-
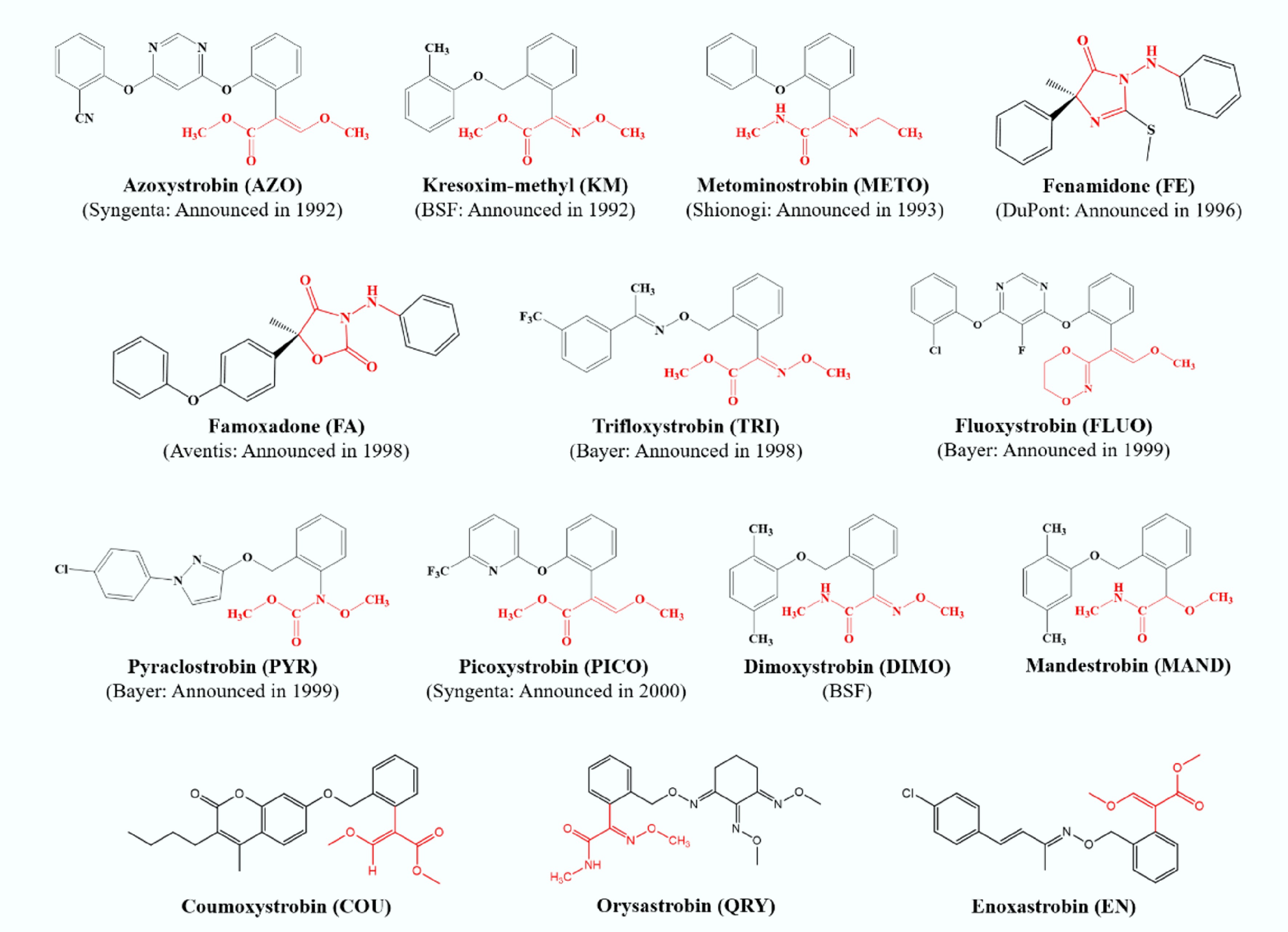
Figure 1.
Announcement time and chemical structures of 14 strobilurin fungicides.
