-
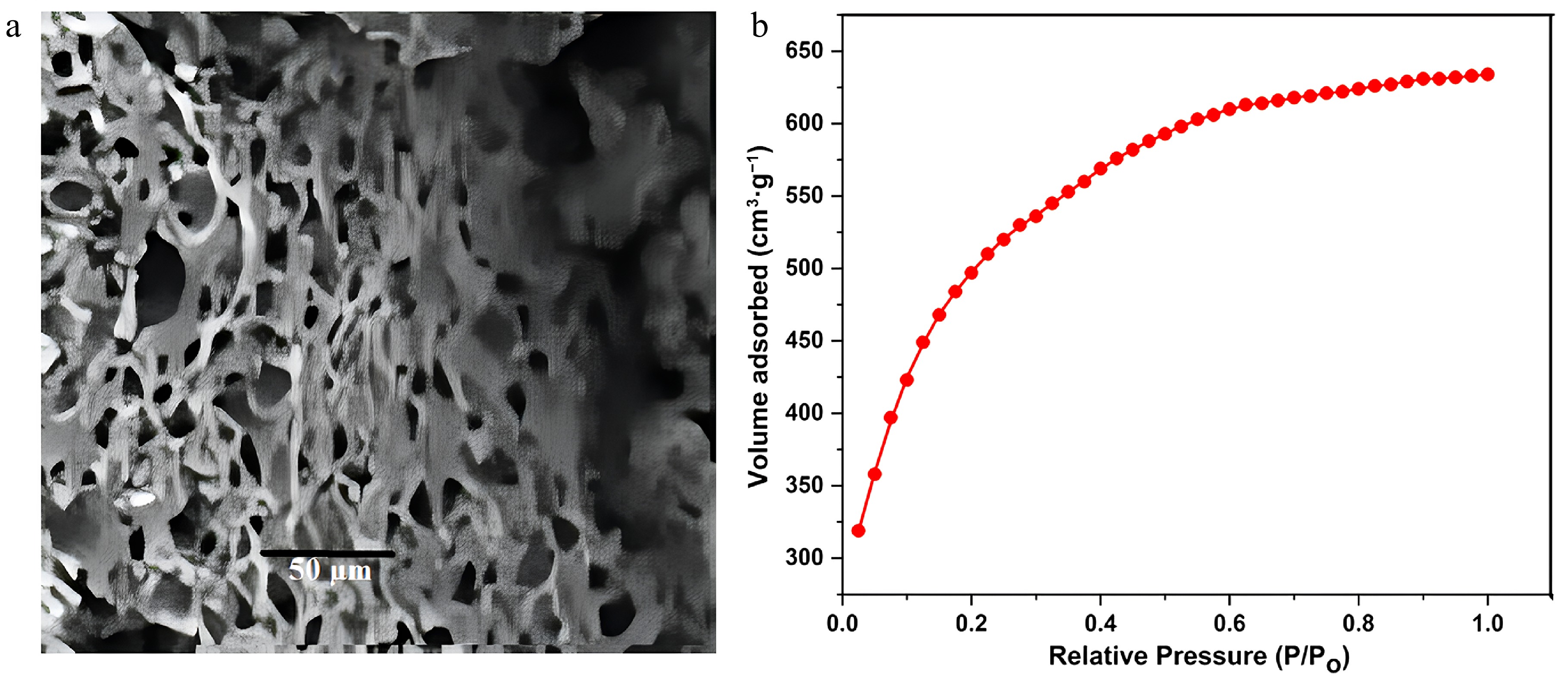
Figure 1.
(a) FE-SEM image of FNAC, (b) nitrogen adsorption isotherms for prepared FNAC.
