-
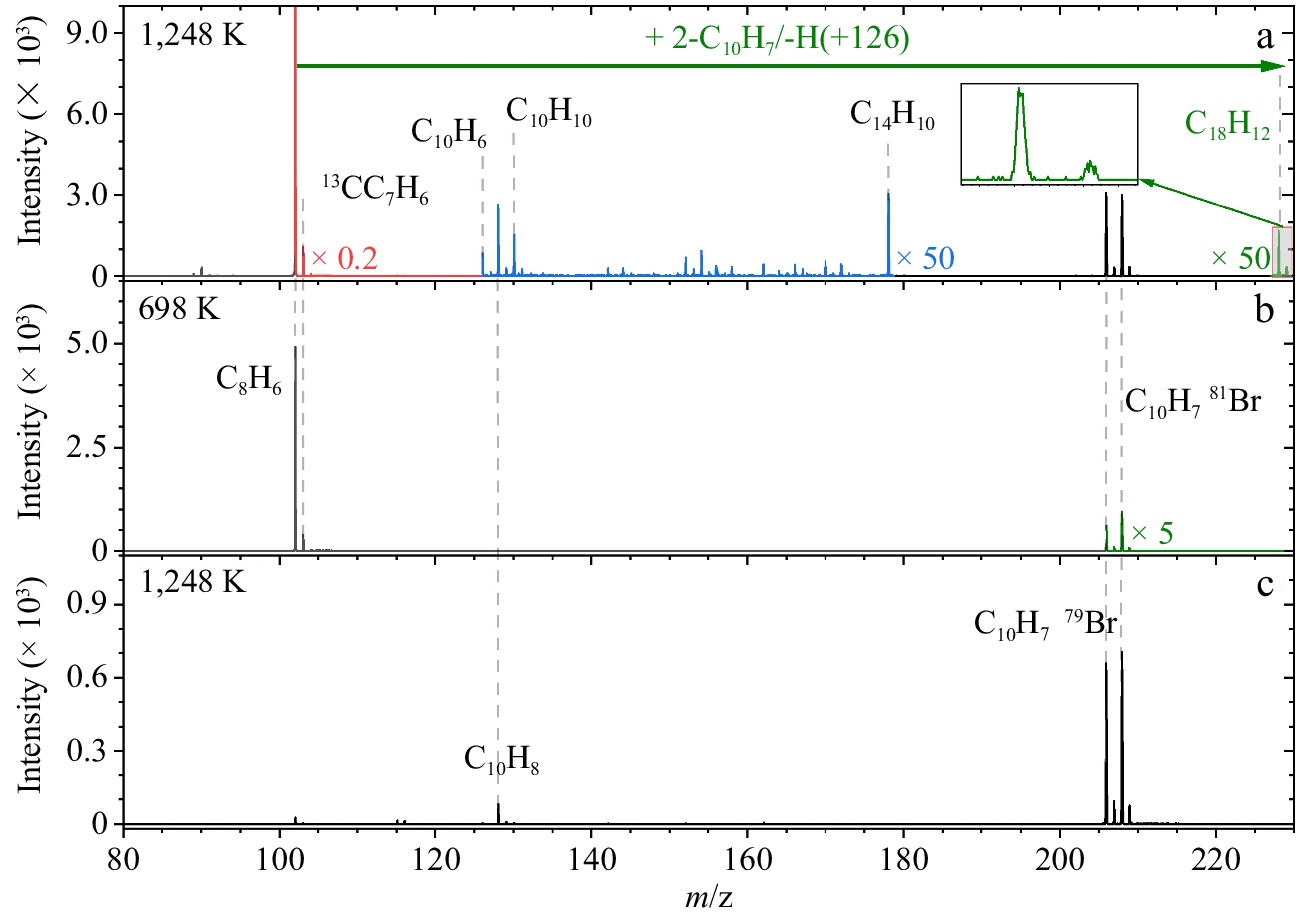
Figure 1.
Mass spectra of the products in flow reactor recorded at a photon energy of 9.5 eV. (a) The reaction of 2-naphthyl and phenylacetylene recorded at 1,248 K. Strong ion peaks (red) and weak ion peaks (blue and green) are multiplied by factors of 0.2 and 50 for clarity, respectively. (b) 2-Naphthyl/phenylacetylene reaction recorded at 698 K. Weak ion peaks (green) are multiplied by a factor of 5 for clarity. (c) 2-Bromo-naphthalene only, recorded at 1,248 K.
