-
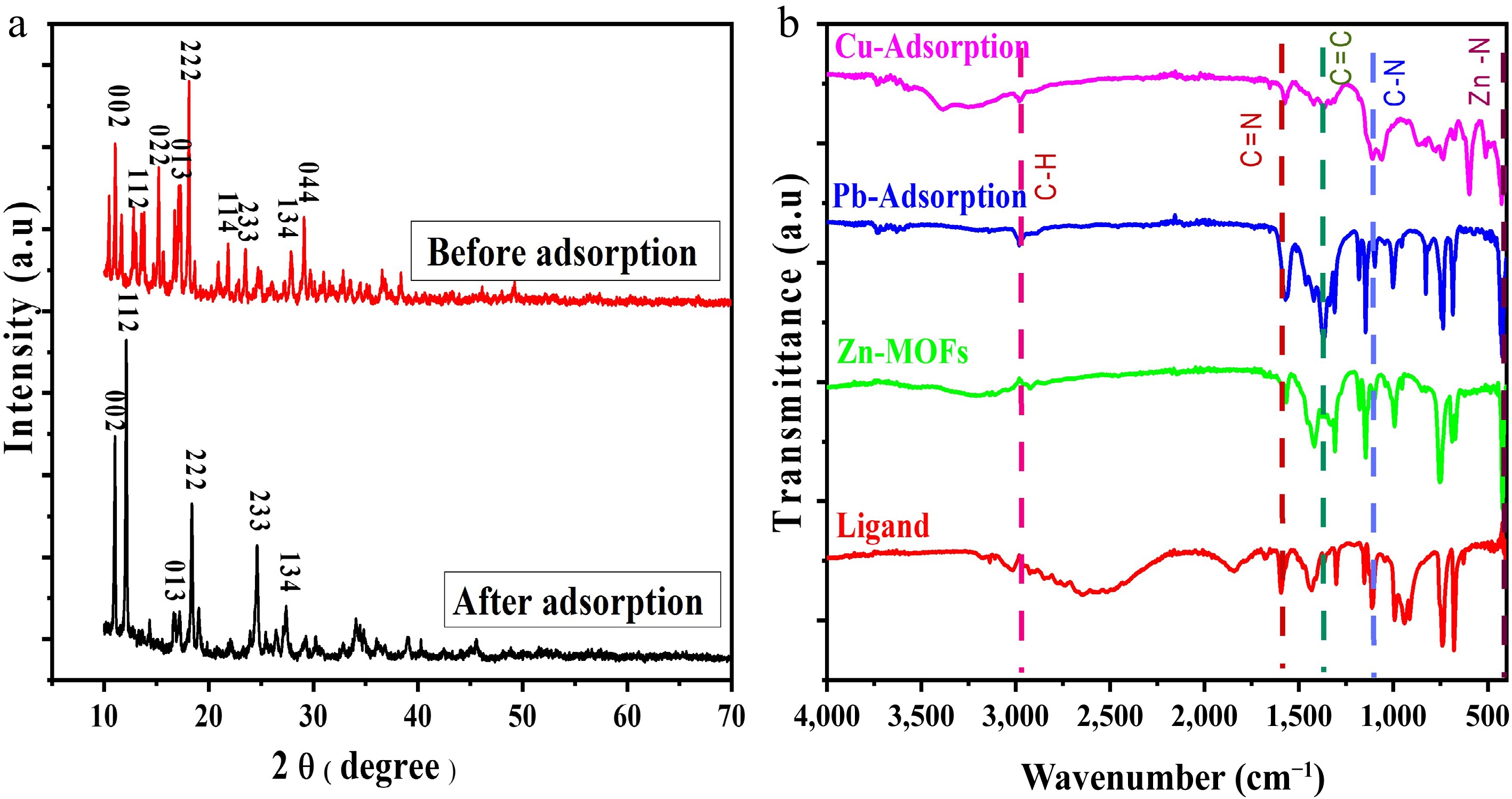
Figure 1.
(a) XRD. (b) FTIR spectra of Zn-MOFs before and after adsorption.
