-
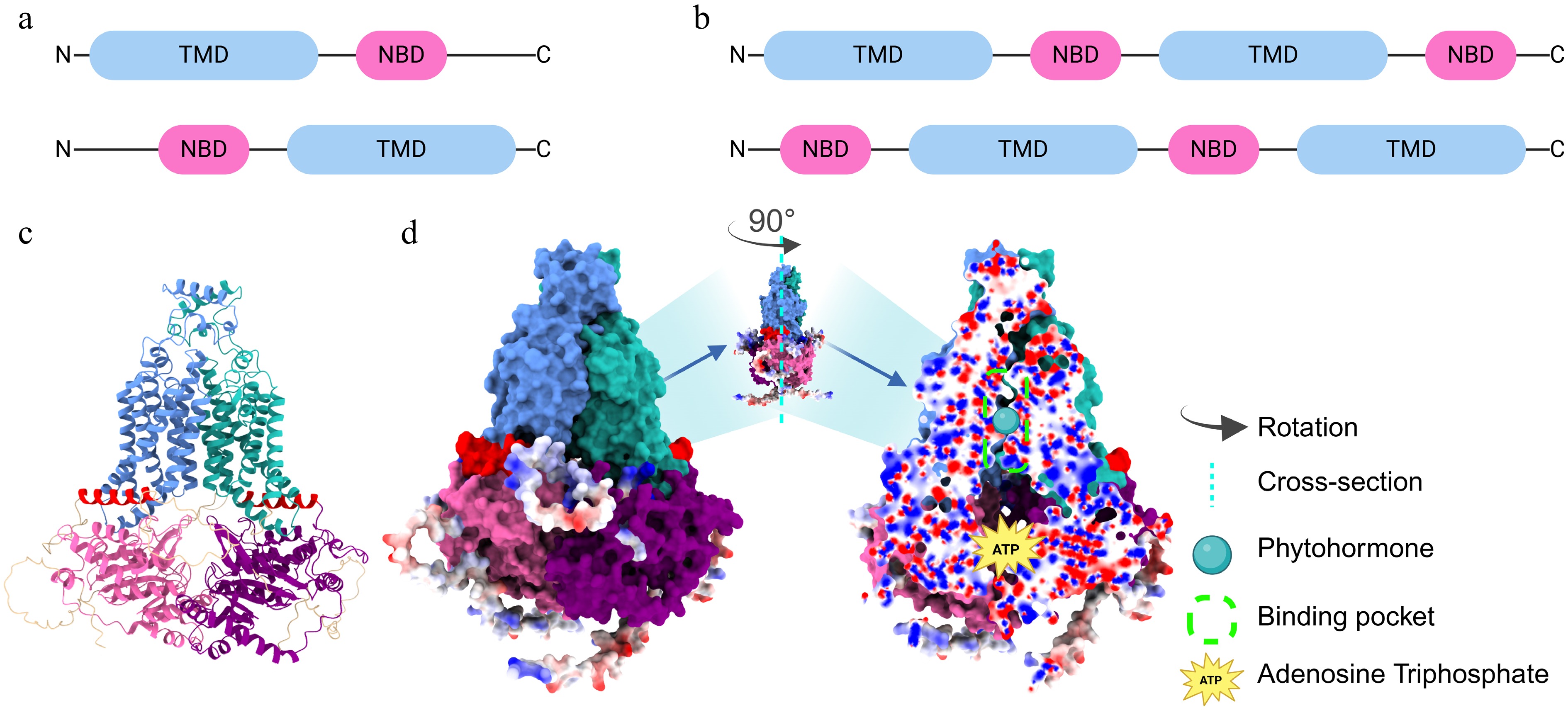
Figure 1.
Structural features of the half-size and full-size ABCG protein. Domain organization of (a) half-size, and (b) full-size ABCG members and their inverted topology. Light blue rounded rectangles represent TMD, while pink ones represent NBD. (c) Structural prediction of the two half-size ABCG proteins using AlphaFold3. The panel shows the predicted 3D structures. (d) Structural prediction of the full-size ABCG protein using AlphaFold3. The left panel shows the surface representations. Color-coding: light sea green/cornflower blue, TMD; pink/purple, NBD; red, coupling helices. Red and blue spheres along the outside of the domains represent dangling amino acid residues. The middle panel shows the full-size ABCG protein rotated 90° counterclockwise to expose the complete binding pocket in longitudinal section. The right panel shows a cross-sectional view of the predicted full-size ABCG structure, showing the hormone-binding and ATP-binding pockets. Red and blue spheres in the cross-section indicate positive and negative electrostatic states, respectively. NBD: Nucleotide-binding domain, TMD: Transmembrane domain.
