-
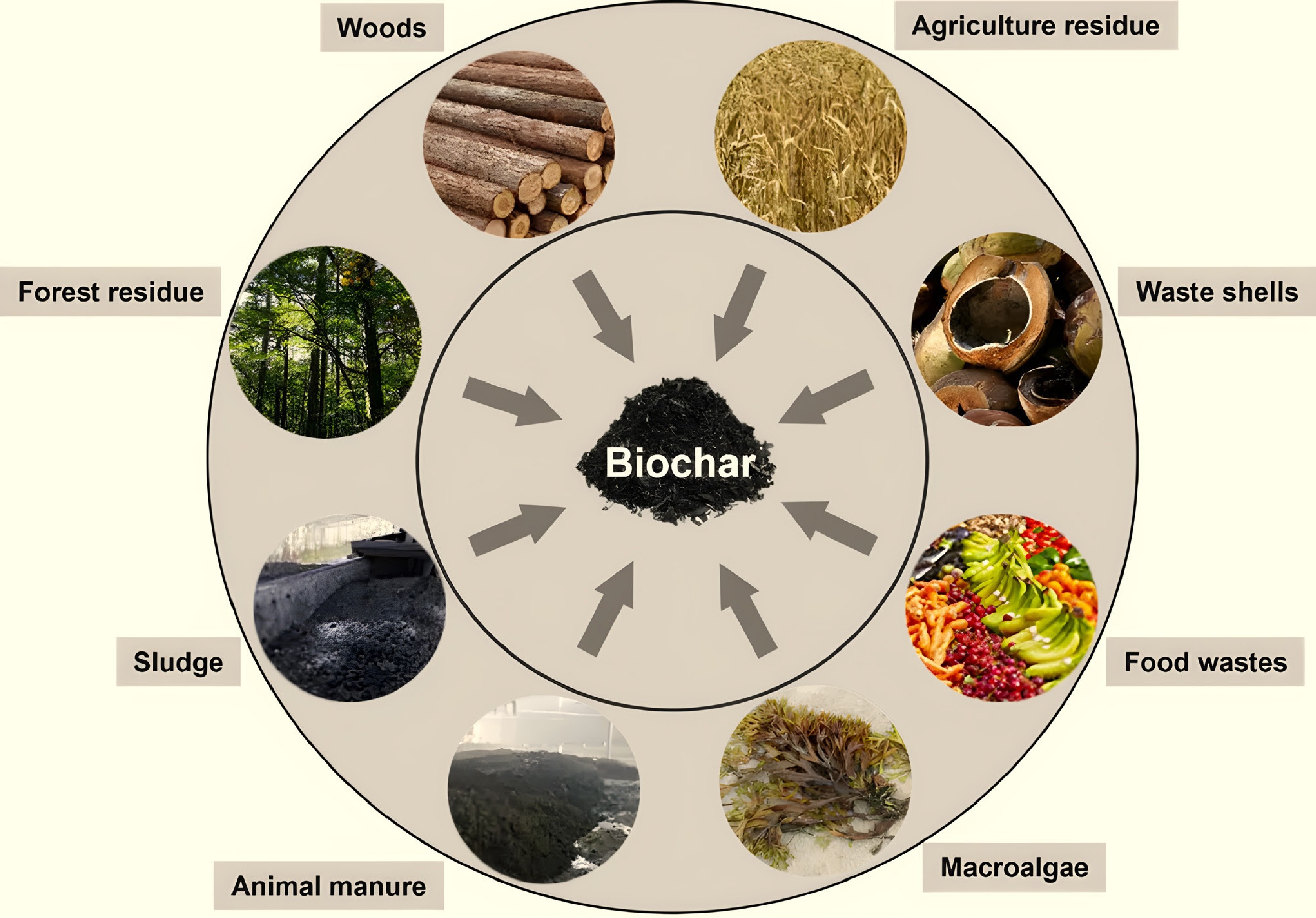
Figure 1.
Biochar is produced from various feedstocks through pyrolysis.