-
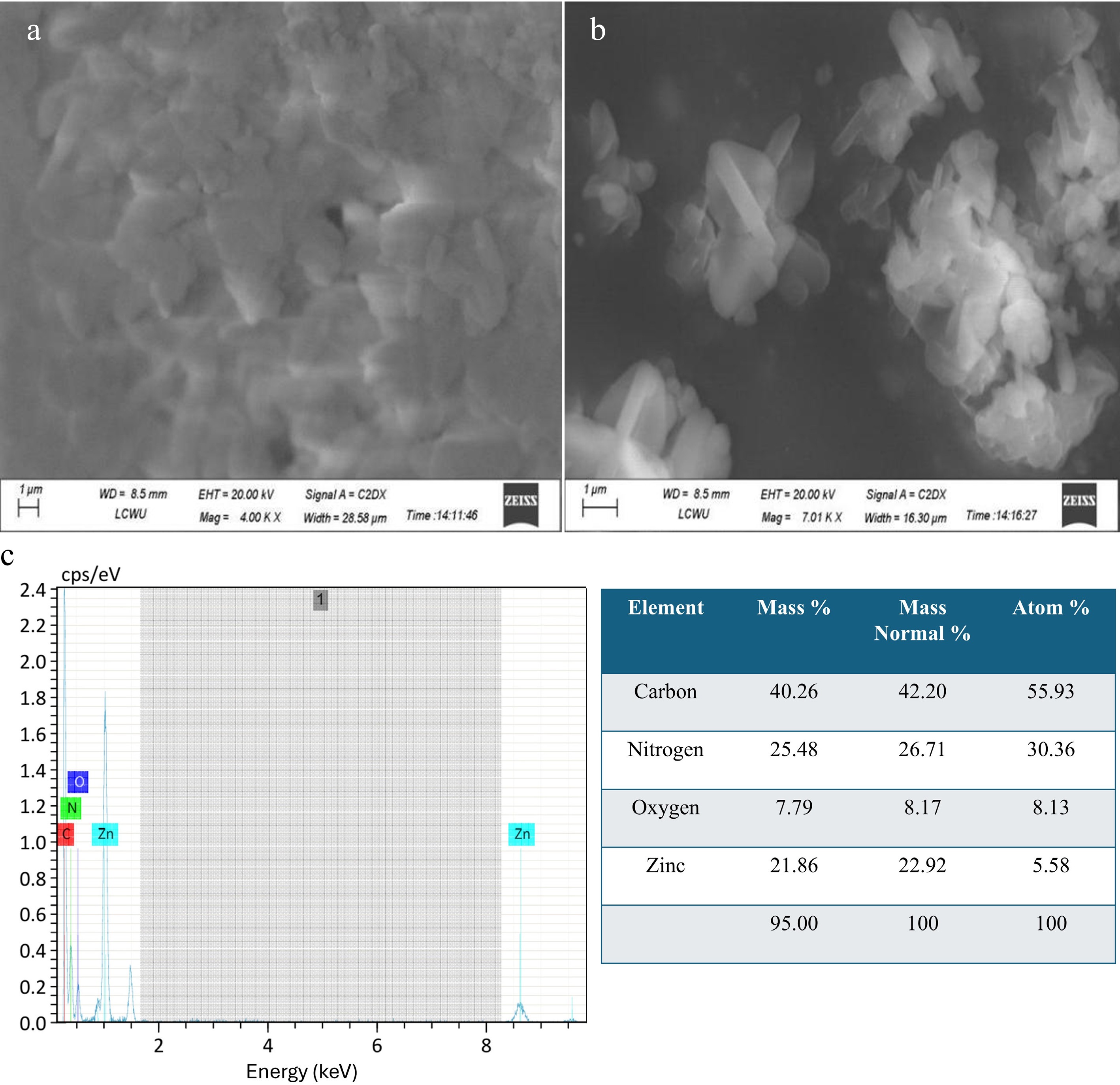
Figure 2.
(a), (b) SEM analysis of Zn-MOFs. (c) EDX spectra of Zn-MOFs.