-
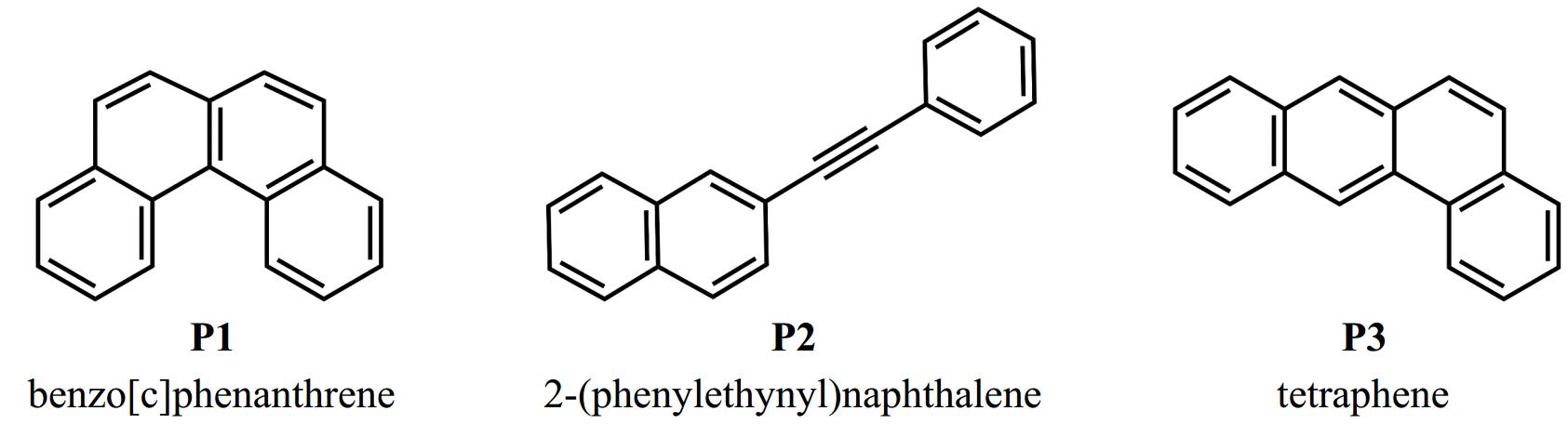
Figure 2.
Candidate molecular structures for C18H12 products observed in 2-naphthyl/phenylacetylene reaction.
-
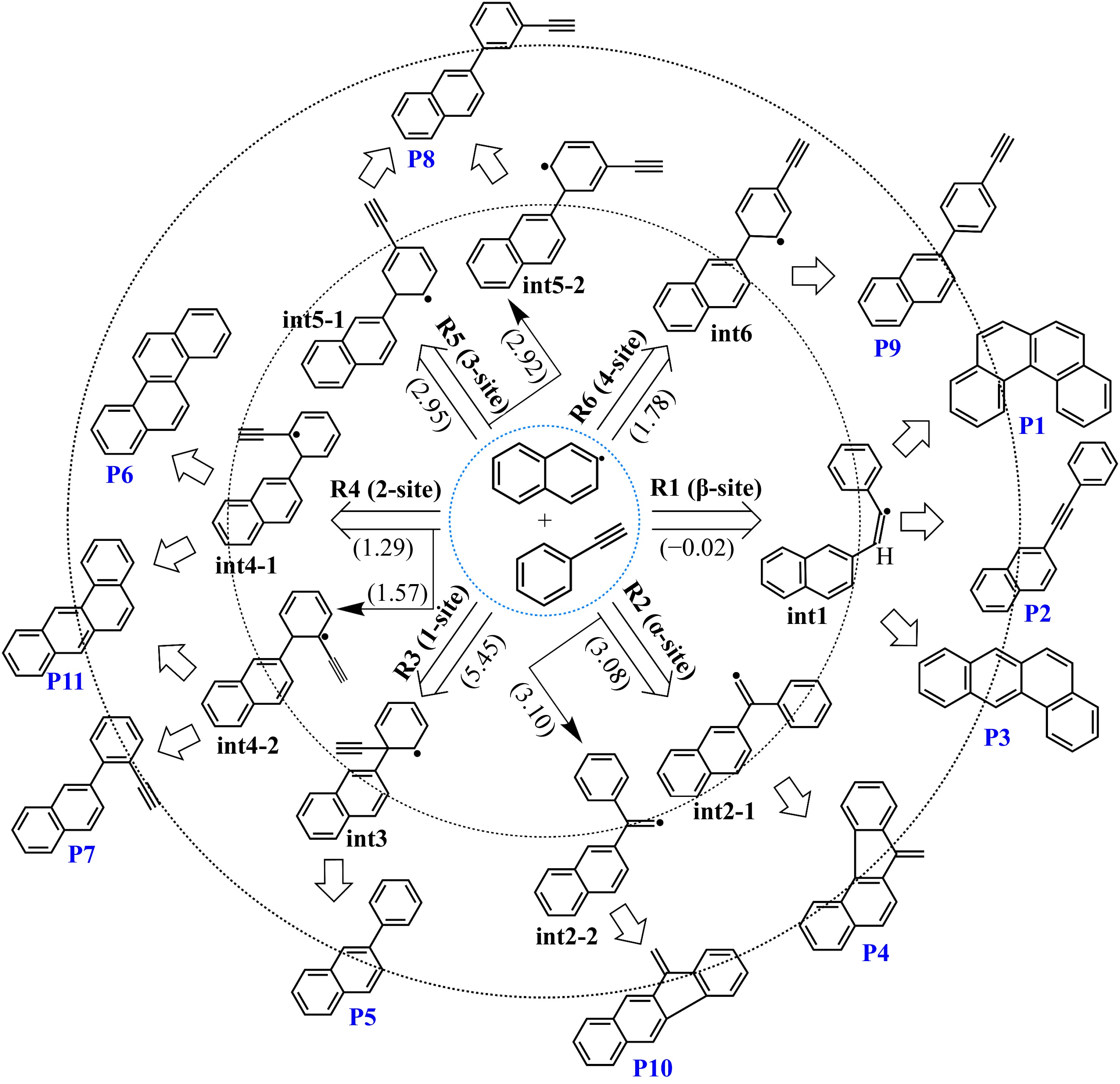
Figure 3.