-
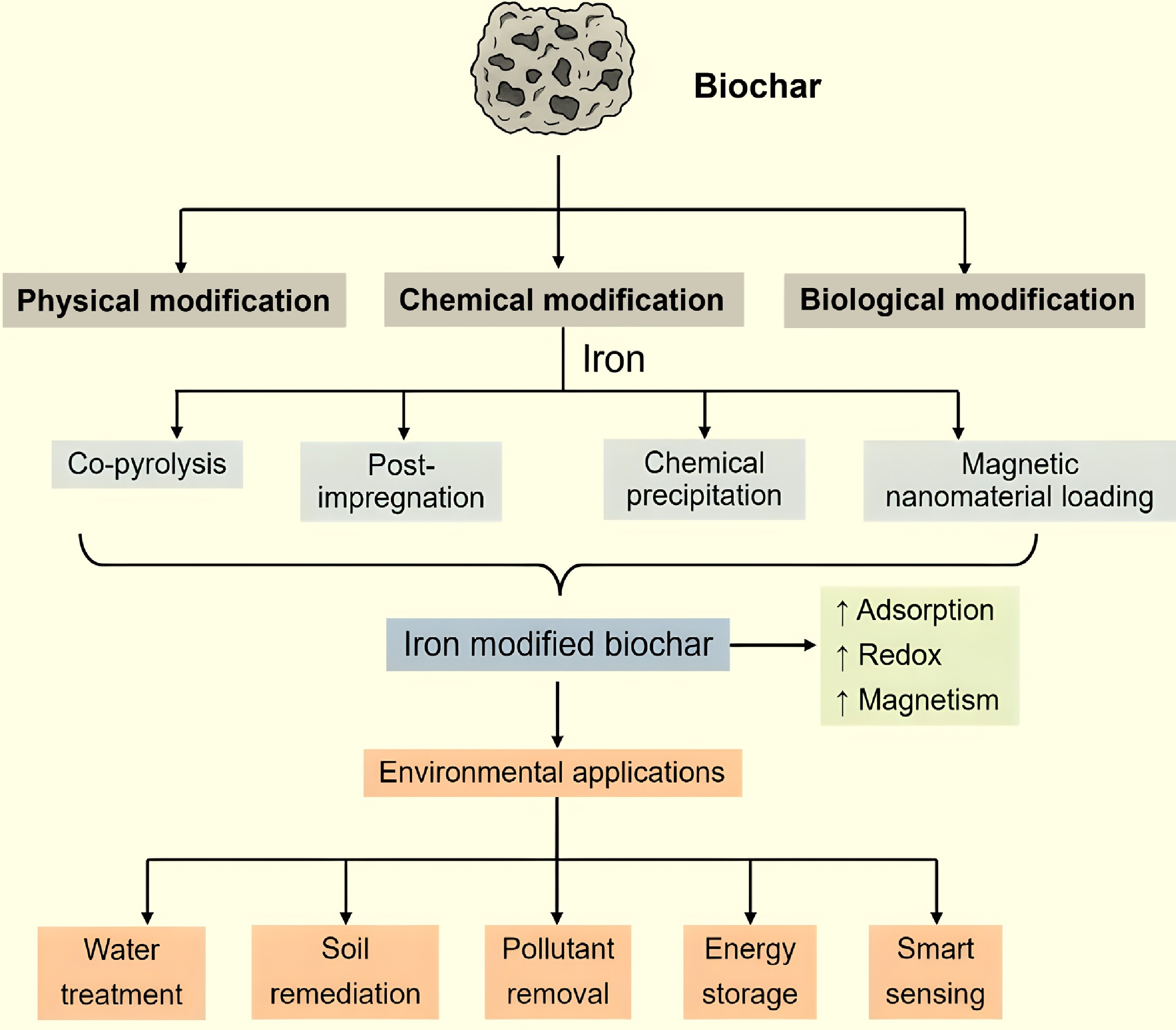
Figure 2.
Preparation, modification methods, and environmental applications of iron-modified biochar.