-
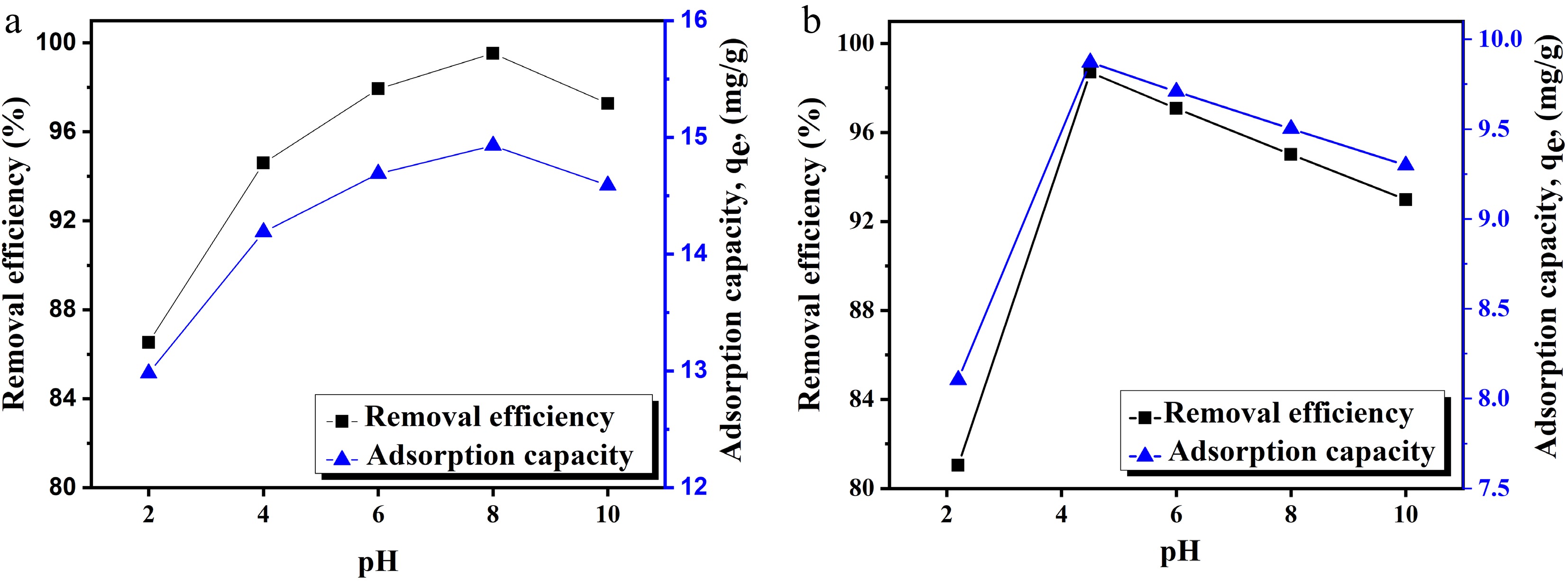
Figure 3.
Effect of pH on the adsorption of (a) Pb(II) and (b) Cu(II) ions.
-
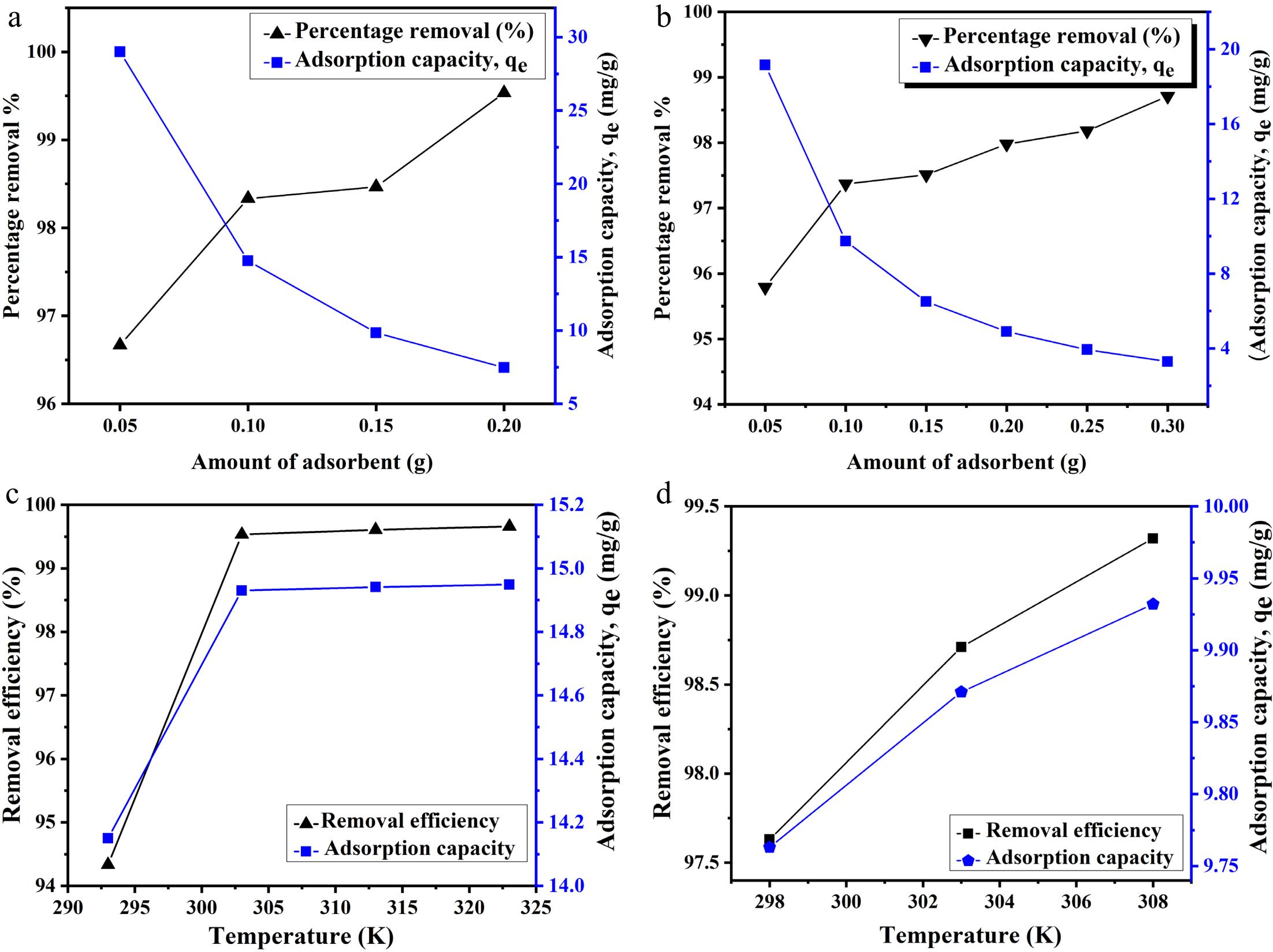
Figure 4.