Initial adducts and major products of the 2-naphthyl/phenylacetylene reaction at different sites. The numbers in brackets represent the transition state energies of initial addition steps.
-
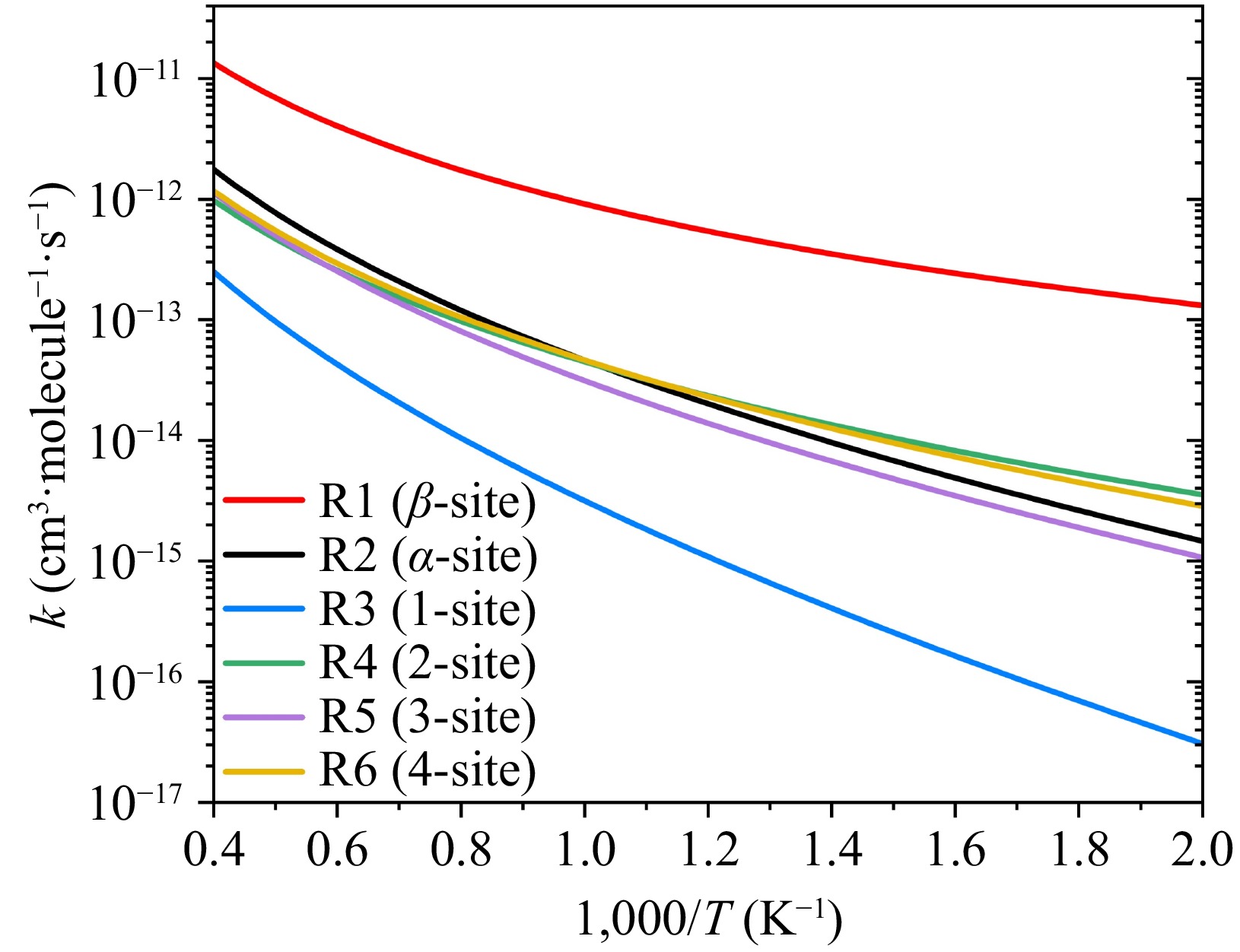
Figure 4.
Six initial addition channels for the reaction of 2-naphthyl with phenylacetylene at high-pressure limit.
