-
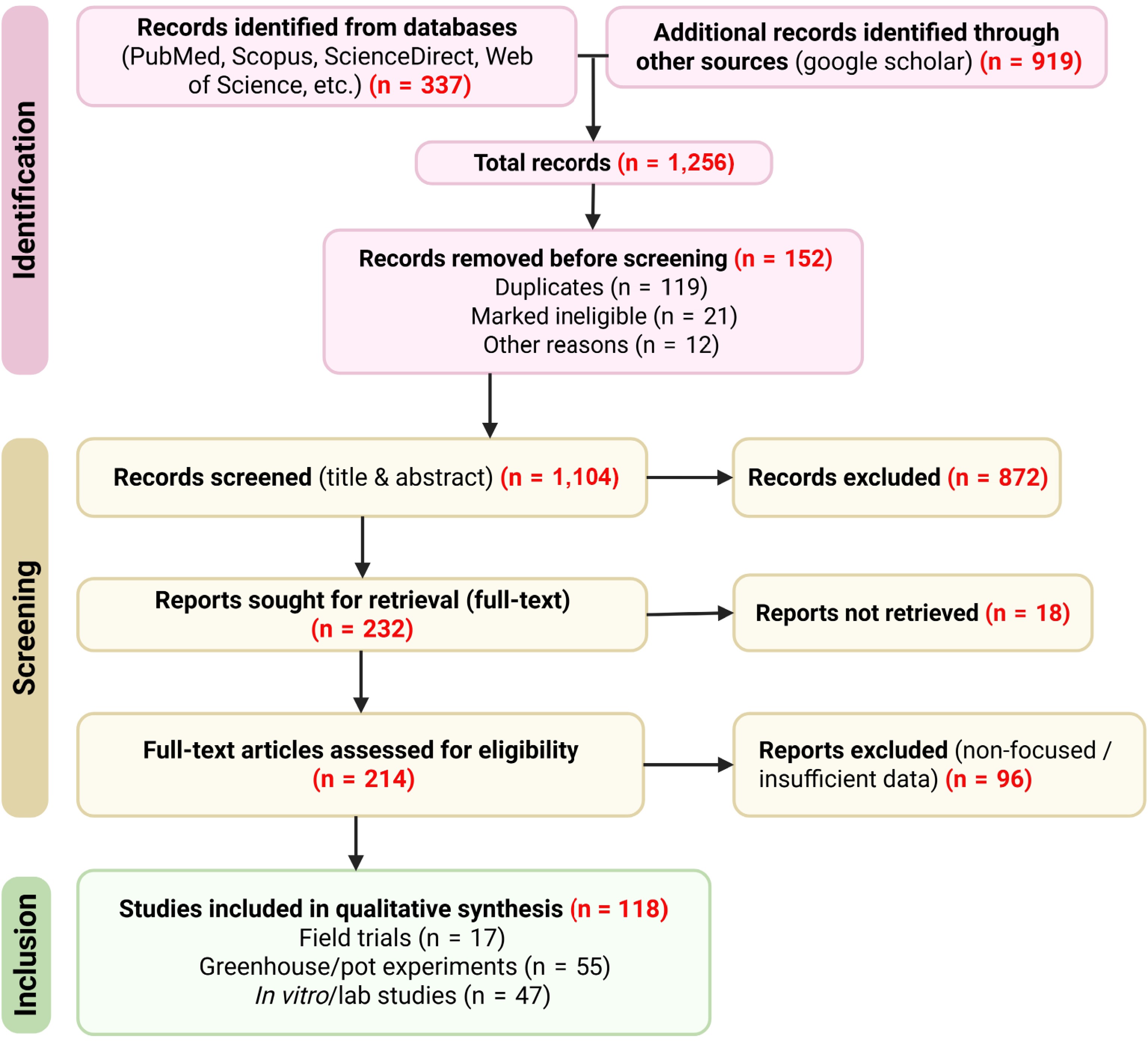
Figure 1.
PRISMA flow diagram illustrating the study selection process.