Effect of adsorbent dosage on removal efficiency and adsorption capacity for (a) Pb and (b) Cu ions. Effect of temperature on the adsorption of (c) Pb and (d) Cu ions.
-
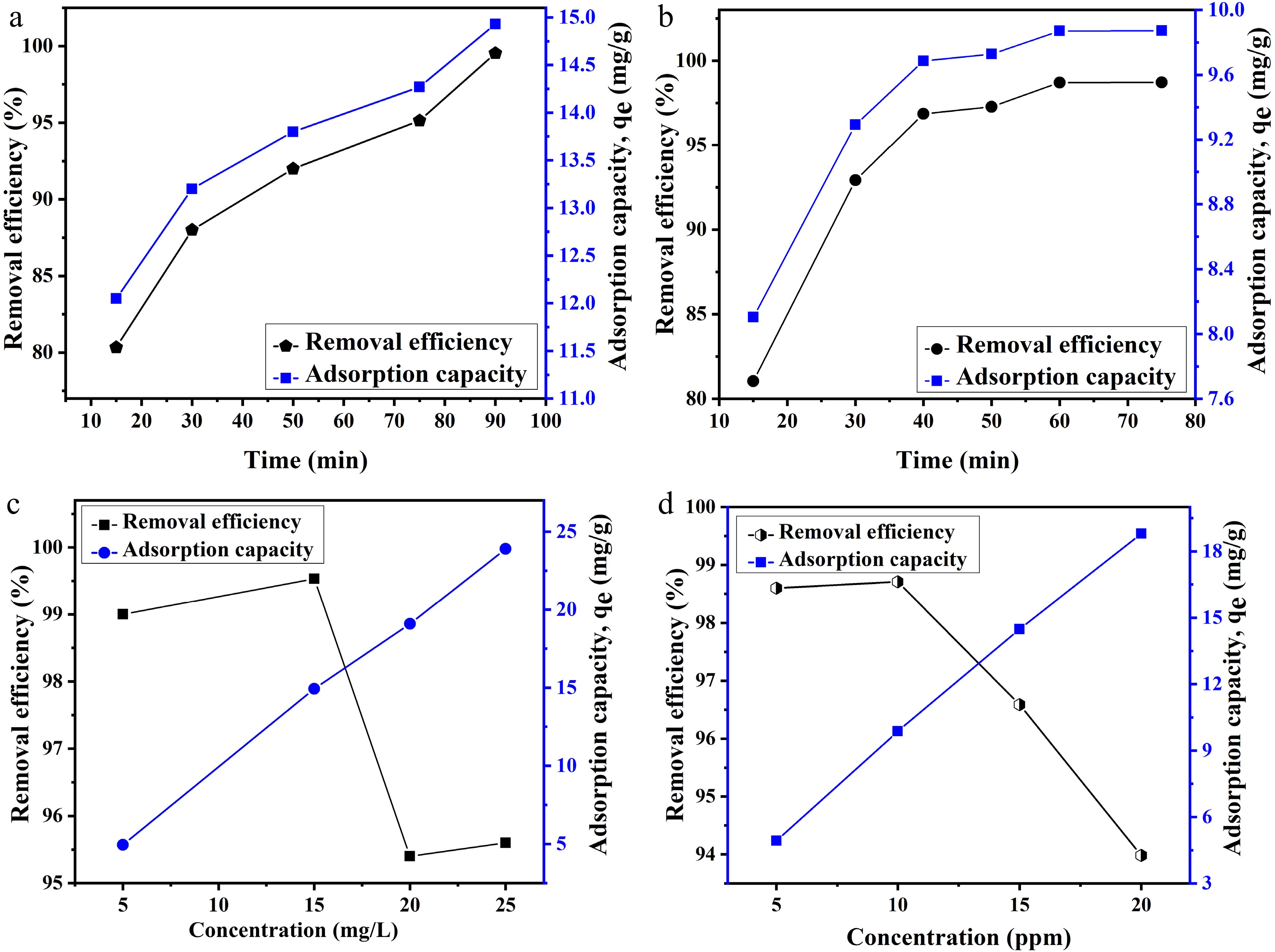
Figure 5.