-
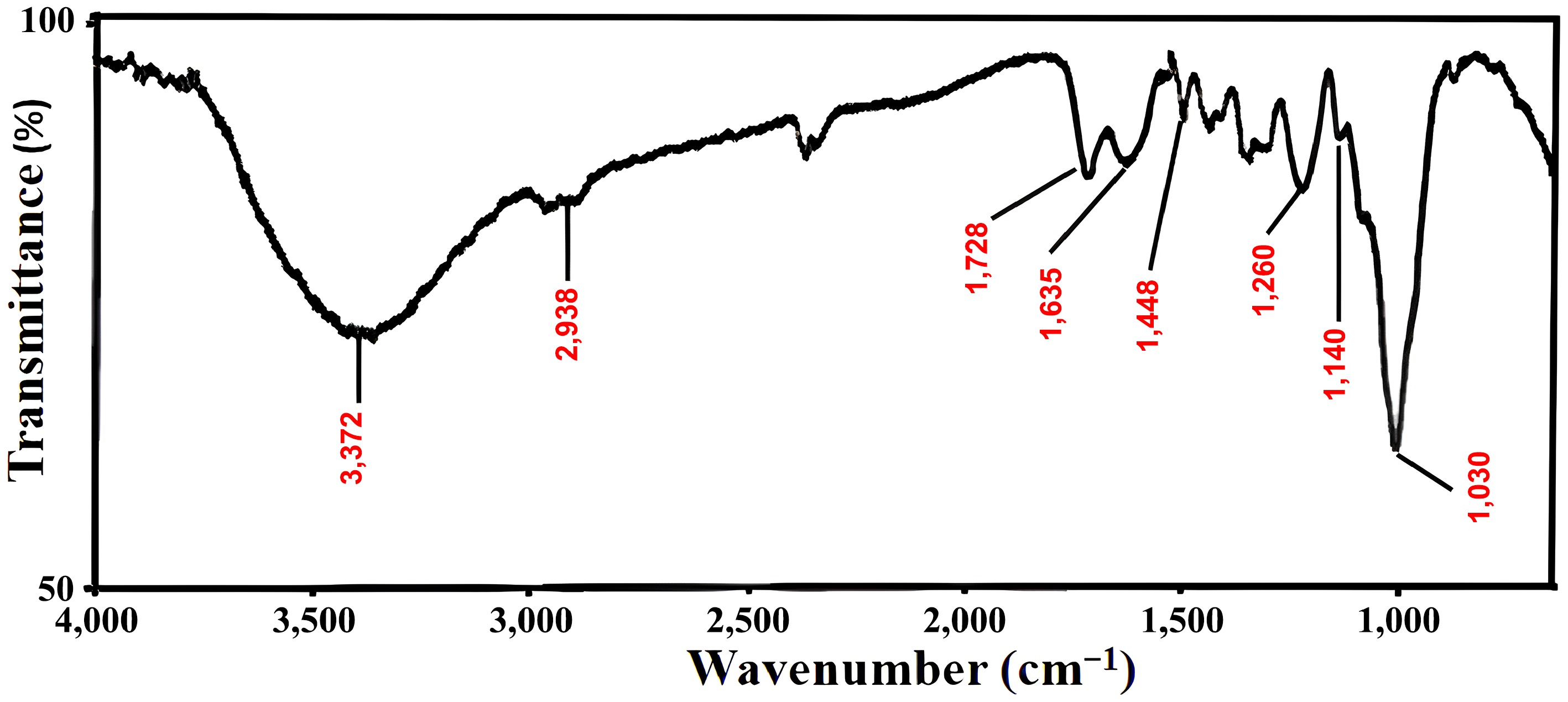
Figure 2.
FT-IR Spectra of FNAC.