-
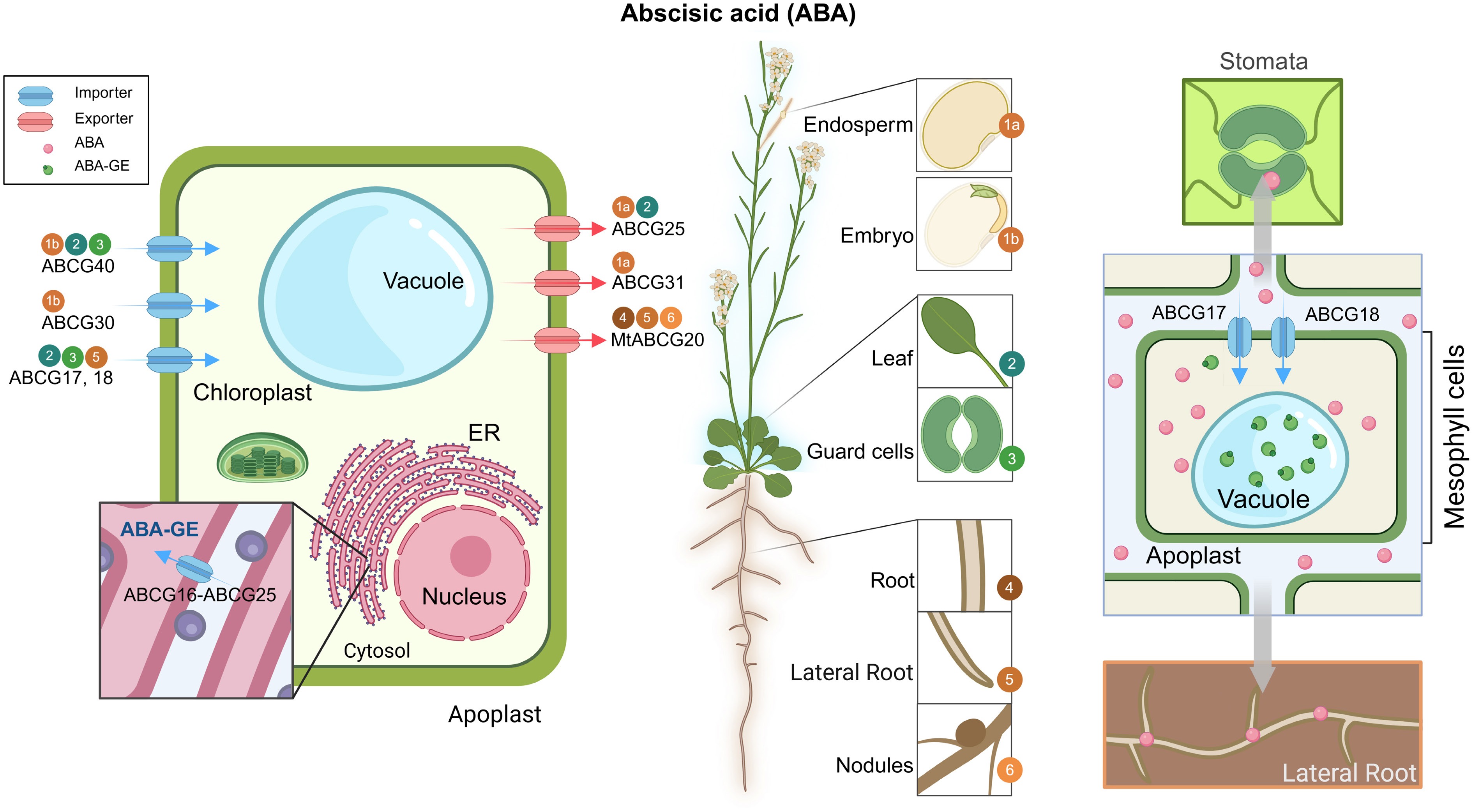
Figure 2.
Overview of ABCG transporters involved in ABA transport. A schematic representation of an Arabidopsis plant highlighting major tissues/organs involved in ABA synthesis and transport (middle), accompanied by subcellular localization of characterized ABCG transporters (left) and a simplified mechanism model (right). Blue arrows represent ABA or ABA-GE importers; red arrows indicate ABA exporters. Gray arrows indicate the direction of ABA movement. Numbers in colored circles correspond to tissue-specific expression sites shown in the plant diagram. Most transporters mediate the movement of bioactive ABA unless otherwise indicated (e.g., ABA-GE). All transporters shown were characterized in Arabidopsis thaliana unless a species prefix is provided. ABA, abscisic acid; ABA-GE, ABA-glucose ester; ER, endoplasmic reticulum; Mt, Medicago truncatula.