-
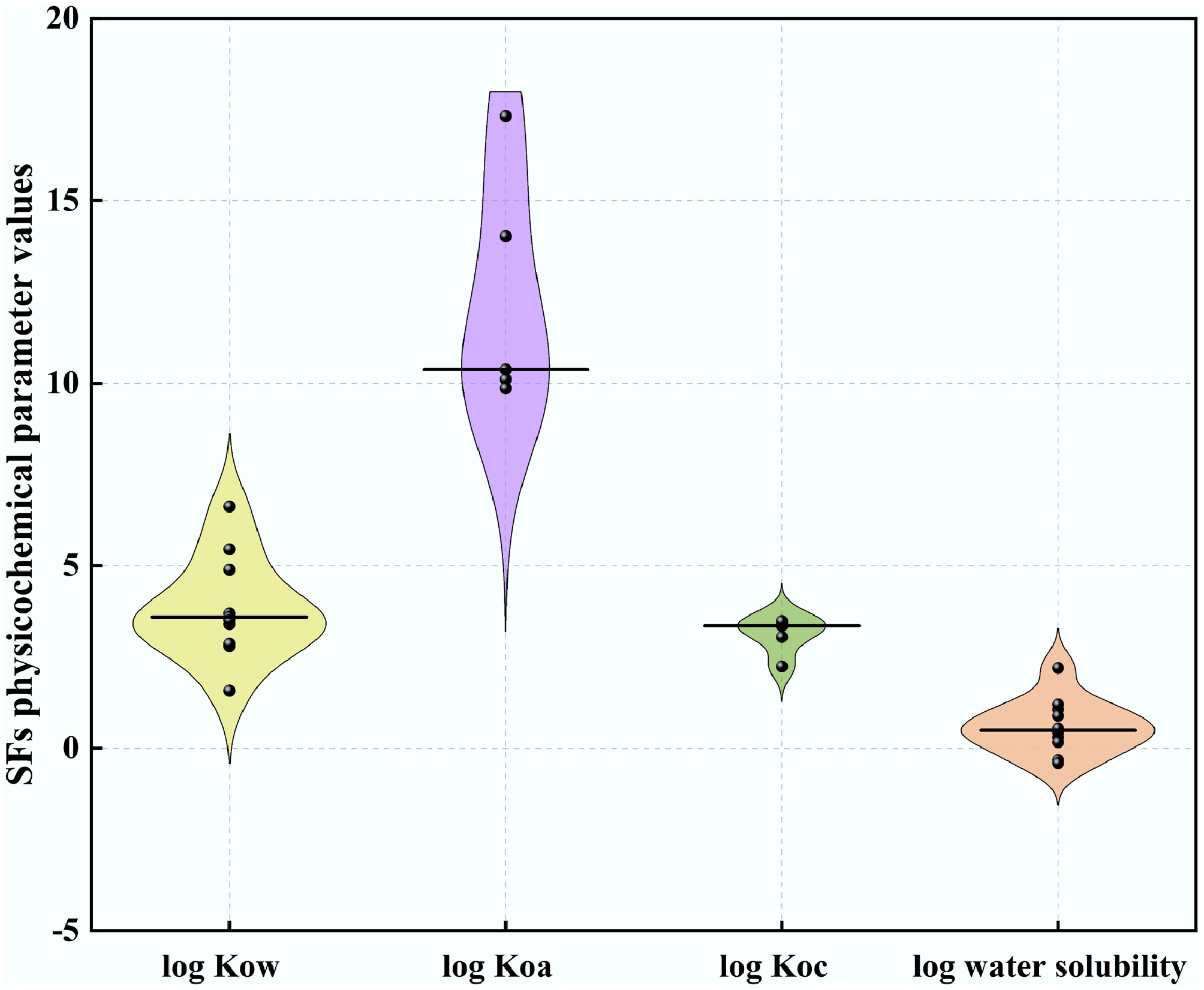
Figure 2.
Violin plots of physico-chemical properties related to mobility and dissipation for strobilurins fungicides currently registered for use in the EU (log Kow: n = 11, log Koa: n = 5, log Koc: n = 5, log water solubility (20 °C): n = 5). Black bars within violins represent medians.