-
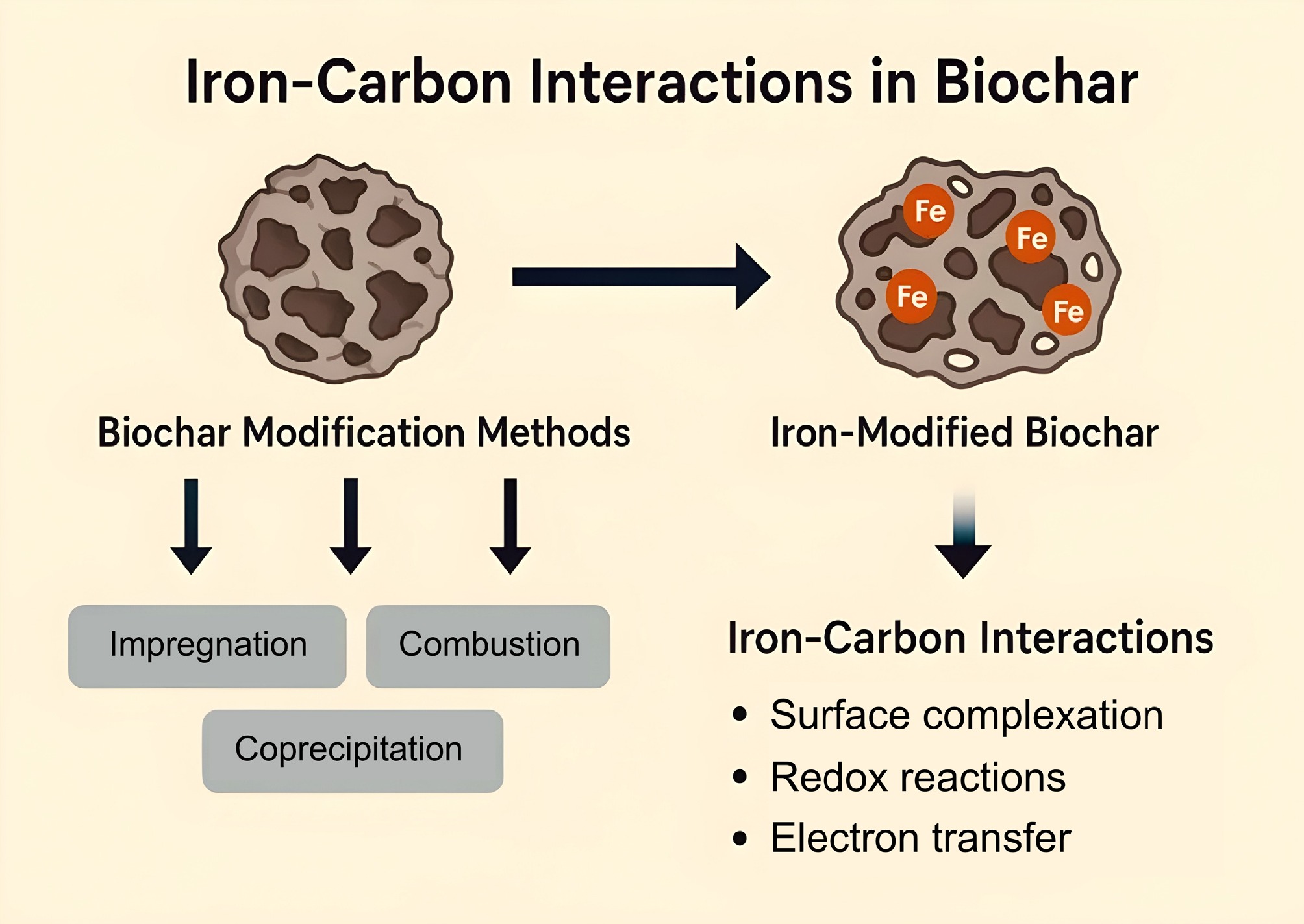
Figure 3.
Iron–carbon interactions in biochar.