-
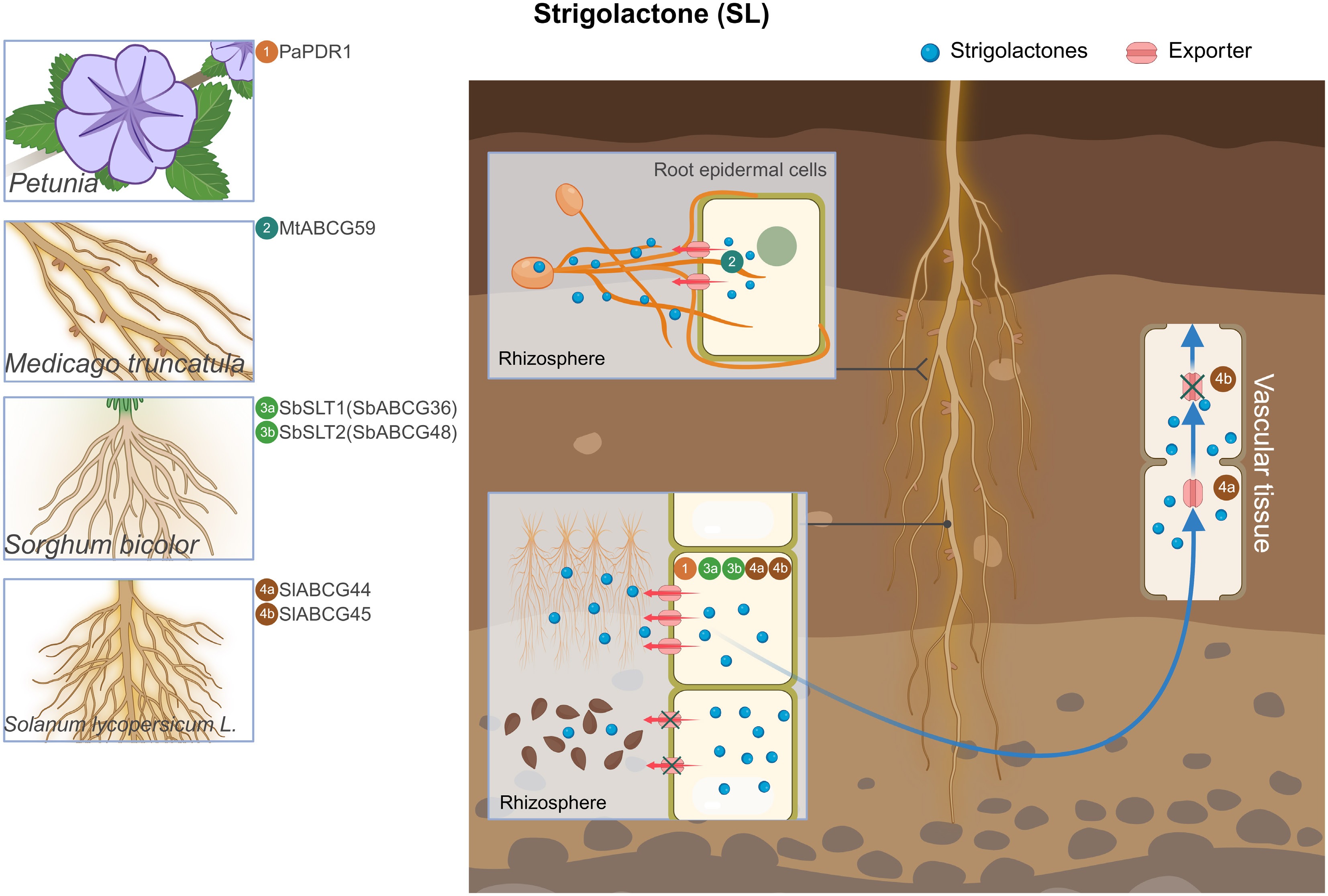
Figure 3.
Overview of ABCG transporters involved in SL transport. Illustration of representative plant species with magnifications highlighting root tissues where ABCG transporters have been characterized. The central root schematic shows SL export sites, with red arrows representing SL export by ABCG transporters. Blue long arrows indicate the direction of ABCG long-distance transport. '×' indicates that ABCG protein transport is repressed. The numbers in circles indicate the names of ABCG transporters. SL, strigolactone; Pa, Petunia; Mt, Medicago truncatula; Sb, Sorghum bicolor; Sl, Solanum lycopersicum.