-
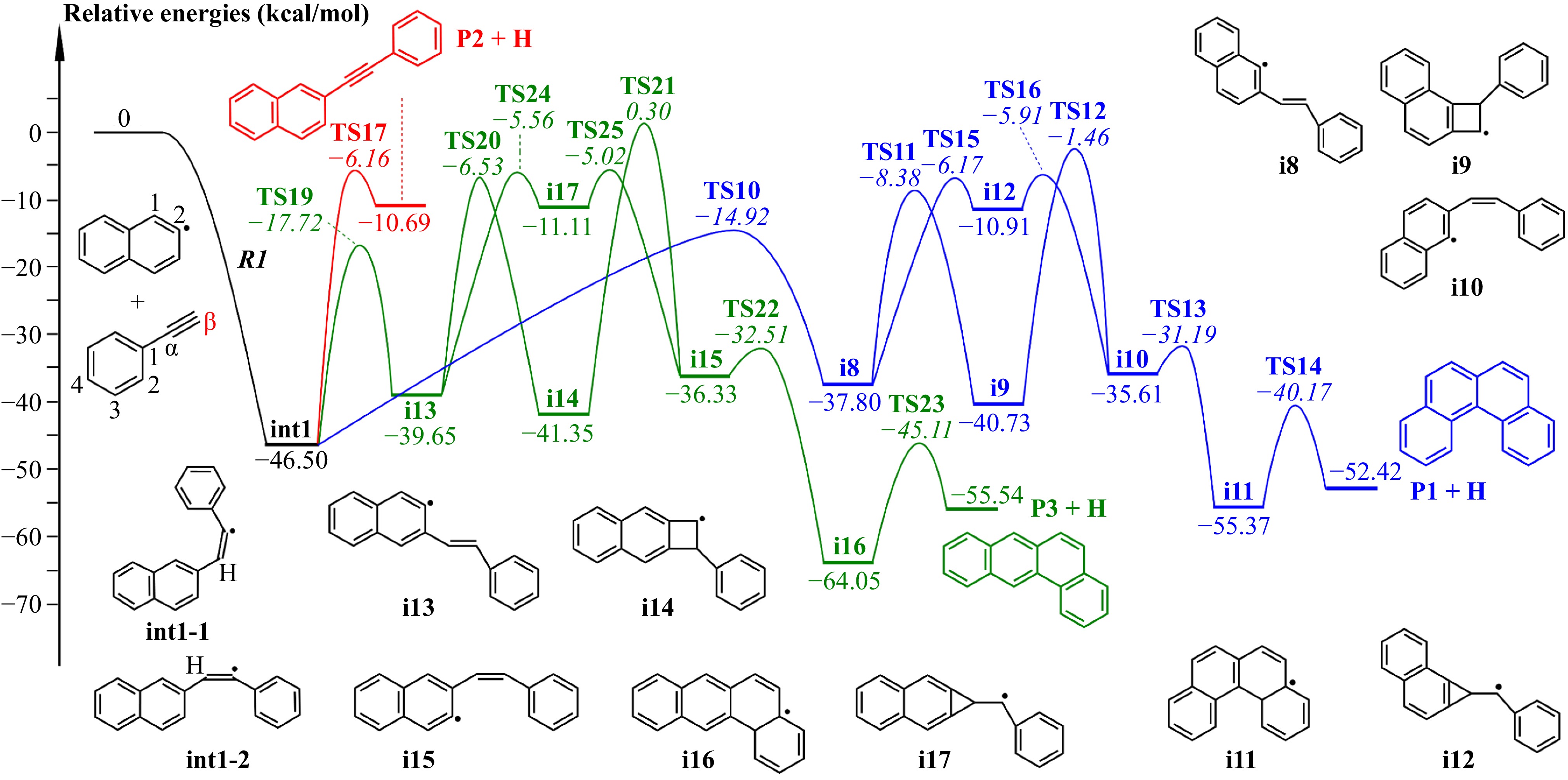
Figure 5.
Potential-energy profile of 2-naphthyl/phenylacetylene reaction at β-carbon. Numbers in normal type represent relative energies of intermediates and products; numbers in italic type represent transition states energies relative to the reactants. All pathways shown here are included in the master equation calculations.