Effect of time on the adsorption of (a) Pb and (b) Cu ion. Effect of the concentration of metal ions on adsorption of (c) Pb and (d) Cu ions.
-
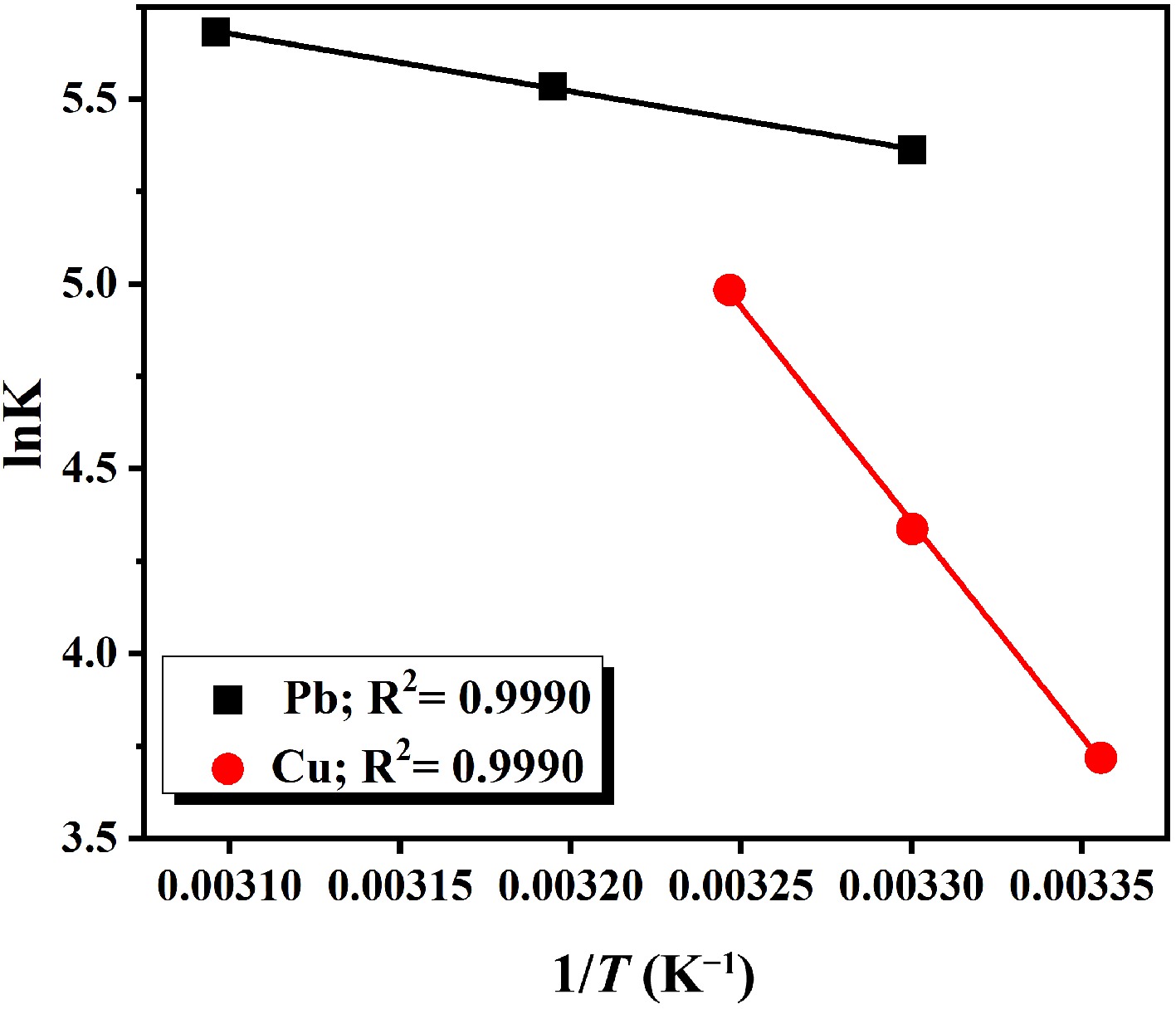
Figure 6.