-
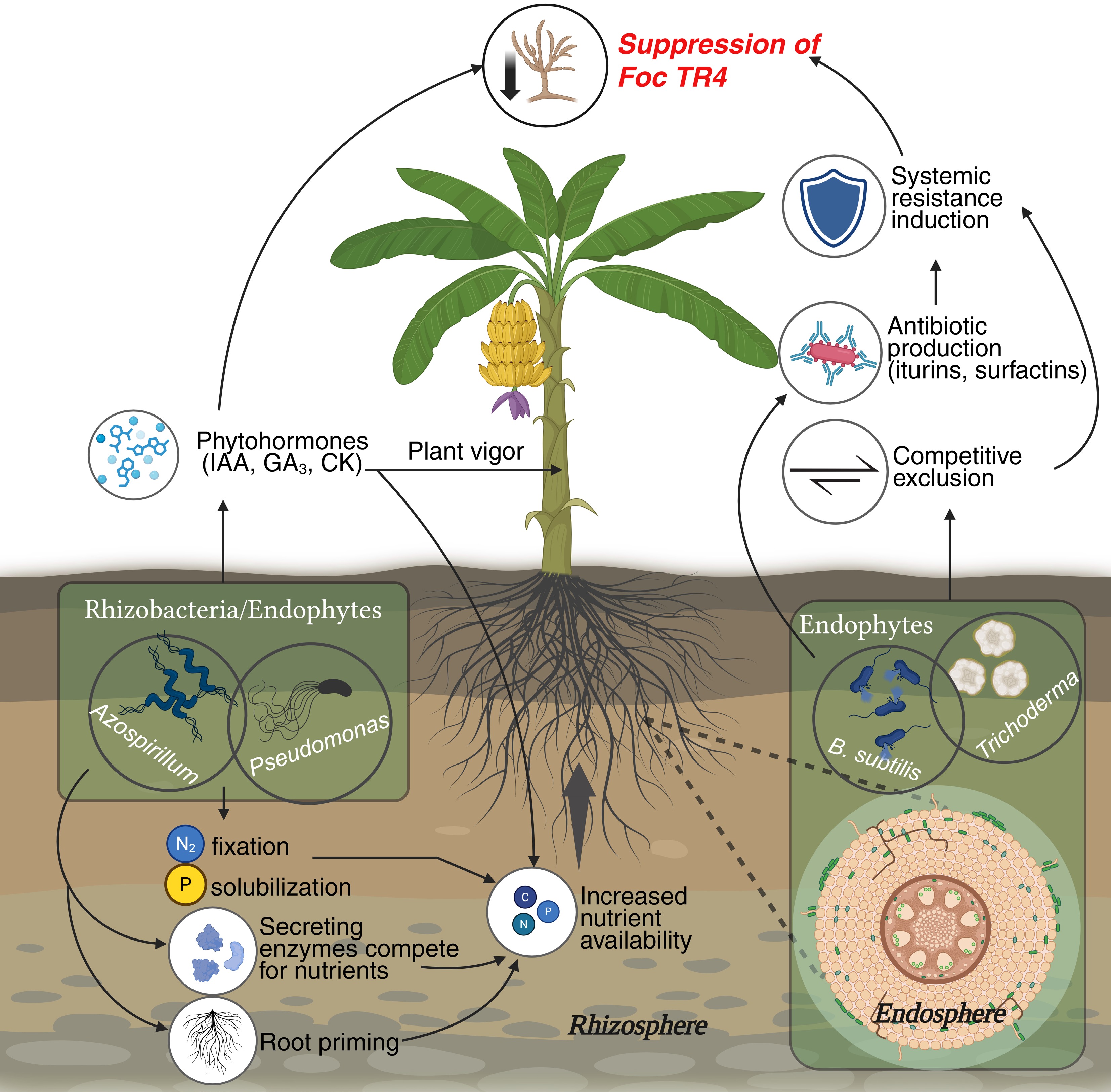
Figure 2.