-
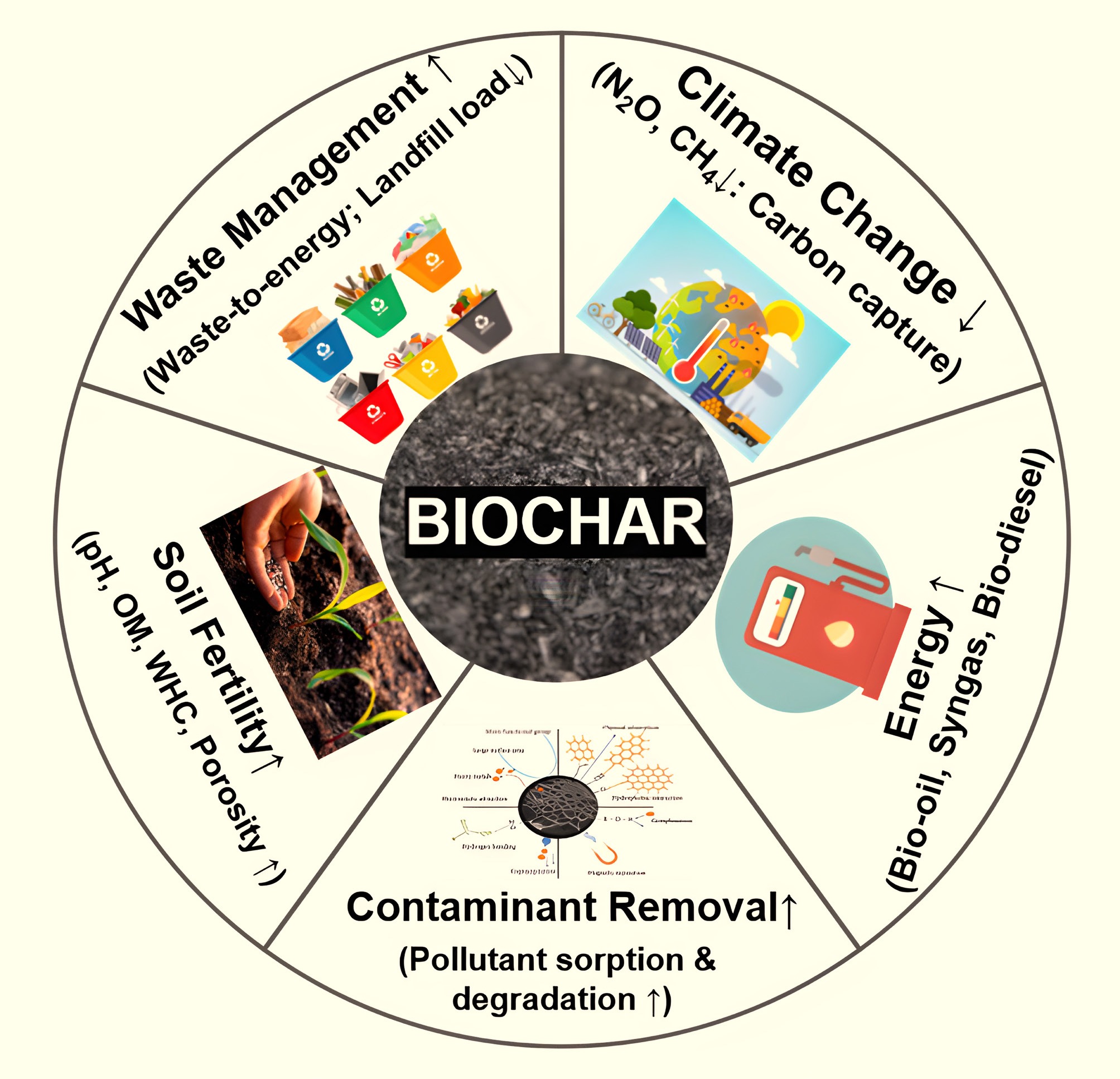
Figure 4.
Biochar application to the environment.
-
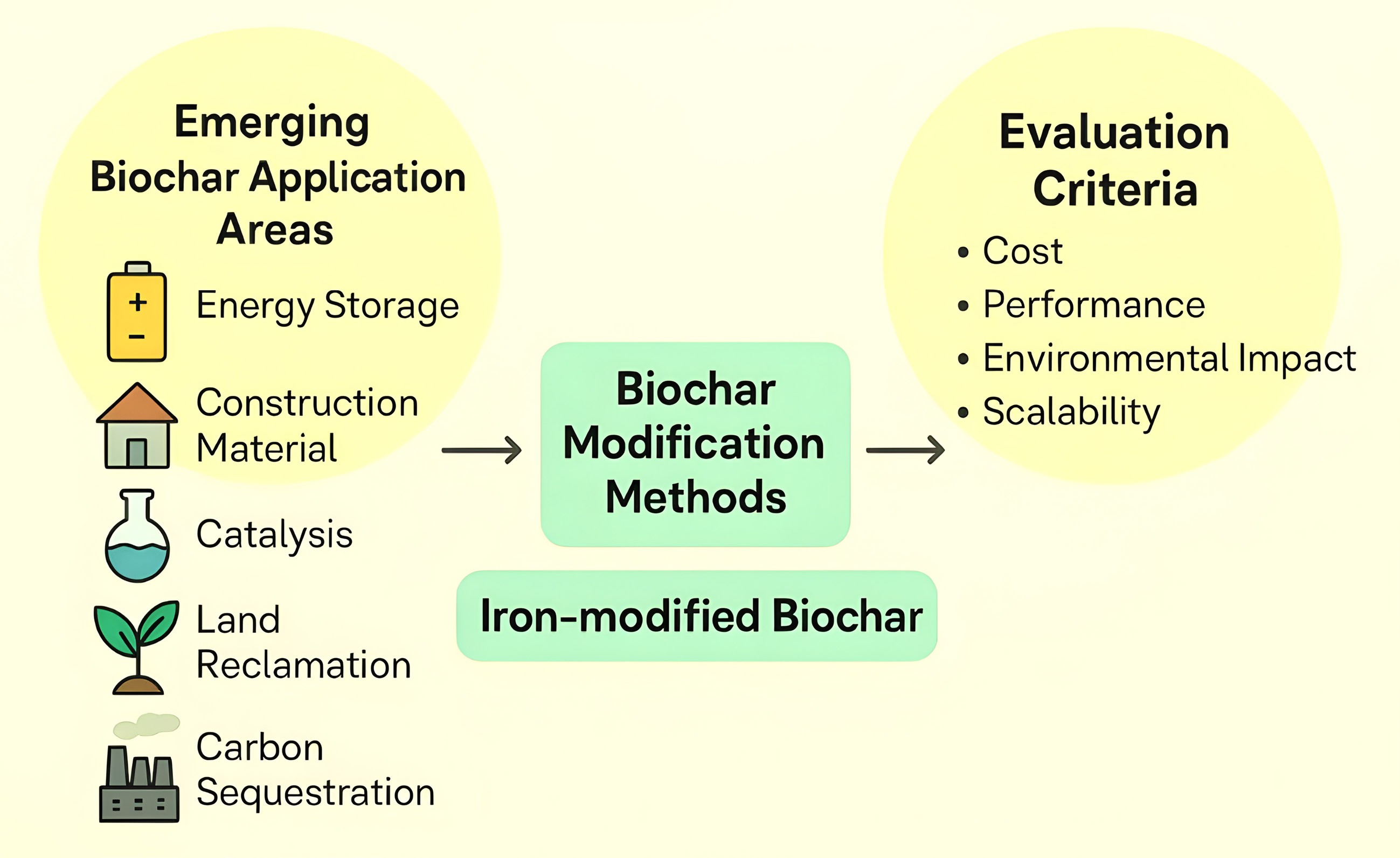
Figure 5.
Emerging biochar application areas and evaluation criteria.
-
Modification method Description Key feature Target contaminant/
applicationRef. Physical activation Steam, CO2, N2, or air at high temperatures activate biochar Increases surface area and porosity Adsorption of dyes, heavy metals [24] Ball milling Utilizes the kinetic energy of moving balls to alter particle morphology Improves specific surface area, pore volume, and functional groups Heavy metals, PFAS removal [25,26] Gas filling Introduces oxidizing gases to react with carbon in biochar Increases surface area, micropore area, micropore capacity, and formation of surface oxides Heavy metals removal [27] Steam Thermal treatment without oxygen, reacts with carbon to form CO and H2 Enhances mesopore structures at higher temperatures, while reducing micropore structure Heavy metals, tetracycline, sulfamethazine removal [13,28] Chemical activation Uses activating agents like KOH, H3PO4, or ZnCl2 Enhances functional groups and pore structure Pollutant removal, energy storage [29] Acid Treatment with hydrochloric, sulfuric, nitric, phosphoric, oxalic, or citric acid effectively removes metallic impurities while introducing acidic functional groups Increases the acidic functional groups and improving the pore structure to provide more cation exchange active sites Heavy metals removal, prepare biochar-based fertilizers, improvement of saline-alkali soil [30−33] Alkali Common alkaline agents include potassium hydroxide and sodium hydroxide. Increases the number of functional groups, improve the specific surface area and pore volume, provide better anion attachment sites Heavy metals, antibiotics, VOCs removal [34,35] Oxidizer Use oxidizing-agents like H2O2 or KMnO4 Provides additional redox potential by increasing the number of oxygen-containing functional groups Soil amendment, heavy metals, organic pollutants removal [29,36,37] Metal salts Pyrolyzed together with the