-
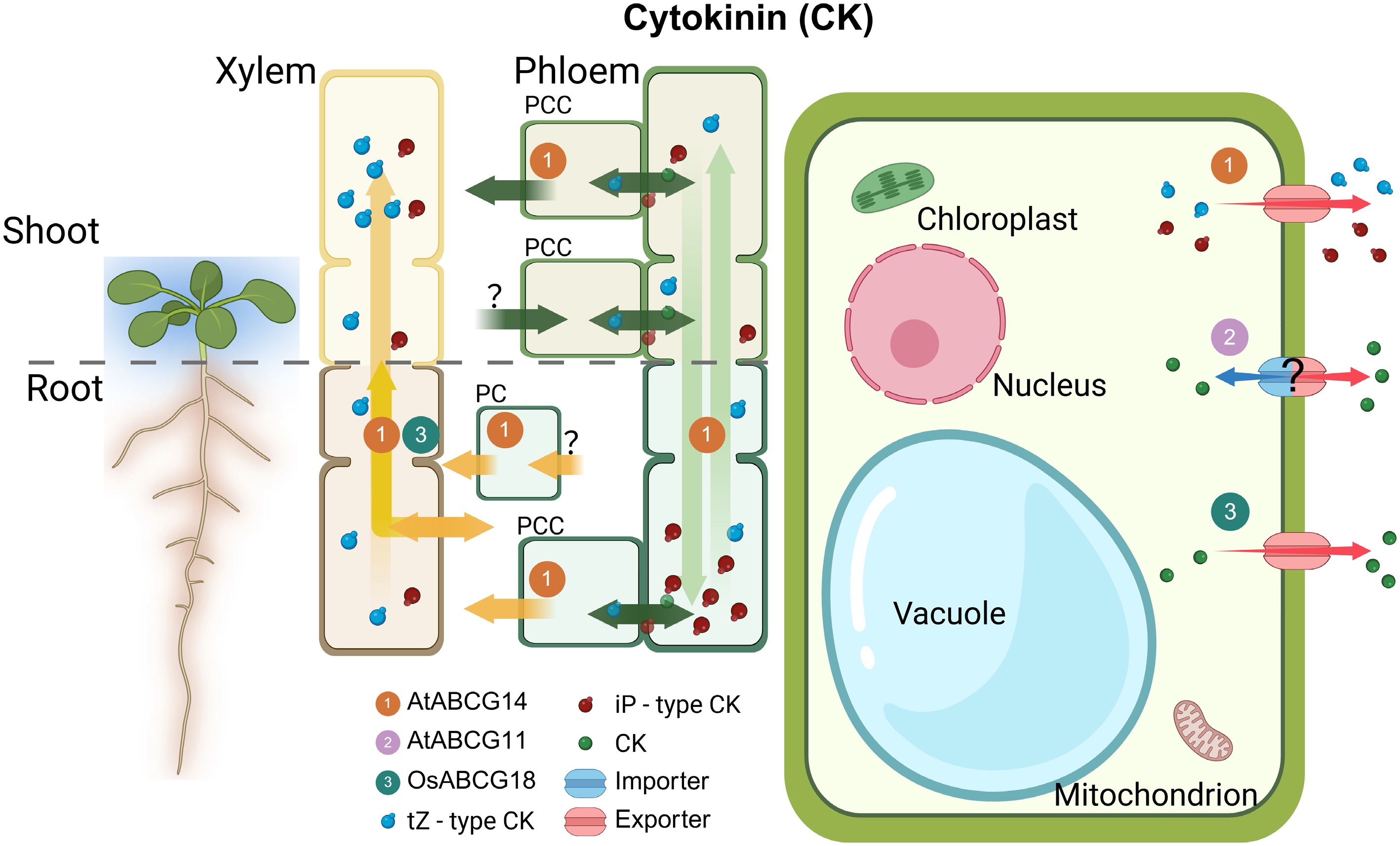
Figure 4.
Overview of ABCG transporters involved in CK transport. Illustration of ABCG transporters in the plant shoot and root with magnifications highlighting tissues and cells where CK transporters are active. Characterized CK exporters (red arrows) are shown in relevant tissues. Yellow and green long arrows indicate the direction of tZ-type and iP-type CK, respectively. The numbers in circles indicate the names of ABCG transporters. PCC, phloem companion cells; R-PC, root pericycle; ?, unknown mechanism; CK, cytokinin; ER, endoplasmic reticulum; At, Arabidopsis thaliana; Os, Oryza sativa.