Schematic overview of banana–microbiome interactions influencing Fusarium wilt resistance. Beneficial microbes such as Bacillus, Pseudomonas, and Trichoderma colonize the rhizosphere and endosphere, enhancing nutrient uptake and triggering defense responses against Fusarium oxysporum f. sp. cubense Tropical Race 4 (Foc TR4). These interactions suppress pathogen growth through antibiosis, competition, biofilm formation, and activation of Induced Systemic Resistance (ISR) in host tissues.
-
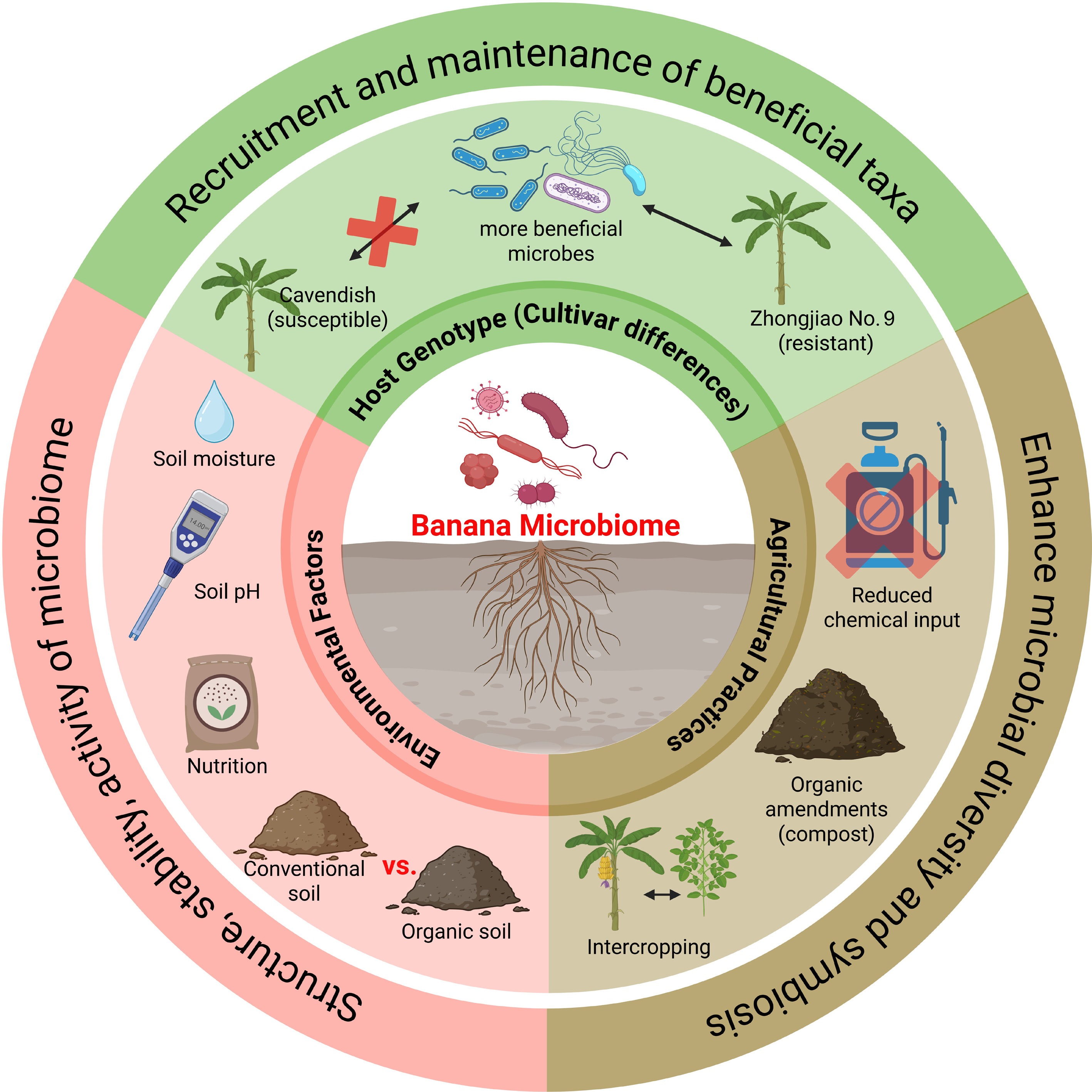
Figure 3.