Van't Hoff plot for lead and copper ions.
-
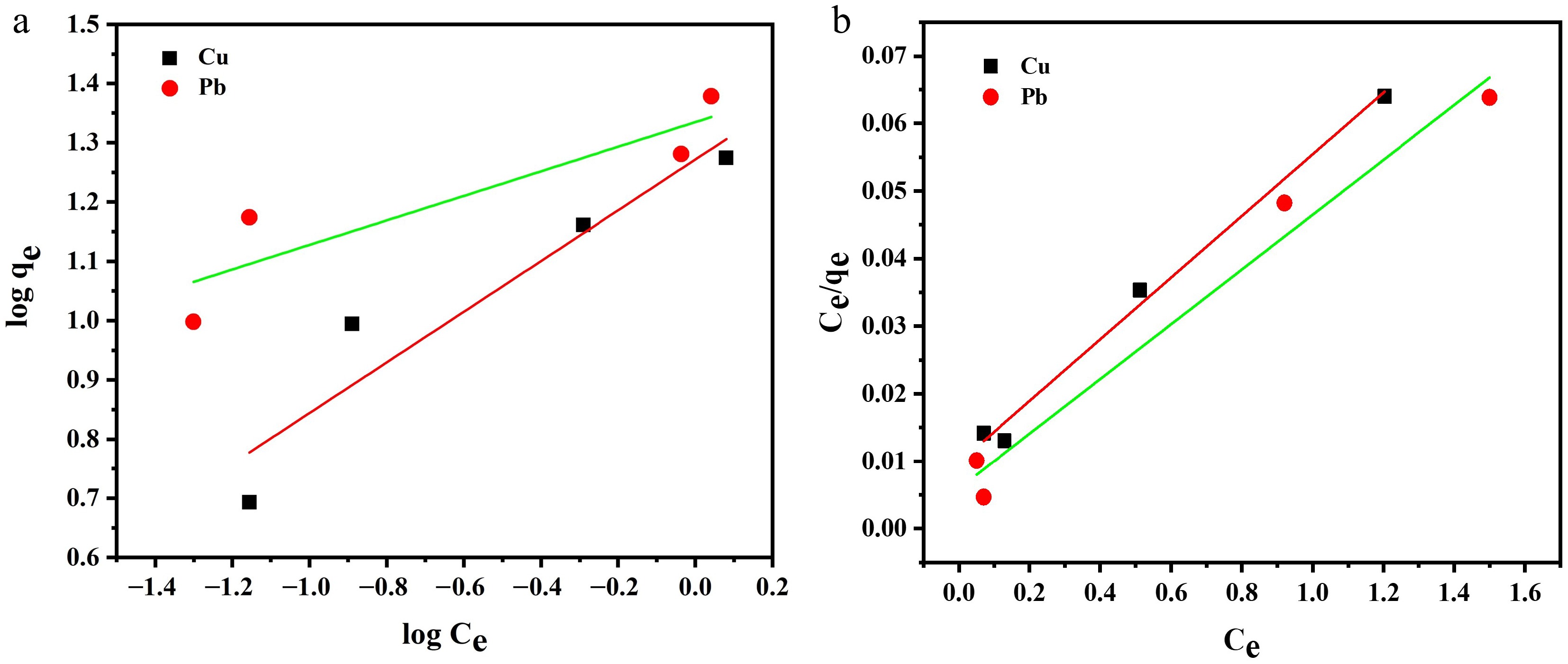
Figure 7.
(a) Freundlich adsorption isotherm. (b) Langmuir adsorption isotherm.