-
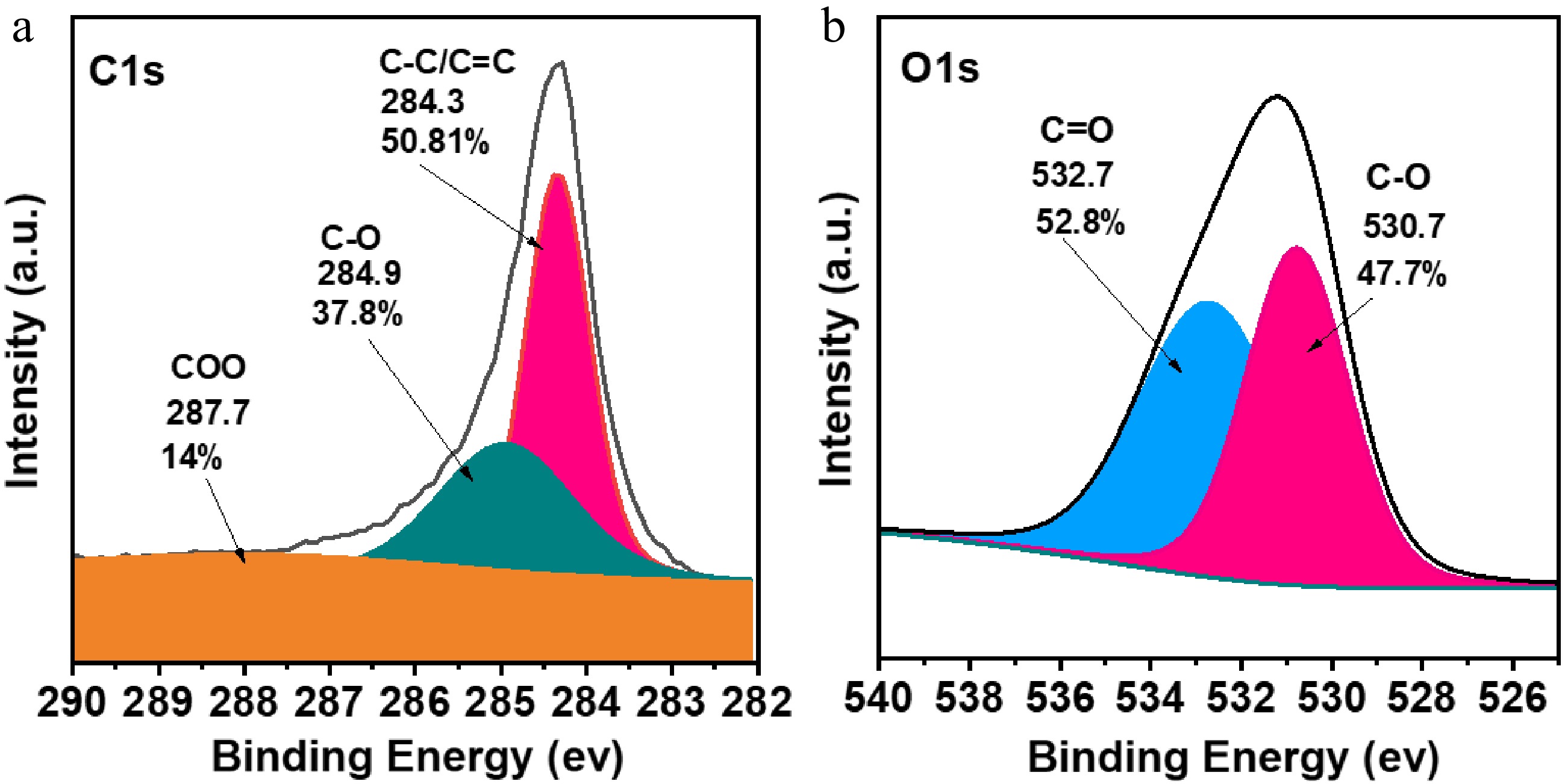
Figure 3.
(a) XPS spectra of FNAC [C 1s], (b) XPS spectra of FNAC [O 1s].
-

Figure 4.
Influence of pH on % removal of BB3 at [BB3] = 100 mg/L, FNAC dose = 0.10 g/100 mL, temperature = 298 K, and contact time = 140 min.