Determinants of banana microbiome composition and functionality. The banana microbiome is shaped by multiple interacting factors. Host genotype influences the recruitment and maintenance of beneficial microbial taxa, with resistant cultivars (e.g., Zhongjiao No.9) supporting more beneficial populations than susceptible ones (e.g., Cavendish). Environmental factors, including soil pH, moisture, nutrient availability, and soil management type (organic vs. conventional), strongly affect the structure, stability, and activity of microbial communities. Agricultural practices, such as reduced chemical inputs, organic amendments, and intercropping, further enhance microbial diversity and promote beneficial plant-microbe symbioses.
-
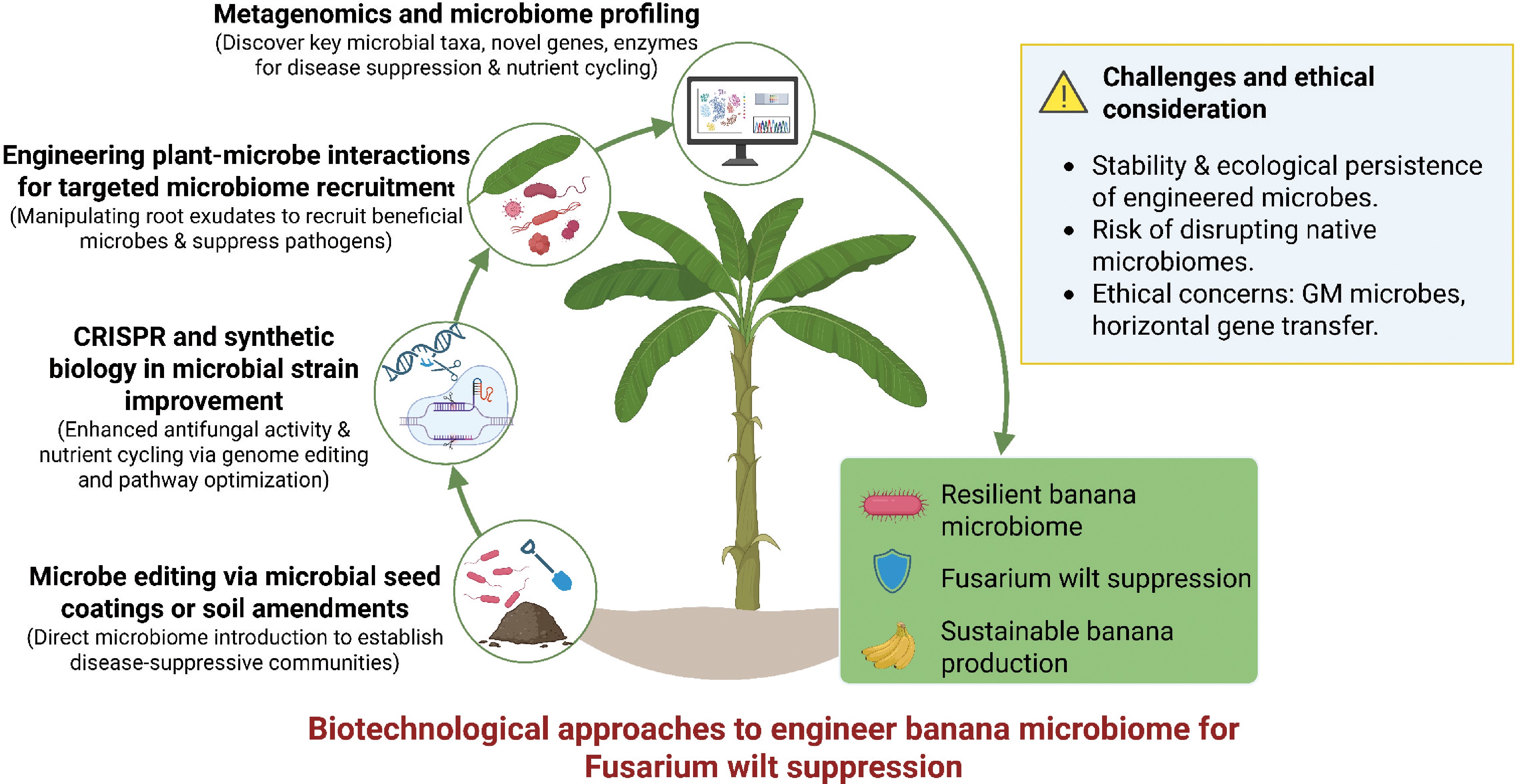
Figure 4.