-
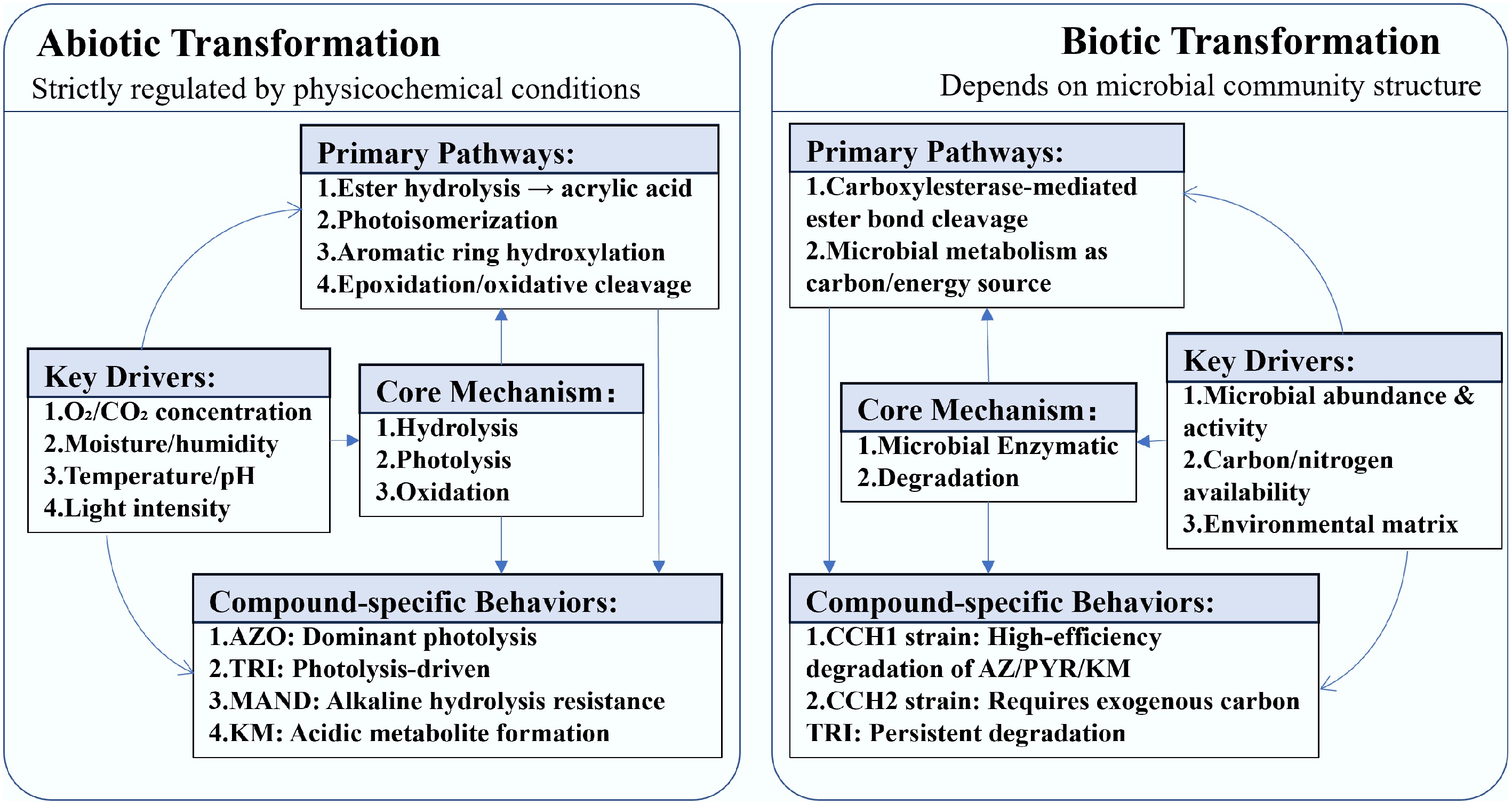
Figure 3.
Comparison of abiotic vs biotic transformation pathways for strobilurin fungicides.