-
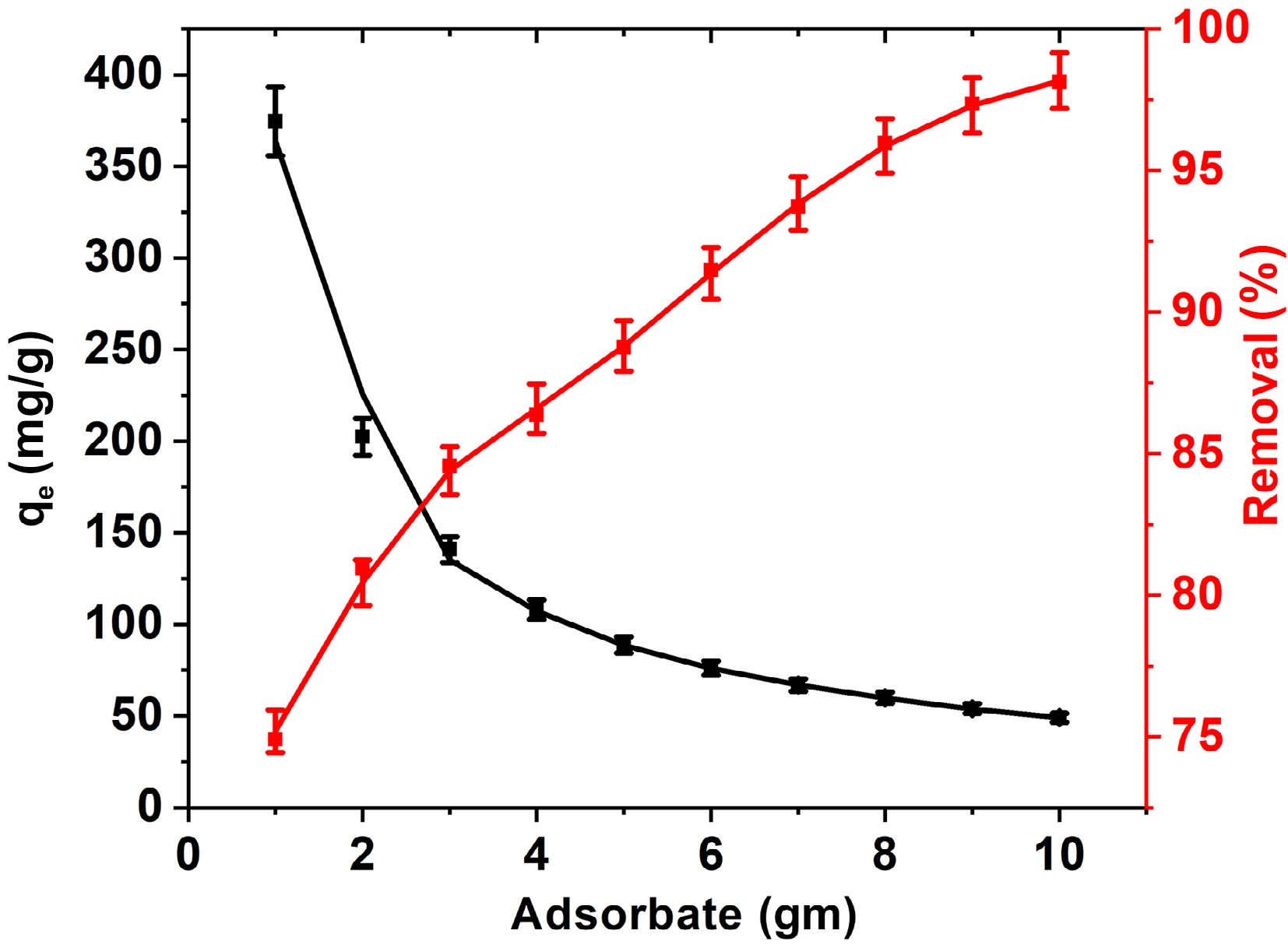
Figure 5.
Influence of FNAC dose on % removal and adsorption capacity of BB3 at [BB3] = 500 mg/L, pH = 6.5 ± 0.1, temperature = 298 K, and contact time = 140 min.