-
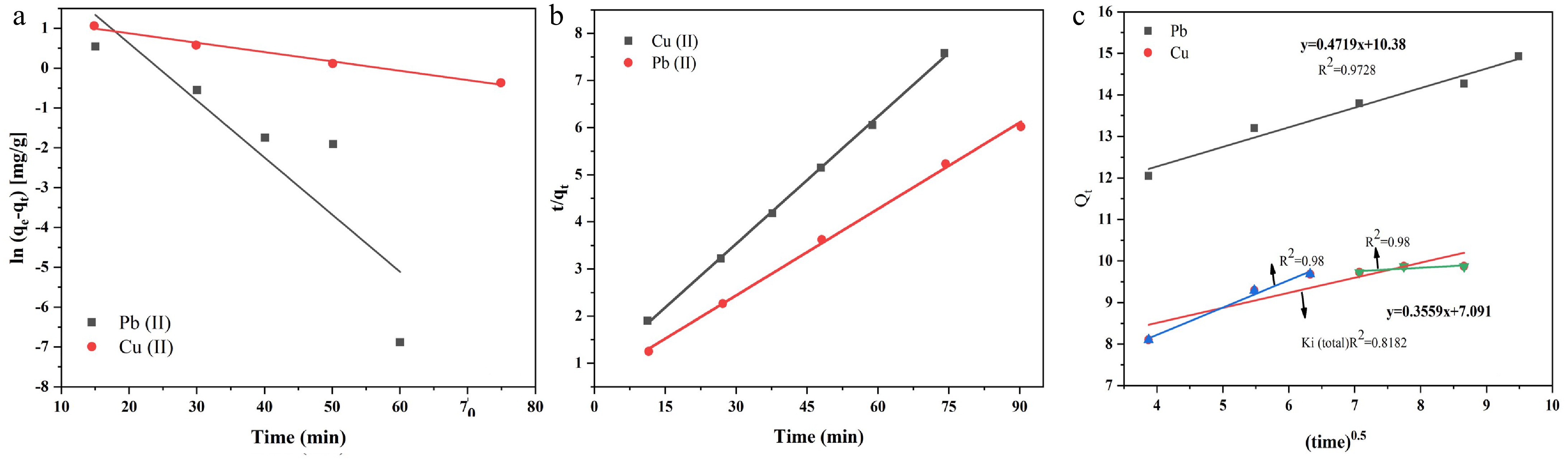
Figure 8.
(a) Pseudo-first-order kinetic model. (b) Pseudo-second-order kinetic model. (c) Intraparticle diffusion model.
-
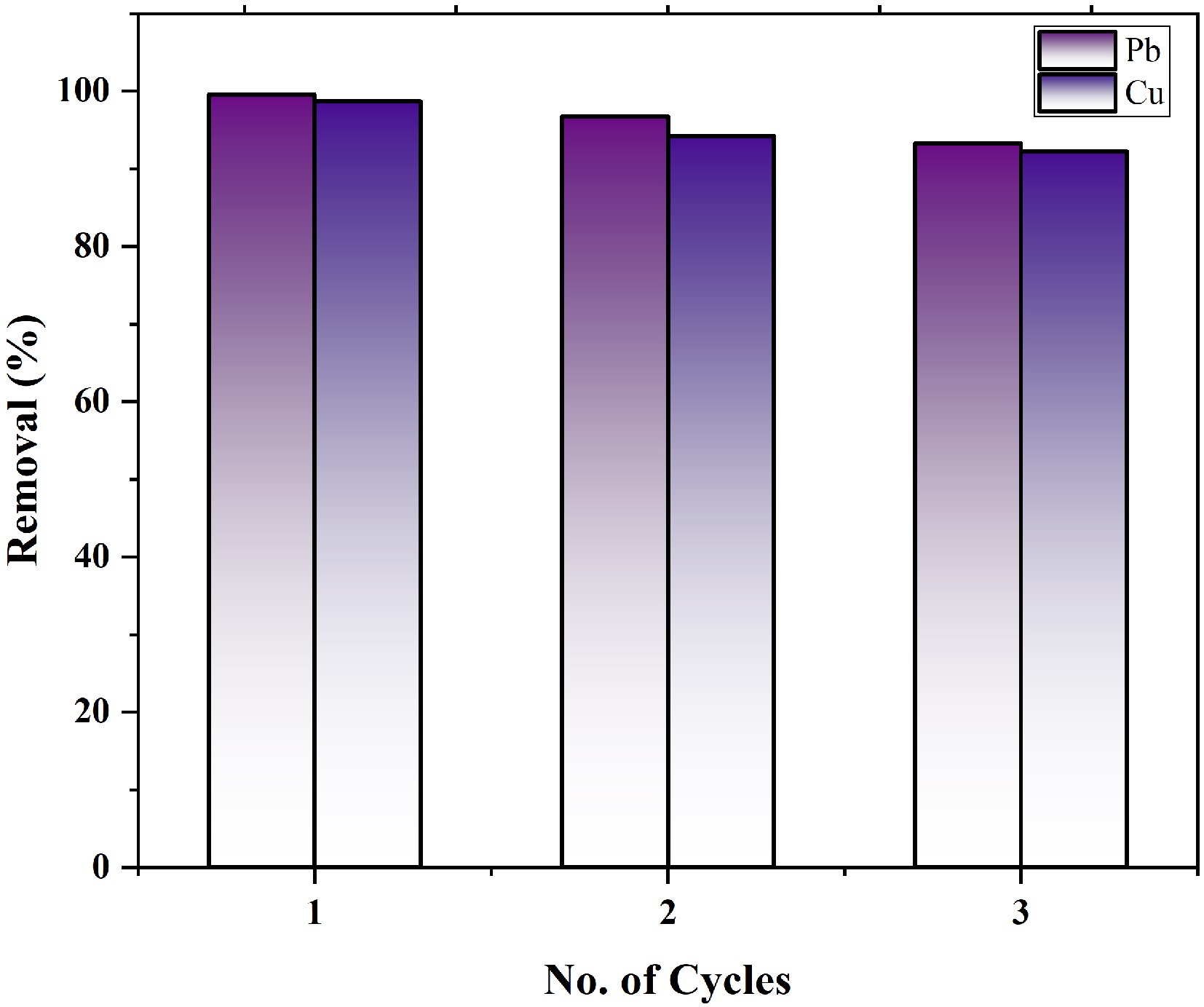
Figure 9.
Reusability of MOFs for the adsorption of copper and lead ions.
-
Copper ions Lead ions Temperature (K) 298 303 308 303 313 323 Δ G (kJ/mol) −9.2 −10.5 −12.7 −13 −14.4 −15.2 Δ H (kJ/mol) 96.6 12.9 Δ S (kJ/mol K−1) 0.35 0.08 Table 1.
Thermodynamic parameters.
-
Adsorbate Freundlich isotherm Langmuir adsorption isotherm Kf n R2 KL qm R2 Pb 21.60 ± 0.05 4.830 ± 0.10 0.7339 6.76 ± 0.003 24.68 ± 0.004 0.9672 Cu 18.66 ± 0.07 2.341 ± 0.06 0.8510 4.65 ± 0.001 21.9 ± 0.002 0.9881 Table 2.
Best fitting parameters for the adsorption isotherm for removal of copper and lead ions using Zn-MOFs
-
Copper Lead C0 (mg/L) RL ± 0.001 C0 (mg/L) RL ± 0.003 5 0.041 5 0.0287 10 0.021 15 0.00976 15 0.014 20 0.0073 20 0.010 25 0.0058 Table 3.
Calculations for the separation factor at different initial concentrations.