-
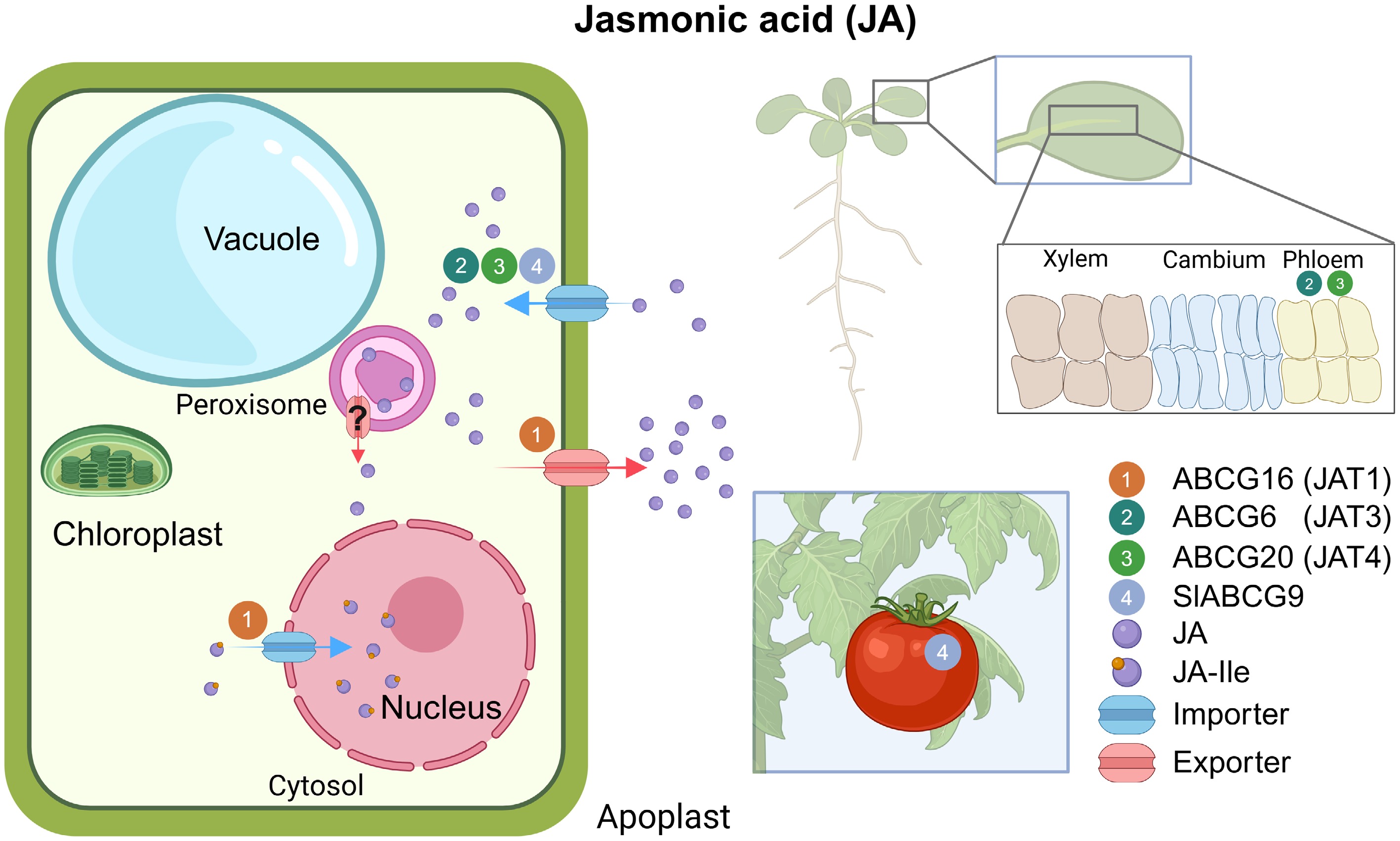
Figure 5.
Overview of ABCG transporters involved in JA transport. (Left) Illustration of intracellular JA transport. (Right) Schematic of tissue expression of JA transporters. The numbers in circles indicate the names of ABCG transporters. Inset boxes are magnifications of the indicated tissues. Blue arrows represent importers; red arrows represent exporters. All transporters were characterized in Arabidopsis unless the transporter name begins with a species abbreviation. ?, unknown mechanism; JA, jasmonic acid; JA-Ile, jasmonoyl-isoleucine.
-
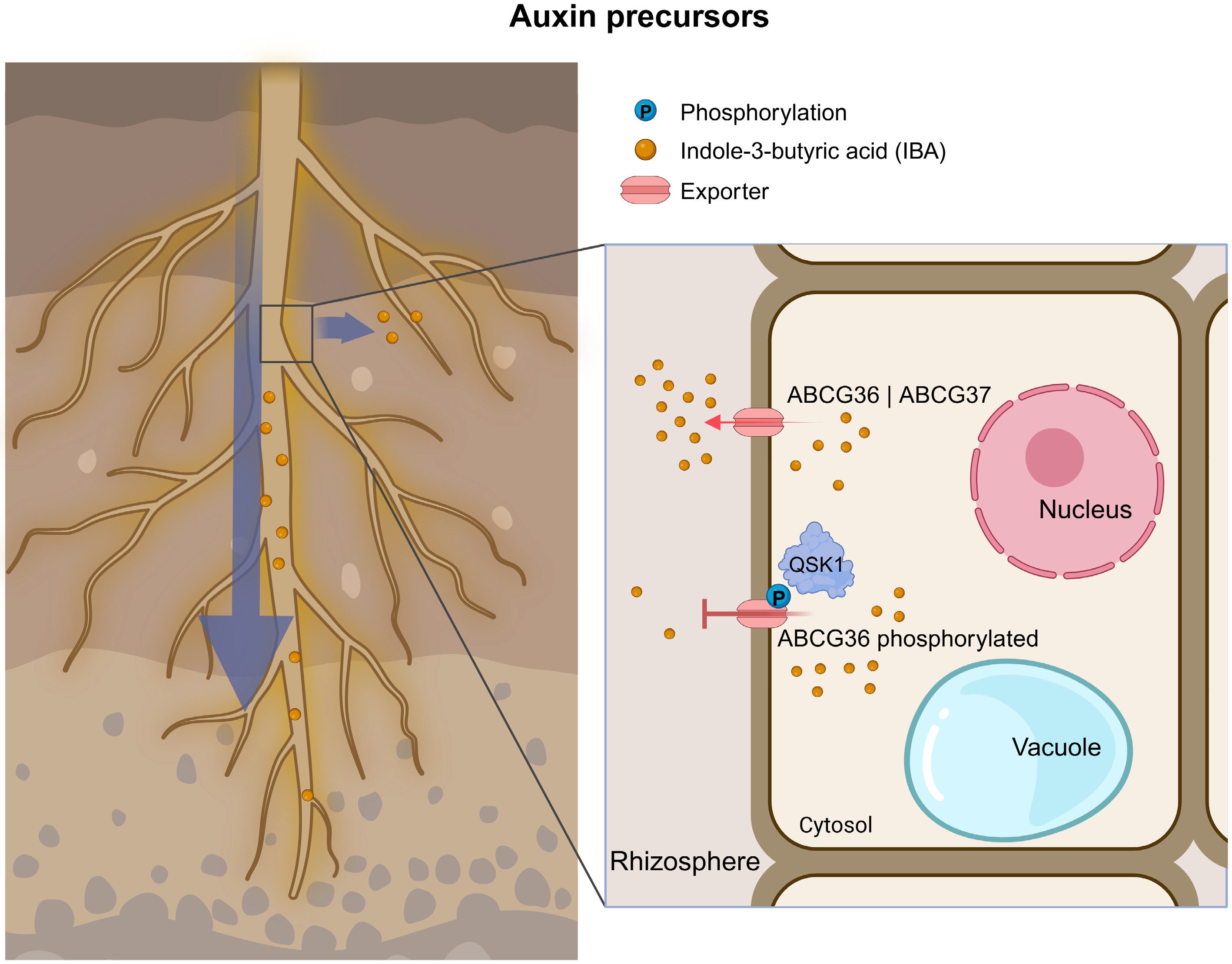
Figure 6.
