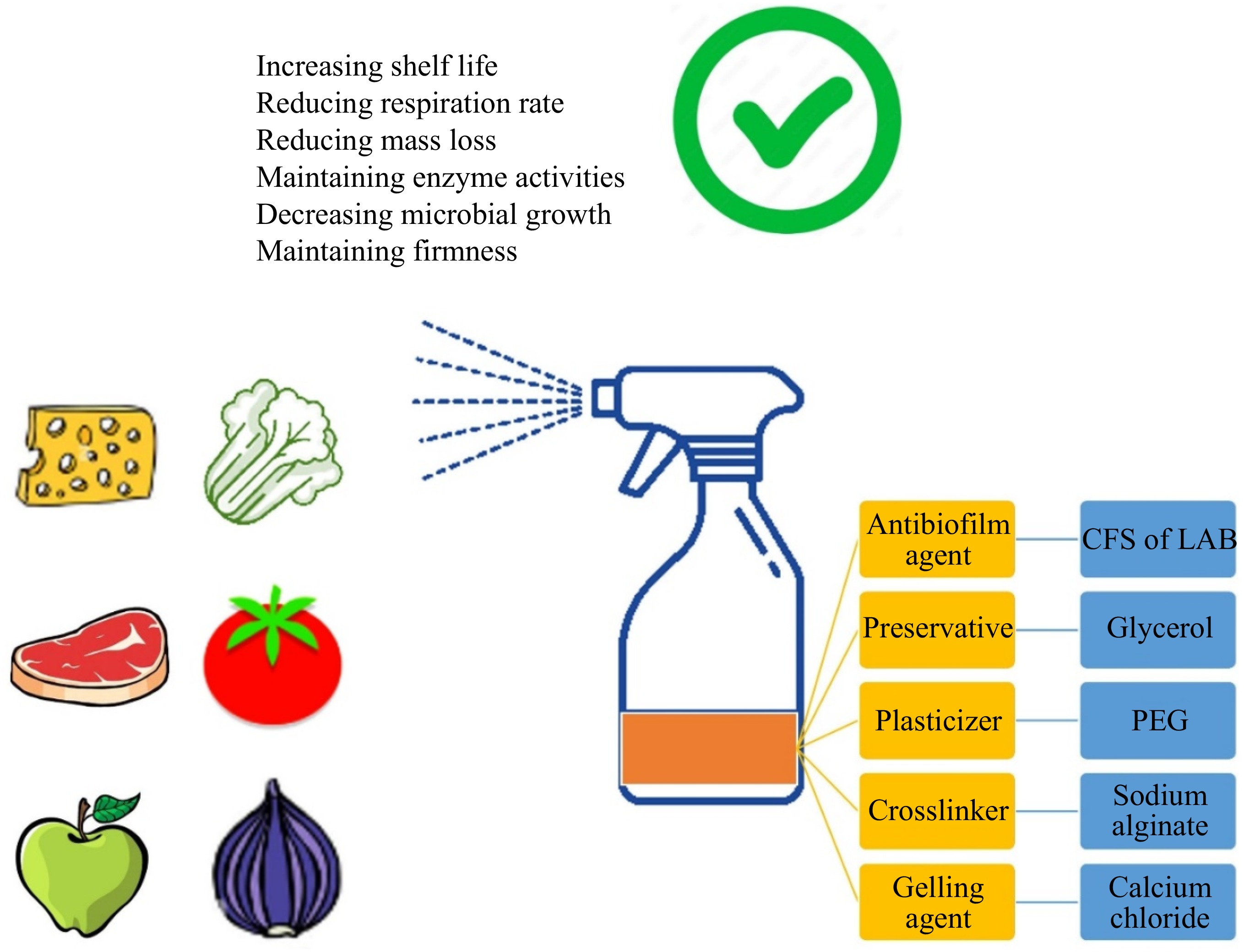
Figure 5.
LAB-based edible coating for biofilm control. CFS: cell-free supernatant; LAB: lactic acid bacteria; PEG: polyethylene glycol.
-
LAB species Antibiofilm activities Identified bioactive compounds Ref. Lactobacillus plantarum and Lactobacillus rhamnosus L. plantarum, L. rhamnosus and their CFS exhibit antimicrobial and antibiofilm activity against E. coli, Acinetobacter baumannii, and Proteus mirabilis. − [16] Lactobacillus plantarum and Bifidobacterium bifidum Strong inhibitory action against multi-drug resistant E. coli. Hydroxyacetone, 3-Hydroxybutyric acid, and Oxime-methoxy-phenyl [17] LAB strains isolated from the healthy human volunteers Inhibit the growth and biofilm formation by E. coli (ATCC 35218) and S. aureus (ATCC 25923) DL-3 phenyllactic acid, DL-p-hydroxyphenyllactic acid, and succinic acid [18] Lentilactobacillus kefiri LK1 and Enterococcus faecium EFM2 Exhibit anti-microbial and anti-biofilm activities by modulating hydrophobicity, auto-aggregation, and exopolysaccharide (EPS) production phenotypes and genotypes of bovine mastitis pathogens − [19] Lactococcus lactis NJ414 The cell-free supernatant (CFS) of the strain demonstrates effectiveness in inhibiting and eradicating biofilm formation by L. monocytogenes, with respective rates of 43.40% ± 0.58% and 38.90% ± 0.46%. − [20] Pediococcus pentosaceus and Enterococcus faecium All LAB develop biofilms to prevent biofilm formations of all tested pathogens through the co-aggregation process. Organic acids, diacetyl, hydrogen peroxide, ethanol, reuterin, and bacteriocins. [11] Lactobacillus helveticus CFS of Lactobacillus prevents the attachment between K. pneumoniae cells and reduced the cell viability of K. pneumoniae. − [21] Lactobacillus plantarum (7), Lactobacillus helveticus (3), Pediococcus acidilactici (1), and Enterococcus faecium (1) species Exhibit antibiofilm activities against S. aureus CMCC26003 and/or E. coli CVCC230. − [22] Pediococcus pentosaceus FB2 and Lactobacillus brevis FF2 Inhibit biofilm formation of B. cereus ATCC14579 (MBIC50 = 28.16%) and S. salivarius B468 (MBIC50 = 42.28%). − [23] L. plantarum strains MiLAB393 and MiLAB14 Antifungal activities against Pichia anomala, Penicillium roqueforti, and Aspergillus fumigatus are observed. But no antibiofilm activities are studied. 3-hydroxydecanoic acid, 2-hydroxy-4-methylpentanoic acid, benzoic acid, catechol, hydrocinnamic acid, salicylic acid, 3-phenyllactic acid, 4-hydroxybenzoic acid, (trans, trans)-3,4-dihydroxycyclohexane-1-carboxylic acid, p-hydrocoumaric acid, vanillic acid, azelaic acid, hydroferulic acid, p-coumaric acid, hydrocaffeic acid, ferulic acid, and caffeic acid. [24] Table 1.