-
Compound Abbreviation Structure Molecular formula CAS M.W. Log Kow Log Koa Log Koc Solubility in water (mg L−1) at 20 °C Azoxystrobin AZO 
C22H17N3O5 131860-33-8 403.40 1.58 14.03 3.05 11.61 Kresoxim-methyl KM 
C18H19NO4 143390-89-0 313.40 3.40 − − 2.00 Pyraclostrobin PYR 
C19H18ClN3O4 175013-18-0 387.80 5.45 17.32 3.48 1.43 Trifloxystrobin TRI 
C20H19F3N2O4 141517-21-7 408.40 6.62 9.86 3.35 0.39 Fluoxystrobin FLUO 
C21H16ClFN4O5 361377-29-9 458.80 2.86 − − 2.56 Picoxystrobin PICO 
C18H16F3NO4 117428-22-5 367.30 3.60 − − 3.10 Dimoxystrobin DIMO 
C19H22N2O3 149961-52-4 326.40 3.59 − − 3.50 Metominostrobin METO 
C16H16N2O3 133408-50-1 284.30 3.69 10.11 2.24 158.00 Mandestrobin MAND 
C19H23NO3 173662-97-0 313.40 3.51 − − 15.80 Fenamidone FE 
C17H17N3OS 161326-34-7 311.40 2.80 − − 7.80 Famoxadone FA 
C22H18N2O4 131807-57-3 374.40 4.89 10.38 3.45 0.47 Table 1.
Physico-chemical properties and structural characteristics of strobilurin fungicides
-
Matrix Target SFs Sample preparation Analytical technique Recovery (RSD) LOQ Ref. Vegetable, fruit, and food Bean AZO, KM, TRI, DIMO,
FLUO, PICO, PYRLLME (ACN/H2O 9:1b) HPLC/UV-AD 61.6%–98.8% (< 10%) 0.004–0.005 mg·kg–1 [34] Rice ORY LLME (DCM/n-Hexane 20:80)
Florisil Column Chromatography (EA/DCM 10:90)HPLC-UV
LC-MS/MS83.9%–92.3%
80.6%–114.8%0.02 mg·kg–1
0.002 mg·kg–1[35] Apple, grape, wheat AZO, KM, TRI Extracted: EA/Cyclohexane
Clean-up: GPCGC-EC
GC-NP
GC-MS70%–114% −a [36] Pomegranate AZO, PYR d-SPE (ACN) LC-MS/MS 78.7%–98% (< 20%) 0.005 mg·kg–1 [17] Pepper PYR, PICO QuEChERS (ACN) UPLC-MS/MS 91%–107%
(3.7%–9.6%)0.12–0.61 μg·kg–1 [21] Watermelon FA LLextraction: DCM
Clean-up: Acetone/petroleum 1:9)HPLC-UVD 84.91%–99.41%
(0.06%–4.93%)− [37] Cucumber PYR QuEChERS UHPLC-MS/MS 89.8%–103.6%
(3.6%–7.5%)8.1 μg·kg–1 [18] Grape, must, wine AZO SPE (MeOH)
SPE (DCM)PIF-FI-SPS 84.0%–87.6%,
95.5%–105.9%,
88.5%–111.2%21 μg·kg–1
18 μg·L–1
8 μg·L–1[31] Jujube PYR, AZO QuEChERS (ACN) LC-MS/MS 87.5%–116.2%
(3.2%–14.7%)0.01–0.2 mg·kg–1 [24] Watermelon TRI QuEChERS (ACN) GC-MS/MS 78.59%–92.66% 0.01 mg·kg–1 [23] Apple tree bark PYR QuEChERS (ACN-Ammonia) HPLC-VWD 86.1%–101.4% 0.028–0.080 mg·kg–1 [20] Green bean, pea AZO LLME (ACN) HPLC-UV
GC-MS81.99%–107.85%
(< 20%)
76.29%–100.91%
(< 20%)0.1 mg·kg–1 [38] Banana AZO QuEChERS-Citrate (ACN) GC-SQ/MS − 0.022–0.199 mg·kg–1 [19] Pomegranate AZO, PYR QuEChERS (ACN);
d-SPE (n-Hexane /acetone 9:1)LC-MS/MS