-
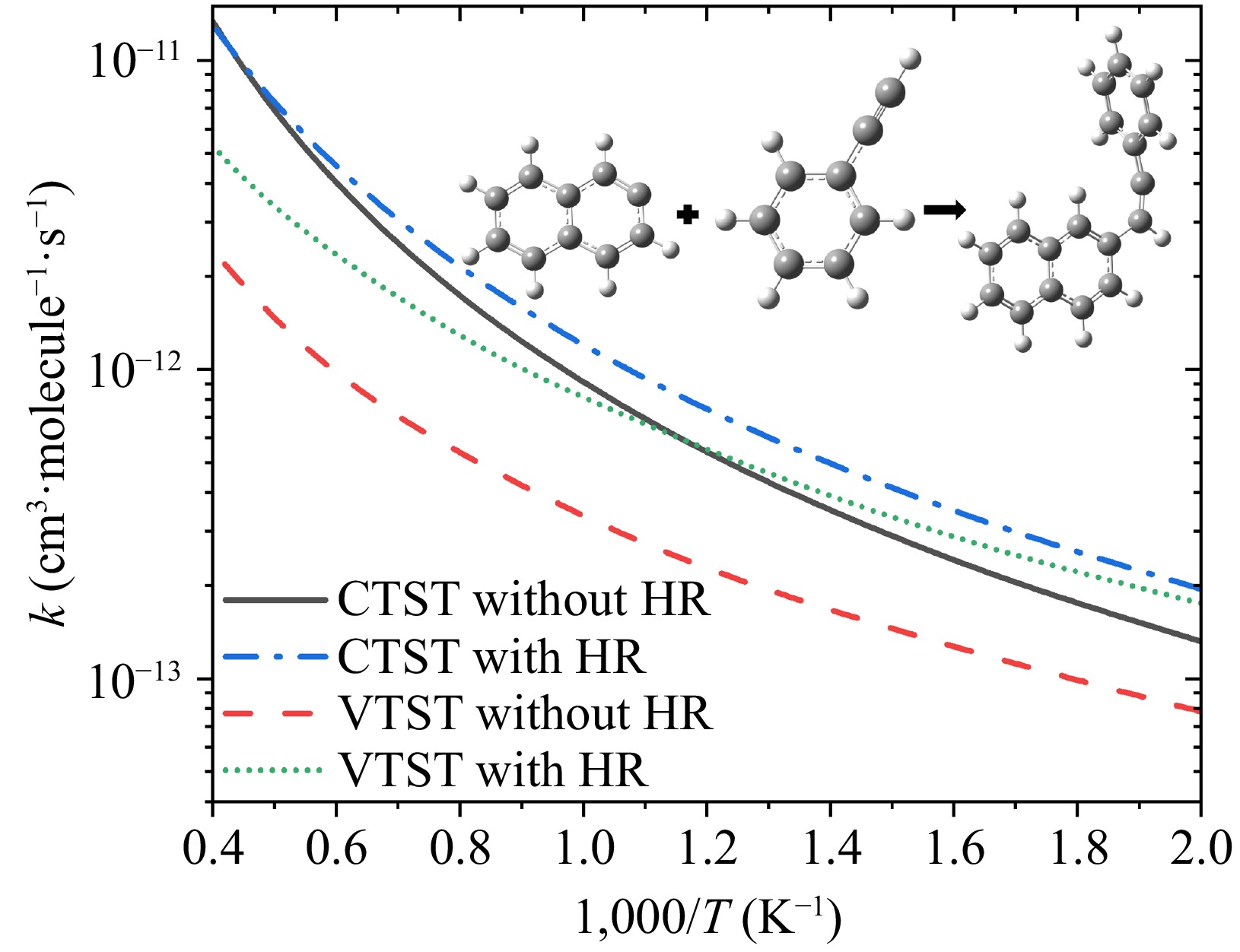
Figure 6.
Comparison of high-pressure limit rate coefficients for the entrance channel R1, with the four lines representing: (a) CTST without HR: CTST without hindered rotor treatment, black solid line; (b) CTST with HR: CTST with hindered rotor treatment, blue dash dot line; (c) VTST without HR: VTST without hindered rotor treatment, red dashed line; and (d) VTST with HR: VTST with hindered rotor treatment, green short dot line.
-
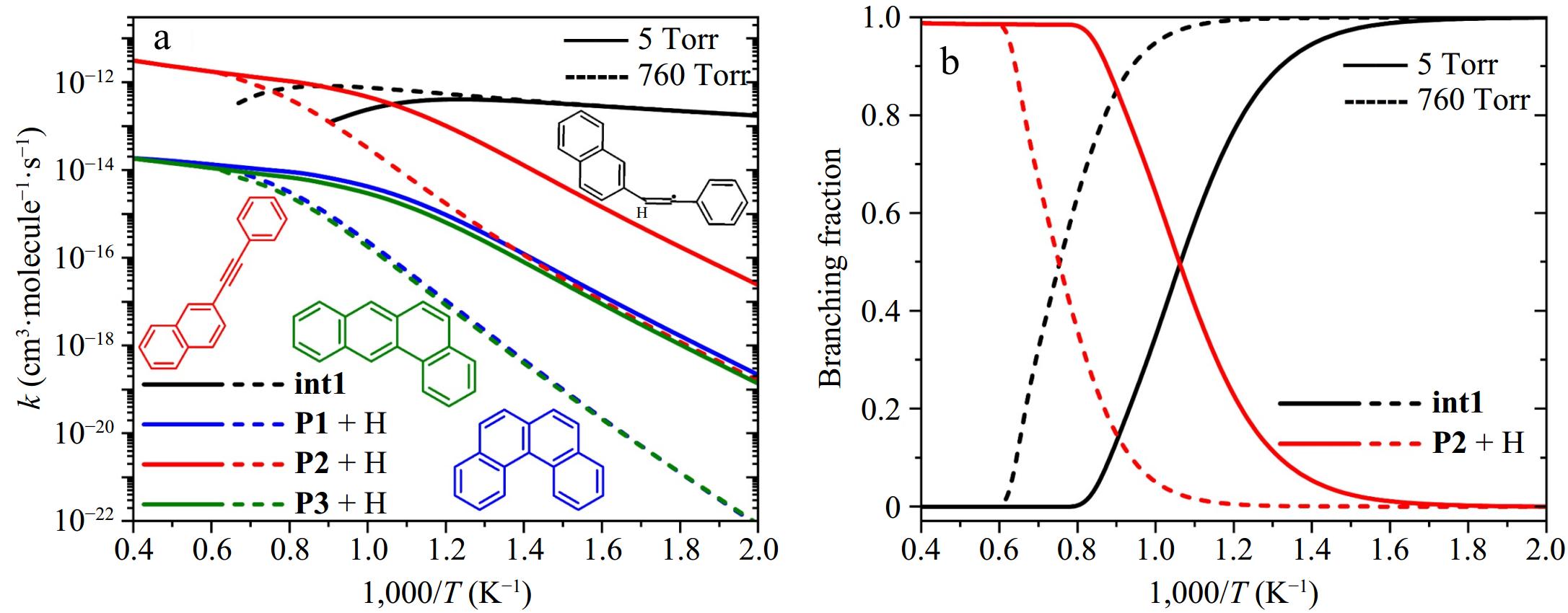
Figure 7.