Antibiofilm efficacy of LAB against pathogenic bacteria.
-
LAB species Antibiofilm mode of action Ref. LAB isolated from the milk sample of bovine mastitis. Inhibits the physiological traits of the S. aureus biofilm, including hydrophobicity, motility, eDNA, and PIA associated to the biofilm. Important metabolic pathways such amino acids and carbohydrates metabolism are among the most noticeably altered metabolic pathways.The expression levels of biofilm-related genes clfA, fnbA, icaD, icaA, and clfB of S. aureus biofilm decreases after LAB-CFS treatment when compared with the control group. [29] Lactococcus lactis strain CH3 SEM shows evidence of structural deformation and loss of membrane integrity of bacterial cells treated with bacteriocin. [36] Lactobacullus paracasei L2 and L20 strains LAB has anti-QS activities in different concentrations. [37] Lactobacillus brevis L. brevis KCCM 202399 CFS inhibits the bacterial adhesion of S. mutans KCTC 5458 by decreasing auto-aggregation, cell surface hydrophobicity, and EPS production (45.91%, 40.51%, and 67.44%, respectively). [32] Pediococcus pentosaceus and Enterococcus faecium All LAB could develop biofilms to prevent biofilm formations of all tested pathogens through the co-aggregation process. [11] Lactobacillus rhamnosus and
L. paracaseiBoth live and heat-killed LAB strains, particularly L. rhamnosus and L. paracasei, demonstrate the ability to interfere with S. mutans and S. oralis biofilm development through competition and displacement mechanisms. [38] Table 2.
Antibiofilm mode of action of LAB species.
-
LAB species Coating material Efficacy Ref. Pediococcus pentosaceus 147 Chitosan Chitosan coatings plus CFS (5.72 ug/ml) of P. pentosaceus 147 inhibit the growth of L. monocytogenes during the storage of cheese contaminated after production. [46] Lacticaseibacillus paracasei TEP8, Lactiplantibacillus pentosus TEJ4 and Lactiplantibacillus plantarum TEP15 Chitosan The films with 75%, 50% and 25% CFS from the three strains exceed 50% inhibition. L. pentosus TEJ4 and L. plantarum TEP15 maintain high inhibition levels (> 79%) at low CFS concentrations. [47] Lactobacillus rhamnosus NRRL B-442 Whey protein Noticeable antimicrobial activity (about 3 mm) is observed against E. coli, L. monocytogenes, S. aureus, or S. typhimurium when 18 mg/ml of cell-free supernatant are added. [48] Lactobacillus paracasei Glycerol and starch as well as gelatin Edible coatings give better results than untreated fruits such as diminishing weight loss, decay percentage, higher ascorbic acid, lycopene, titratable acidity, total sugars and total soluble phenols concentration as well as lower bacterial load, T.S.S./T.A. than uncoated fruits. [49] Lactiplantibacillus plantarum A6 Exo-polysaccharide L. plantarum A6 remain viable both in the solution and on the surface of the fruit after coating, protecting the fruit against two of the three evaluated fungi (Fusarium sp. and Rhizopus stolonifer). The edible coating controls weight loss, maintained firmness, and slowed the respiration rate of cherry tomato; the other physicochemical properties and the appearance of the fruit are not negatively affected. [50] Lactococcus lactis L3A21M1 and Lc. garvieae SJM17 Alginate, maltodextrin and glycerol The application of coating with immobilized Lactococcus cells on cheeses reduces significantly (p < 0.05) the contamination by L. monocytogenes on surface and prevents the growth of mesophilic bacteria by the 6th and 8th day of storage at 4 °C. [51] Lacticaseibacillus paracasei ALAC-4 Chitosan CS-CFS films exhibit strong antifungal activities against molds and yeasts, especially C. albicans, and also have excellent mechanical properties. Additionally, FTIR spectroscopy indicates that hydrogen bonds between the CFS and CS formed, and there is a smooth surface, compact cross-section observed in SEM morphologies of CS-CFS films. [52] Lactobacillus gasseri Pea protein isolates and psyllium mucilage Cell-free supernatants (CFS, so-called postbiotics) and whole-cell postbiotics (WCP) of probiotics exhibit antioxidant and antibacterial activities and are significant sources of phenolic compounds. [53] Table 3.
Edible coatings formulated with CFS of LAB.
Figures
(5)
Tables
(3)