GC-MS78.7%–98% (< 20%) 0.005 mg·kg–1
0.01 mg·kg–1[17] Water, juice, wine, vinegar PICO, PYR, TRI d-LLME (DES: thymol, octanoic acid) HPLC 77.4%–106.9%
(0.2%–6.8%)− [39] Rice TRI Extraction solvent (ACN) LC-MS/MS 74.3%–103.0% (0.5%–6.8%%) − [40] Cereal AZO, PYR,
TRId-LLME-SFOD (Nonanoic acid) HPLC-MS/MS 82.0%–93.2%
(1.6%–7.4%)− [41] Apple, citrus, cucumber, potato, tomato AZO,
KM,
PYR− Electrokinetic capillary chromatography 81.7%–96.1%
86.5%–95.7%
87.3%–97.4%0.005–2.5 mg·kg–1 [32] Tea TRI QuEChERS GC-MS 80.7%–105.8% (<9.3%) 0.05 mg·kg–1 [22] Red wine TRI, KM,
PICOCross-linked poly as a sorbent FPIAs-monoclonal antibodies 80%–104% (<12%) − [33] Cotton seed TRI, PICO,
KM, AZOd-LLME (ACN) GC-ECD 87.7%–95.2%
(4.1%–8.5%)− [42] Grape wine AZO, KM, TRI, PYR, FA, FE LLME (EA/n-Hexane 50:50) LC-ADA
GC-MS95.5% ± 5%
104% ± 6%0.3–0.6 mg·L–1
0.4–0.8 mg·L–1[43] Environmental media Lake, river, tap water AZO, ORY, PICO,
DIMO, KMMagnetic SPE HPLC-MS/MS 80.8%–109% 0.18–0.24 mg·L–1 [25] Soil, water, FA SPE (ACN) UHPLC-Orbitrap-MS 70%–120% (< 20%) 0.1 mg·kg–1
1 μg·L–1[44] Atmosphere AZO,
DIMO,
FLUO,
KM,
PYR,
TRI− LC-MS/MS 91.6% ± 12.2%
99.9% ± 5.6%
100.8% ± 10.4%
101.2% ± 0.2%
99.9% ± 0.7%
97.7% ± 6.3%− [45] Biological Sample Fish gill, blood, liver, muscle, PYR Mixtures of PSA, C18, MgSO4
QuEChERS-PC
Waters Oasis HLB SPEUPLC-MS/MS 112.5%–276.2%
(< 10%)
45.3%–259.7%
(< 10%)
86.94%–229.9%
(< 10%)0.002 mg·kg–1 [26] Human urine AZO, PYR d-LLME (Choline chloride / sesamol 1:3) HPLC-MS/MS 50%–101% 0.03–0.07 ng·mL–1 [28] Human urine AZO SPE (ACN) UHPLC-Orbitrap-MS 90%–103% 0.01 ng·mL–1 Human blood AZO, FA SPE (Dichloromethane /
methanol 9:1)GC-MS/MS 70%–120% (< 20%) < 1.45 ng·mL–1 [30] Indoor dust New dry wall, gypsum, house dust AZO,
PYR,
TRI,
FLUOExtracted: DACM/Hexane,1:1
Clean-up: ENVI-FlorisilLC-MS/MS 92%–96%
73%
36%
38%− [11] Indoor dust AZO, FLUO, TRI, PYR Extracted: ACN
Clean-up: ENVI-FlorisilUHPLC-MS/MS 91.2%–108% 0.005–0.01 ng·mL–1 [46] a: '–' indicates that the sample preparation method or recovery data were not recorded in the related literature. b: Extraction solvent ratio, indicated by the volume ratio. Abbreviation: d-LLME (Dispersive Liquid-Liquid Microextraction), d-SPE (Dispersive Solid Phase Extraction), GPC (Gel Permeation Chromatography), DES (Deep Eutectic Solvents), SFOD (Solidification floating organic droplets), ACN (Acetonitrile), DCM (Dichloromethane). Table 2.