(a) Rate coefficients, and (b) branching fractions for elementary reactions with 2-naphthyl + phenylacetylene as reactants: the stabilization of 2-styrylnaphthalene radical (int1), the formation of benzo[c]phenanthrene (P1) + H, 2-(phenylacetylene)-naphthalene (P2) + H, and tetracene (P3) + H. Solid and dashed lines represent results at 5 and 760 Torr, respectively.
-
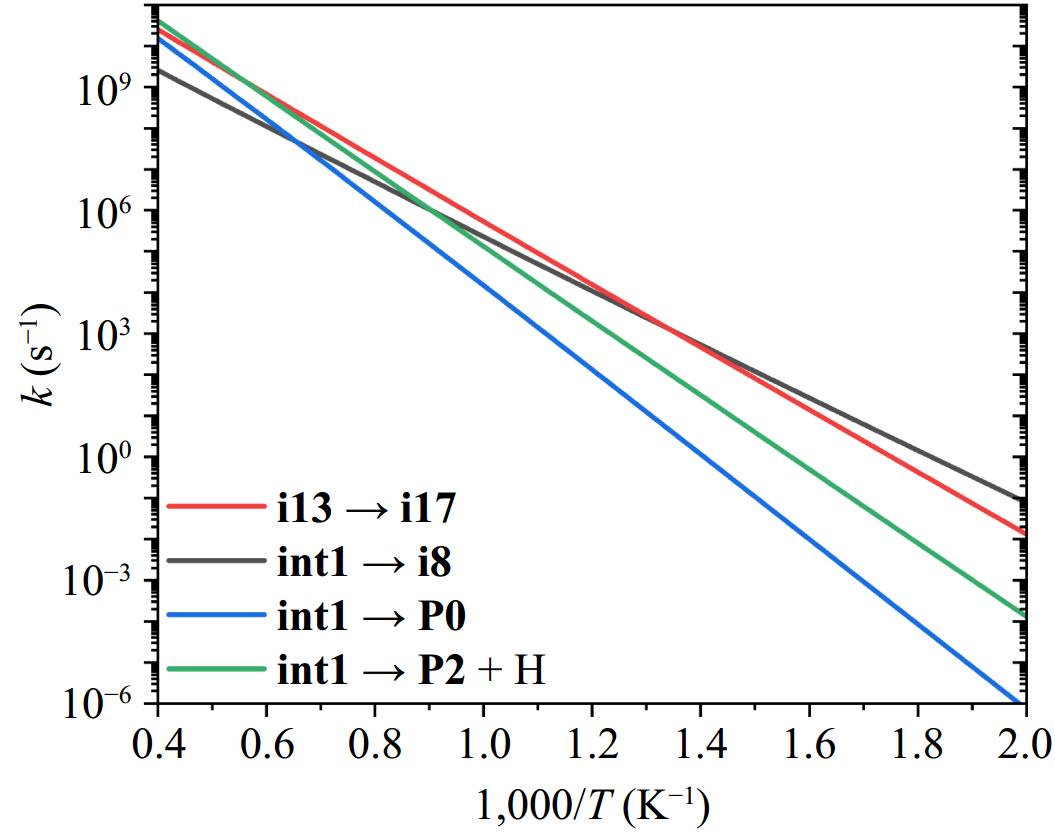
Figure 8.
The rate coefficients for the decomposition pathways of int1 at the high-pressure limit.