-
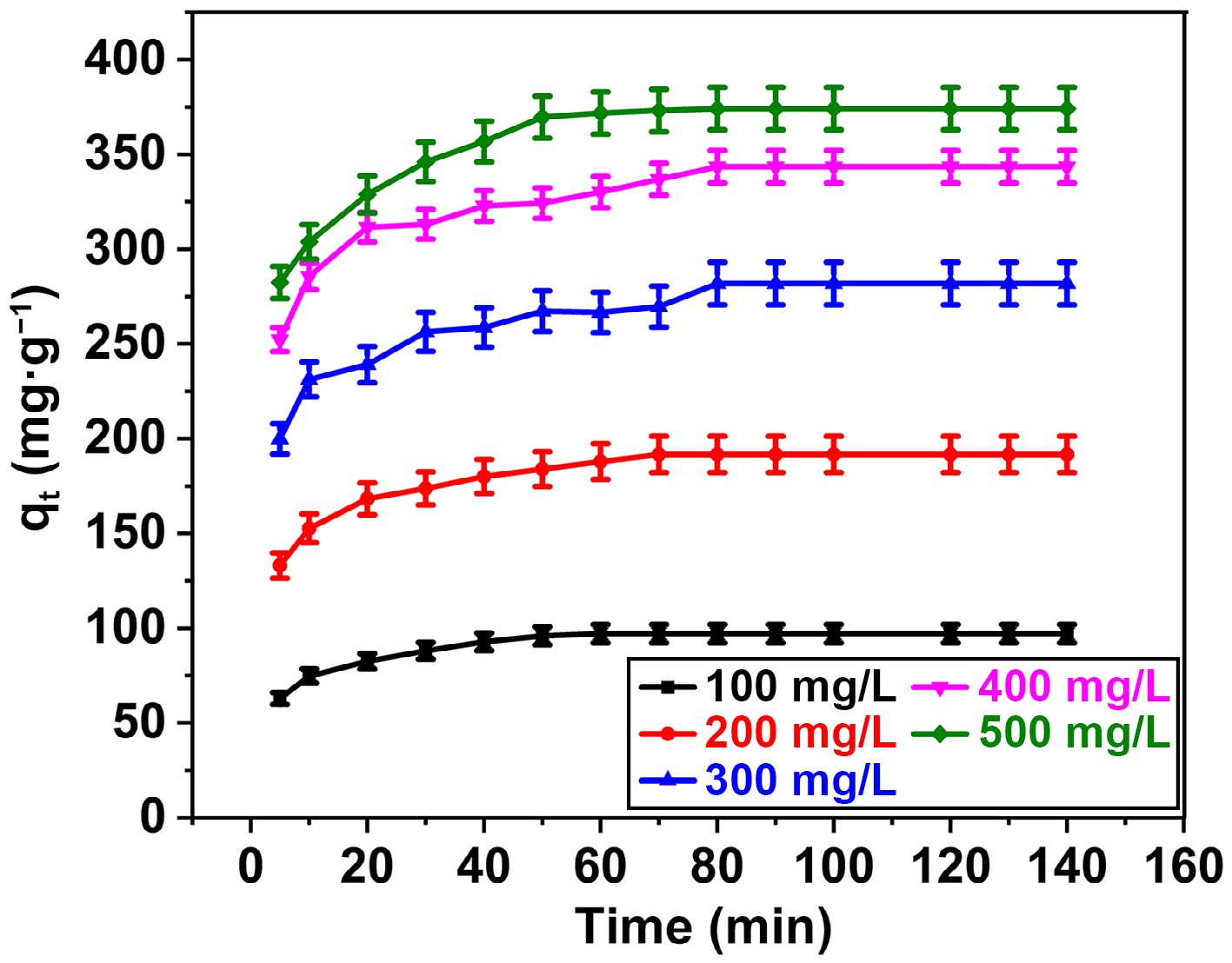
Figure 6.
Influence of BB3 and contact time on % removal of BB3 at FNAC dose = 0.10 g/100 mL, pH = 6.5 ± 0.1, temperature = 298 K, and contact time = 140 min.