Extraction, purification, and analytical methods of strobilurin fungicides
-
Matrix Target SFs Concentration Region Ref. Foodstuff Wheat PYR 0.08–9.91 mg·kg–1 China [51] Ginseng root, Ginseng stem and leaf AZO 0.343–9.40 mg·kg–1 China [52] Pepper PYR 1.68–3.27 mg·kg–1 China [21] PICO 2.79–2.80 mg·kg–1 Coffee bean AZO < 1.43 μg·kg–1 Brazil [34] DIMO < 1.46 μg·kg–1 KM < 1.48 μg·kg–1 PICO < 1.33 μg·kg–1 TRI < 1.54 μg·kg–1 Watermelon leaf Fa 19.695 mg·kg–1 China [37] Banana AZ 0.05–2.0 mg·kg–1 Brazil [19] Corn AZO 0.01–0.024 mg·kg–1 China [53] TRI < LOQ PYR 0.013–0.065 mg·kg–1 Apple PYR 0.01–0.070 mg·kg–1 China [54] Dried grape PYR 0.01–0.024 mg·kg–1 Turkey [55] Dried apricot TRI 0.01–0.162 mg·kg–1 Environment Soil AZO 0.726 mg·kg–1 China [52] Soil AZO 8.9–15.7 μg·kg–1 Vietnam [56] Sediment 5.5–35.0 μg·kg–1 Drinking water AZO 0.37–3.66 ng·L–1 China [50] FLUO < MDL ~0.011 ng·L–1 PYR 0.01–0.25 ng·L–1 TRI 0.07–1.03 ng·L–1 Suspended solid AZO 0.02–0.01 ng·L–1 China [50] FLUO < MDL PYR 0.04–0.48 ng·L–1 TRI < MDL ~ 0.007 ng·L–1 Drinking water AZO 0.036–2.41 μg·L–1 Vietnam [57] TRI 0.003–0.56 μg·L–1 Stream AZO 0.008–1.13 μg·L–1 the United States [58] PYR 0.006–0.054 μg·L–1 Groundwater AZO 0.2–0.9 ng·L–1 the United States [49] PYR 0.1–4.8 ng·L–1 Surface water AZO 30.6–59.8 ng·L–1 the United States [49] PYR 15.2–239 ng·L–1 Surface water AZO 0.01–47.3 ng·L–1 China [59] FLUO < MDL ~0.10 ng·L–1 PYR 0.01–0.52 ng·L–1 TRI < MDL ~0.21 ng·L–1 Indoor dust AZO < MDL ~21.9 ng·g–1 China [46] FLUO < MDL ~1.91 ng·g–1 PYR < MDL ~1,946 ng·g–1 TRI < MDL ~9.52 ng·g–1 Table 3.
Concentrations of strobilurin fungicides in foodstuffs and environmental media
-
Target SFs Research content Conclusion Ref. PYR Investigated the toxicological risks of PYR toward HepG2 cells and the mechanisms of intoxication in vitro. PYR induced DNA damage and mitochondrial dysfunction, leading to excessive generation of intracellular ROS, which ultimately resulted in mitochondrial-mediated cell apoptosis and toxic effects on human hepatocarcinoma HepG2 cells. [81] PYR, TRI, FA, FE Identified transcriptomic signatures shared with neurological disorders by exposing mouse cortical neuron-enriched cultures to PYR, TRI, FA, and FE. PYR, TRI, FA, and FE induced transcriptional changes in vitro. By inhibiting mitochondrial complex III, and they upregulated Nrf2-targeted antioxidant response genes and Rest. These changes are associated with human brain aging and neurodegeneration. [79] TRI Explored the mechanism of TRI-mediated mitophagy in human skin keratinocytes exposed to TRI. Mitochondrial damage and mitophagy likely contribute to TRI-induced toxicity in human keratinocytes, suggesting a potential mechanism for cutaneous diseases developed upon exposure. [70] AZO Explored the effects of AZO on human esophageal squamous cell carcinoma KYSE-150 cells and investigated the underlying mechanisms. AZO effectively induced apoptosis in esophageal cancer cells via mitochondrial-mediated apoptotic pathways. [72] PYR Using a multi-analytical approach that integrates toxicological database mining, protein-protein interaction (PPI) network analysis, and molecular docking, we studied the molecular mechanisms of PYR toxicity. PYR exposure was significantly associated with pathways related to prostate cancer and renal dysfunction, indicating its potential role as an inducer of these diseases. [82] Table 4.
Human related toxicity studies of strobilurin fungicides
Figures
(3)
Tables
(4)