-
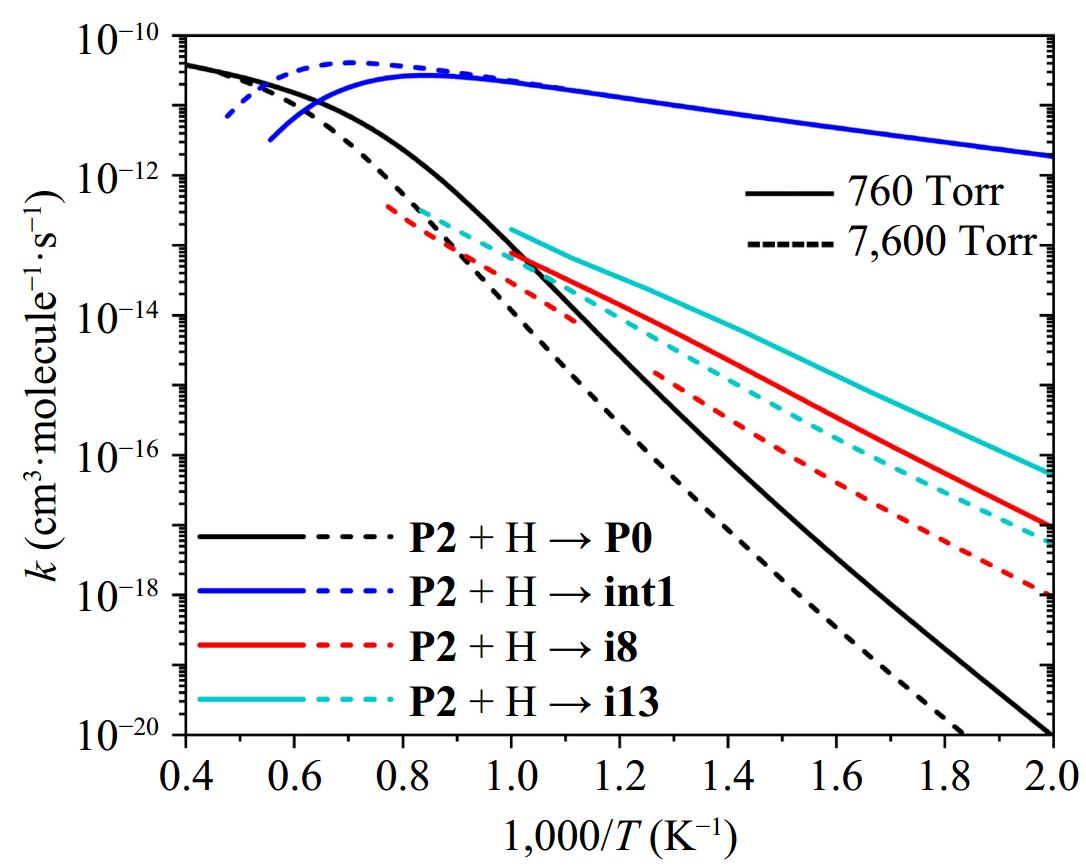
Figure 9.
Reaction rate constants of major channels of the P2 + H reaction at 760 and 7,600 Torr.
-
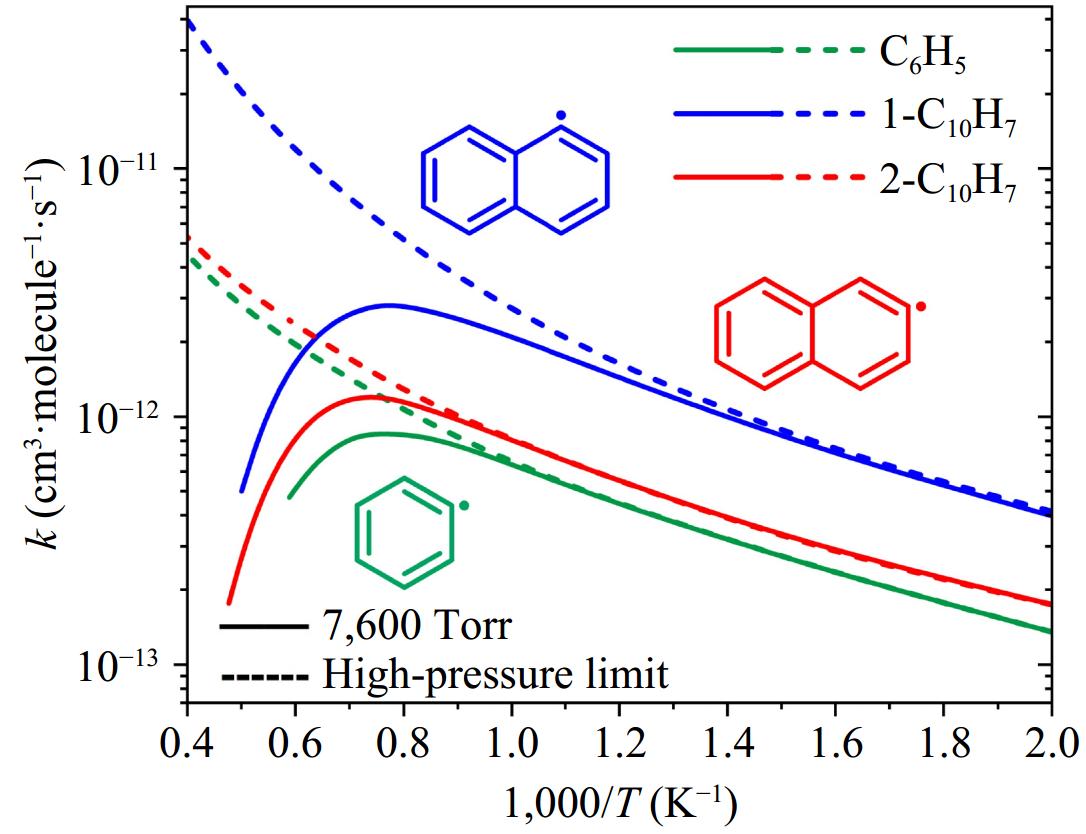
Figure 10.