-
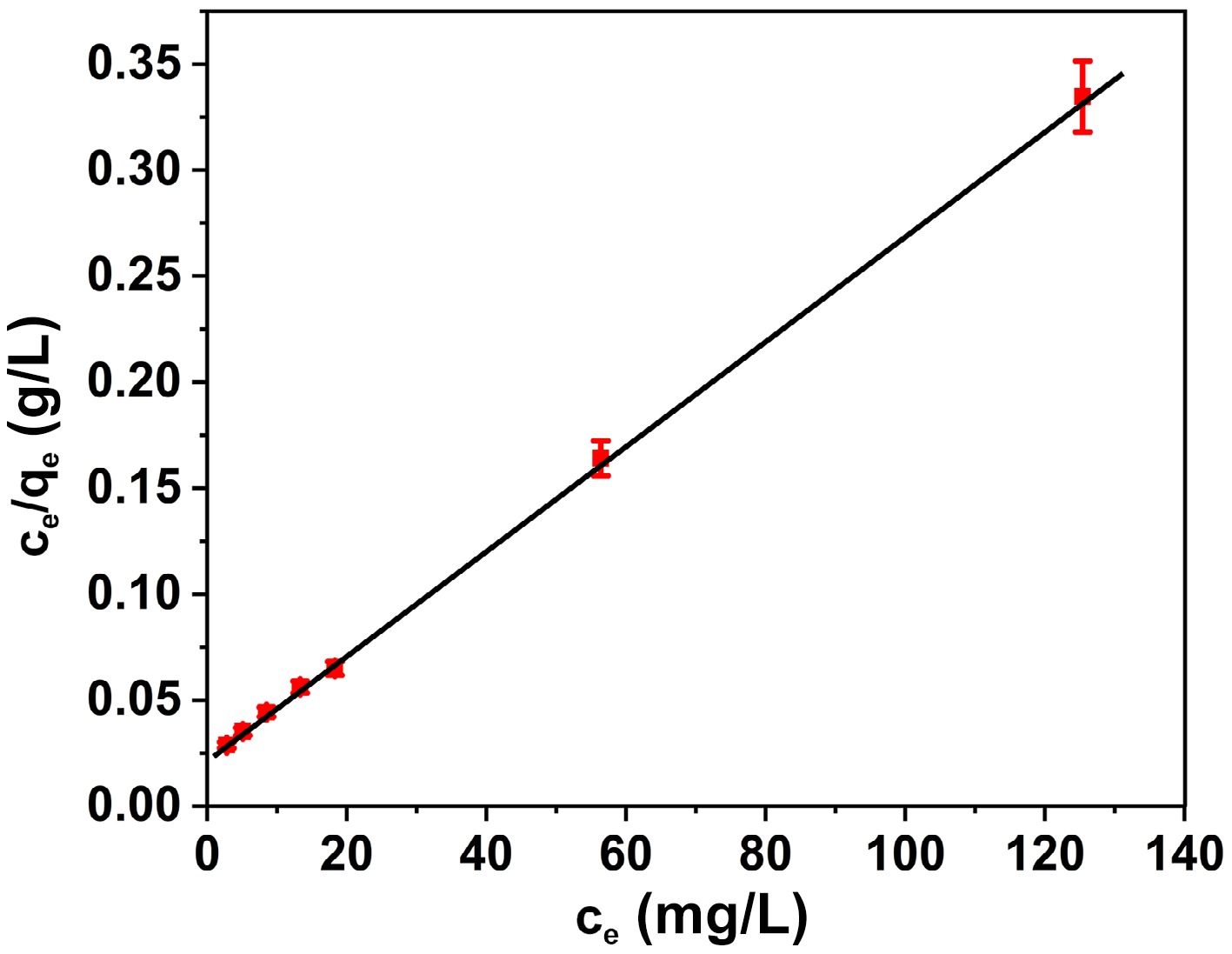
Figure 7.
Plot of the Langmuir isotherm model for the adsorption of BB3 on FNAC.