feedstock, or treating pre-formed biochar under specific conditions. Form the porous structure, oxygen-containing functional groups, catalytic capacity, recycled biochar Heavy metals, dyes, antibiotics removal [30,38−40] Iron impregnation Post-pyrolysis treatment with FeCl3, Fe(NO3)3, or FeSO4 solutions Introduction of Fe species for redox activity Heavy metals, phosphate, arsenic [41,42] Co-pyrolysis with iron Biomass and iron precursors are pyrolyzed together Strong interaction between Fe and the carbon matrix Enhanced stability and adsorption [43] Precipitation method Iron salts precipitated onto the biochar surface Uniform Fe distribution, nano-Fe formation Nitrate, antibiotics, organics [44] Magnetic modification Embeds magnetite or maghemite (Fe3O4/γ-Fe2O3) nanoparticles Magnetic recovery, reusable sorbents Magnetic separation, water treatment [45] Hybrid composite Blending Fe-biochar with zeolite, silica, graphene, or polymers Multifunctionality and synergistic remediation effects Emerging pollutants, multi-contaminant sites [46,47] Nano-iron functionalization Biochar functionalized with zero-valent iron (nZVI) Enhanced reactivity, Fenton-like activity Organic pollutants, Cr(VI), pesticides [16,48] Red mud incorporation Utilizes industrial waste (iron-rich red mud) as Fe source Sustainable, cost-effective approach Soil remediation, acid mine drainage [49] Smart biochar sensor systems Iron-biochar integrated with sensing agents or responsive materials Environmental sensing and contaminant detection Real-time monitoring, innovative remediation [14] Table 1.
Biochar modification methods and iron-modified biochar
-
Feature Iron-modified biochar Acid/alkali-modified biochar Physical modification Other metal modifications (e.g., Mg, Al, Zn) Magnetic separation Excellent, enables easy recovery None None Usually none (unless with magnetic metals) Adsorption of anions (e.g., As, Cr, F) Very strong (specific adsorption + reduction for Cr) Alkali-modification improves it, but weaker and non-specific Moderate (mainly physisorption) Strong (e.g., Al-modified for As, Mg-modified for P) Adsorption of cations (e.g., Pb2+, Cd2+) Good Excellent (acid-modification increases surface O-groups) Good Excellent (e.g., Mg-modified