-
Adsorbate qe (mg/g) Experimental Pseudo-first-order Pseudo-second-order k1 qe calculated (mg/g) R2 k2 qe calculated (mg/g) R2 Pb 14.93 −0.024 ± 0.08 3.658 ± 0.044 0.9831 0.0125 ± 0.08 15.52 ± 0.001 0.9977 Cu 9.87 −0.014 ± 1.8 34.88 ± 0.001 0.7066 0.024 ± 0.05 10.44 ± 0.001 0.9993 Table 4.
Best fitting kinetic parametres for the removal of copper and lead by Zn-MOFs.
-
Serial no. Adsorbent Adsorbate Adsorption capacity (mg/g) Cost effectiveness Removal percentage Industrial application potential Ref. 1. Almond shells Pb(II) 3.58 Very high 80.3% Moderate [53] 2. Walnut shells Pb(II) 3.59 Very high 82.7% Moderate [53] 3. Neem leaves Pb(II) 22.33 Very high − Moderate [53] 4. Citrus peel Pb(II) 23.04 High 97.08% Low to moderate [54] 5. Rice bran Cu(II) 5.93 High − Moderate [55] 6. Rice husk Cu(II) 10.93 High 85% Moderate [56] 7. Our Zn-MOFs Pb(II) and Cu(II) 29 and 19 Moderate 99.5% and 98.7% High − Table 5.
Comparison of different parameters of various natural adsorbents with Zn-MOFs.
Figures
(9)
Tables
(5)