Comparison of rate coefficients for the formation of initial adducts in the reactions of phenyl/phenylacetylene, 1-naphthyl/phenylacetylene, and 2-naphthyl/phenylacetylene. Solid and dashed lines represent the values at 7,600 Torr and high-pressure limit, respectively.
-
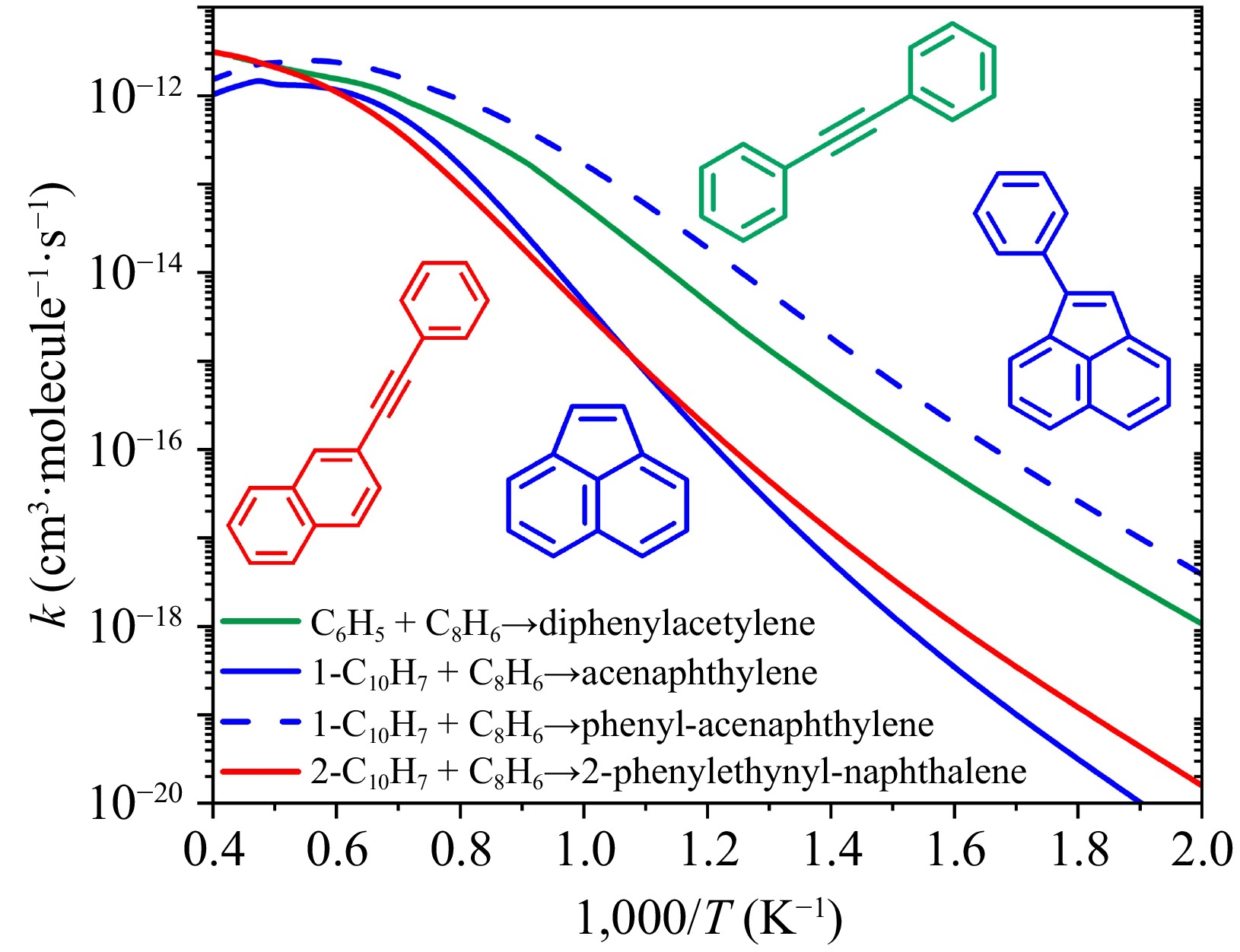
Figure 11.
Rate constants for aromatic formation via formally-direct mechanism in reactions between phenylacetylene (C8H6) and three aryl radicals at 7,600 Torr. Products in green and red correspond to phenyl and 2-naphthyl, which have 'free' edge sites; and products in blue correspond to 1-naphthyl, which has 'zigzag' edge sites.
Figures
(11)
Tables
(0)