Biotechnological strategies for engineering the banana microbiome to combat Fusarium wilt. Metagenomics, CRISPR/synthetic biology, plant–microbe interaction engineering, and microbiome editing strategies converge to enhance disease resistance, suppress Fusarium oxysporum f. sp. cubense, and promote sustainable banana production, while addressing ecological and ethical considerations.
-
Microbiome Approach Key suppressive taxa/indicators Source of isolation Outcome Ref. Bacillus Biofertilizer amendment Sphingobium, Dyadobacter, Cryptococcus Rhizosphere Decreased F. oxysporum; sustained biocontrol effect [38] Piriformospora, Streptomyces Spatiotemporal application of biocontrol agents P. indica, S. morookaensis Roots, rhizosphere Enhanced growth, reduced Fusarium wilt incidence [39] Pseudomonas, Gemmatimonas, Sphingobium Crop rotation (chilli pepper–banana) Gemmatimonas, Penicillium, Mortierella Soil Decreased F. oxysporum; improved diversity [37] Aspergillus Green manure intercropping Aspergillus, reduced Fusarium Soil Reduced disease; changed fungal community [40] Pseudomonas, Bacillus Rotation crops (pepper/eggplant) Antagonistic core taxa Rhizosphere Legacy suppression of wilt [41] Burkholderia Pineapple–banana rotation + biofertilizer Burkholderia Soil Increased α-diversity; suppressed disease [42] Bacillus, Mortierella Microbial consortia application Functionally beneficial core/indicator OTUs Soil Reduced disease, enhanced microbial richness [43] Aspergillus fumigatus, Fusarium solani Pineapple residue incorporation A. fumigatus, F. solani Soil Reduced pathogen; increased antagonistic fungi [44] Pseudomonas spp. Endophyte and rhizosphere microbiome tracking Pseudomonas P8, S25, S36 Root, rhizosphere Suppressed pathogens, promoted beneficial microbes [45] Bacillus, Rhizosphere bacteria Consortia of native isolates Multiple Bacillus spp. Root, corm, rhizosphere Suppressed wilt; increased growth/yield [46] Bacillus High-throughput sequencing + isolate screening Chujaibacter, Bacillus, Sphingomonas Soil B. velezensis YN1910 effective biocontrol [47] Pseudomonas, Bacillus Soil metagenomics + greenhouse validation Pseudomonas enriched in suppressive soils Soil Confirmed suppressiveness via isolates [35] Pseudomonas, Trichoderma P. fluorescens and T. viride formulations Pseudomonas fluorescens Pf1, Trichoderma viride Tv-6 Rhizoplane of crops 80.6% disease reduction [48] Pseudomonas Powder/capsule formulations of P. fluorescens Pf1 Pseudomonas fluorescens Pf1 Rhizoplane of crops Increased yield; better than carbendazim [49] Pseudomonas Liquid P. fluorescens via drip irrigation Pseudomonas fluorescens 60% wilt reduction; 41%–89% nematode reduction [50] Trichoderma viride +
P. fluorescensSucker treatment + soil drenching Trichoderma viride + P. fluorescens Banana rhizosphere Effective combined control [36] Nonpathogenic Fusarium oxysporum EF-1α and IGS sequencing Nonpathogenic F. oxysporum Banana farm soil Evidence of horizontal gene transfer of pathogenicity genes [51] Table 1.
Field-based studies demonstrating microbiome-mediated suppression of Fusarium wilt of banana.
Figures
(4)
Tables
(1)