Overview of ABCG transporters involved in auxin precursor transport. (Left) Illustration of an Arabidopsis root highlighting the spatial context of IBA transport. (Right) Enlarged view of a root cell from the region marked in the left panel, illustrating the subcellular dynamics of IBA export. Red arrows represent exporters. The blue arrow indicates the direction of auxin precursor movement. The long red arrows indicate promotion, red T-shaped lines indicate inhibition, and the blue circle with 'P' represents phosphorylation. All transporters were characterized in Arabidopsis unless the transporter name begins with a species abbreviation. IBA, indole-3-butyric acid.
-
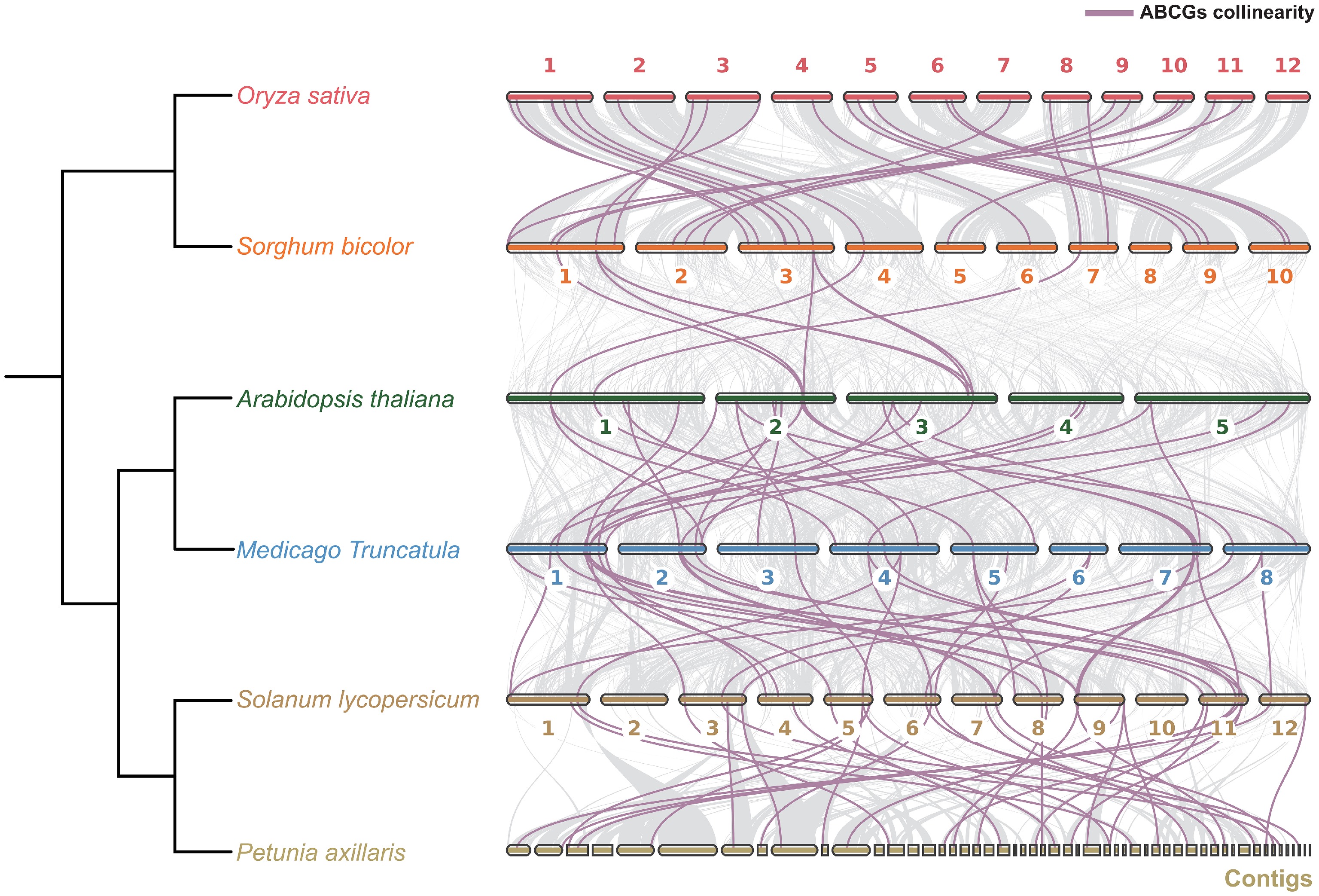
Figure 7.