for Cd)Treatment of organic pollutants Adsorption + catalytic degradation Adsorption (may be enhanced
via porosity)Adsorption (high surface area) Adsorption (some may have catalytic properties) Primary function Adsorption, reduction, catalysis, magnetism Enhancement of ion exchange/
electrostatic adsorptionCreation of porous structure Enhancement of specific adsorption/complexation Application cost Low Low to moderate Moderate (high temperature
& energy)Moderate to high
(depends on metal salt)Key application field Wastewater (As/Cr removal, organic degradation), soil remediation Adjustment of adsorption for cations/anions General-purpose adsorbent, energy storage Targeted pollutant removal (e.g., P, F) Ref. [78−81] [82−85] [86−89] [90−92] Table 2.
The advantages of iron modification compared with other modification methods
-
Modified biochar Target pollutant Modification method Key cost advantage Cost efficiency
(USD/g pollutant)Ref. Na2S-BC Hg(II) One-step pyrolysis with Na2S Use of industrial byproduct Na2S; Simplified one-step process. 1.74 [107] K2FeO4-BC Heavy metals Pyrolysis + K2FeO4 impregnation High regeneration capability; Biomass oil/gas byproducts offset energy cost. Becomes cheaper
after three cycles[108] Fe-BC P Chemisorption with Fe3+ Feedstock is waste with gate fee; modifiers from scrap metal/waste. ~2.00 [109] Ca-BC P Chemisorption with Ca2+ Feedstock is waste with gate fee; modifiers from scrap metal/waste. ~1.50 [109] Struvite precipitation P Chemical precipitation Benchmark for P recovery ~17.29 [109] Ca/Mg-BC P One-step co-modification with CaCl2/MgCl2 Very low-cost waste feedstock; high capacity. 0.66 [110] Amine-modified BC Dimethyl sulfide HNO3/NH3 ammoxidation Free feedstock; modification cost is low. 2.28 [111] Commercial AC Dimethyl sulfide — Benchmark for comparison 2.62 [111] Table 3.
Cost-benefit comparative analysis table of modified biochars for pollutant removal
Figures
(5)
Tables
(3)