-
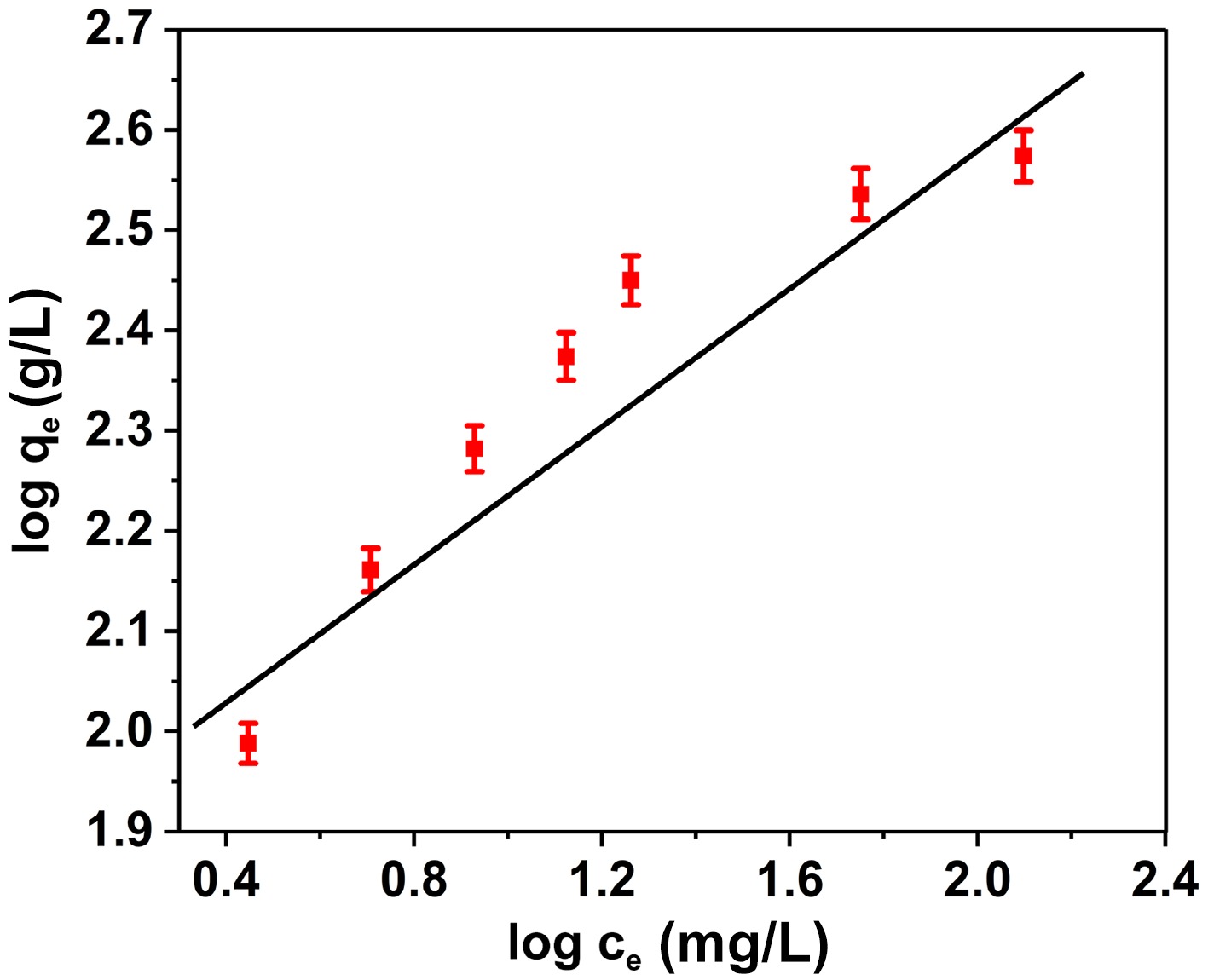
Figure 8.
Plot of Freundlich isotherm model for the adsorption of BB3 on FNAC.