Collinearity analysis of ABCG genes across six representative monocot and eudicot species. Purple Bézier curves represent the collinear relationships of ABCG genes across species.
-
Name Transporter family Identified
substratesTransport direction Subcellular localization Main expression sites Ref. ABCG25 ABCG ABA Efflux PM Leaf vascular vein/root/hypocotyl/endosperm [62,63] ABCG40 ABCG ABA Influx PM Root/leaf guard cells/embryo [65,66] ABCG31 ABCG ABA Efflux PM Endosperm [65] ABCG30 ABCG ABA Influx PM Root/embryo [63,65] MtABCG20 ABCG ABA Efflux PM Lateral root [17] Lr34res ABCG ABA Unclear Unclear Unclear [67] ABCG17 ABCG ABA Influx PM Shoot cortex/leaf mesophyll cells [68,69] ABCG18 ABCG ABA Influx PM Shoot cortex/leaf mesophyll cells [68,69] ABCG16-ABCG25 ABCG ABA-GE Influx PM Unclear [64] ABCC1 ABCC ABA-GE Influx Tonoplasts Unclear [40,80,81] ABCC2 ABCC ABA-GE Influx Tonoplasts Unclear [40,80,81] NPF4.6 (AIT1) NPF ABA Influx PM Seed/vascular of root/leaf/stem [25,126] NPF4.1 (AIT3) NPF ABA Influx PM Unclear [126] NPF4.2 (AIT4) NPF ABA Influx PM Unclear [126] NPF4.5 (AIT2) NPF ABA Influx PM Unclear [25,126] SlAIT1.1 NPF ABA Influx PM Leaf/guard cells/meristem [127] NPF2.12 NPF ABA/GA Influx PM Root meristematic zone/hypocotyl periderm [116] NPF2.13 NPF ABA/GA Influx PM Shoot [116] NPF3 NPF ABA Influx PM Root endodermis [116,128] NPF2.14 NPF ABA-GE/GA Influx Tonoplasts Shoot vasculature/hypocotyl periderm [116] DTX50 MATE ABA Efflux PM Root/leaf/guard cells [27] OsDG1 MATE ABA Efflux PM Node/leaf vascular bundles/rachilla [28] OsPM1 AWPM-19-like ABA Influx PM Vasculature/root tip/guard cells [129] PaPDR1 ABCG SL Efflux PM Root tips/bud/lateral axils [90] MtABCG59 ABCG SL Efflux PM Root/nodule [18] SlABCG44 ABCG SL Efflux PM Root epidermal cells [91] SlABCG45 ABCG SL Efflux PM Root epidermal cells [91] SbABCG36 (SlSLT1) ABCG SL Efflux PM Root epidermal cells [88] SbABCG48 (SlSLT2) ABCG SL Efflux PM Root epidermal cells [88] PUP1 PUP CK Influx PM Flower/leaf margins in hydathodes/mature leaves/major veins/petioles/stem [29] PUP2 PUP CK Influx PM Flower/leaf vascular/major veins/petioles [29] OsPUP7 PUP CK Influx ER Vascular bundle/pistil/stamens [30] PUP14 PUP CK Influx PM Embryo/shoot apical meristem/root [130] OsPUP4 PUP CK Influx PM Vascular tissues [131] OsPUP1 PUP CK Influx ER Root vascular cells [132] PUP8 PUP CK Efflux PM Shoot apical meristem [31] PUP7 PUP CK Influx Tonoplast Shoot apical meristem [31] PUP21 PUP CK Influx Tonoplast Shoot apical meristem [31] OsENT2 ENT CK Influx PM Root [34] ENT3 ENT CK Influx PM Root vasculature [33] HvSWEET11b SWEET CK Efflux PM Nucellar projection/vascular bundle/anther [133] ABCG14 ABCG CK Efflux PM Root pericycle/root vascular tissues/phloem and xylem region of the leaf midrib [19,97,100] OsABCG18 ABCG CK Efflux PM Phloem and xylem region of the leaf midrib [20] ABCG11 ABCG CK Unclear PM Vascular system of root and leaf [101] ABCI19-21 ABCI CK Unclear ER Shoot/root [43] ABCC4 ABCC CK Efflux Unclear Root [41] AZG1 AZG CK Efflux PM Root/flower [134] AZG2 AZG CK Influx PM/ER LR primordia [125,134,135] ABCG6 (JAT3) ABCG JA Influx PM Leaf phloem [103] ABCG20 (JAT4) ABCG JA Influx PM Leaf phloem [103] ABCG16 (JAT1) ABCG JA/JA-lle Efflux/Influx PM/nuclear envelope Vascular tissues of cotyledons [104,122] SlABCG9 ABCG JA Influx PM Red ripe fruit [106] ABCD1 (CTS) ABCD JA precursor( OPDA) Influx Peroxisomal membrane Root/bud/silique/rosette [42] JASSY Unclear JA precursor (OPDA) Efflux Outer chloroplast envelope Unclear [136] PtrOPDAT1 Unclear JA precursor (OPDA) Efflux Plastid inner envelope Leaf/stem (xylem and phloem)/root [137] ABCD1 (PXA1) ABCD Auxin precursors (IBA) Influx Peroxisomal membrane Unclear [138] ABCB4 ABCB Auxin Efflux/Influx PM Root [139,140] ABCB21 ABCB Auxin Efflux/Influx PM Root pericycle/lateral root [139,141] ABCB19 ABCB Auxin/BR Efflux PM Flower/epidermal and mesophyll cells/inflorescence stem/seedling hypocotyl/root endodermal cells [16,45,115] ABCB1 ABCB Auxin/BR Efflux PM Flower/root endodermal cells [5,50,115] ABCB6 ABCB Auxin Efflux PM Leaf/root vascular tissues. [46] ABCB20 ABCB Auxin Efflux PM Root [46] ABCB15, 17, 22 ABCB Auxin Efflux PM Outer tissues of the root meristem/epidermis/lateral root cap [44] ABCB16 ABCB Auxin Efflux PM Outer tissues of the root meristem/epidermis/lateral root cap [44] ABCB18 ABCB Auxin Efflux PM Hypocotyl vascular tissues /mature root tissues [44] ABCG36 ABCG Auxin precursors (IBA) Efflux PM Root epidermal cells/lateral root cap [21,110,142] ABCG37 ABCG Auxin precursors (IBA) Efflux PM Root epidermal cells/lateral root cap [21,142] Long PIN1-4, 7 PIN Auxin Efflux PM Embryo/leaf/root/flower/stem/
root hair cells[124,143] Short PIN5 PIN Auxin Influx ER Hypocotyl/cotyledon vasculature/guard cells/leaf/stem/flower/root hair cells [144] Short PIN8 PIN Auxin Influx ER Leaf vein/ pollen/root epidermal cells/
root hair cells[123] Short PIN6 PIN Auxin Influx PM/ER Flower/shoot/lateral root [145,146] PILS6 PILS Auxin Influx ER Root/cotyledon/hypocotyl [147,148] AUX1/LAX APC Auxin Influx PM Root apical tissues/root epidermal cells [149] WAT1 MSF Auxin precursors (IBA) Efflux Tonoplast Root [150] NPF6.3 (NRT1.1) NPF Auxin Influx PM Shoot/root [151] NPF7.3 (NRT1.5) NPF Auxin Influx PM Root pericycle cells/primary root vascular cylinder/lateral root [26] NPF5.12 (TOB1) NPF Auxin precursors (IBA) Influx Tonoplast Lateral root cap [95] NPF2.10 (GTR1) NPF GA/JA-lle Influx PM Floral organs/shoot/root/around apical meristems/senescent leaves [152] NPF3 NPF GA Influx PM Root endodermis [128] SWEET13 SWEET GA Influx PM Anther/vascular tissues in leaves and roots/axillary buds/embryonic cotyledons [35] SWEET14 SWEET GA Influx PM Anther/vascular tissues in leaves and roots/axillary buds/embryonic cotyledons [35] OsSWEET3a SWEET GA Influx PM Vascular tissue [153] LHT1/2 AAP ETH precursors (ACC) Influx PM Root epidermis/leaf mesophyll [154] EDS5 MATE SA precursors (isochorismate)/SA Efflux Plastid/chloroplast envelope Unclear [155,156] Table 1.
A comprehensive summary of identified phytohormone transporters.
Figures
(7)
Tables
(1)