-
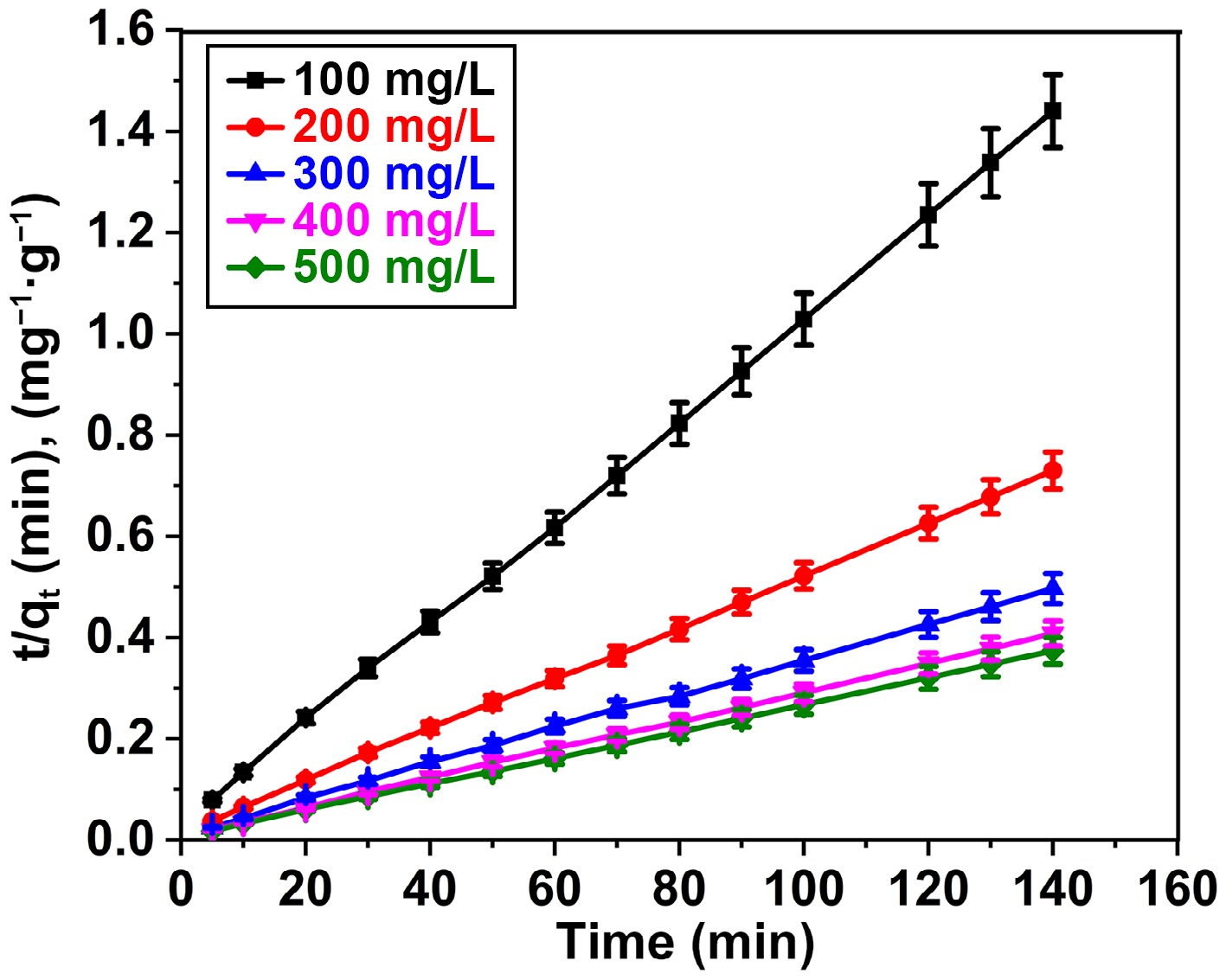
Figure 9.
Pseudo-second order plot for adsorption of BB3 on FNAC at FNAC dose = 0.10 g/100 mL, pH = 6.5 ± 0.1, temperature = 298 K, and contact time = 140 min.
-
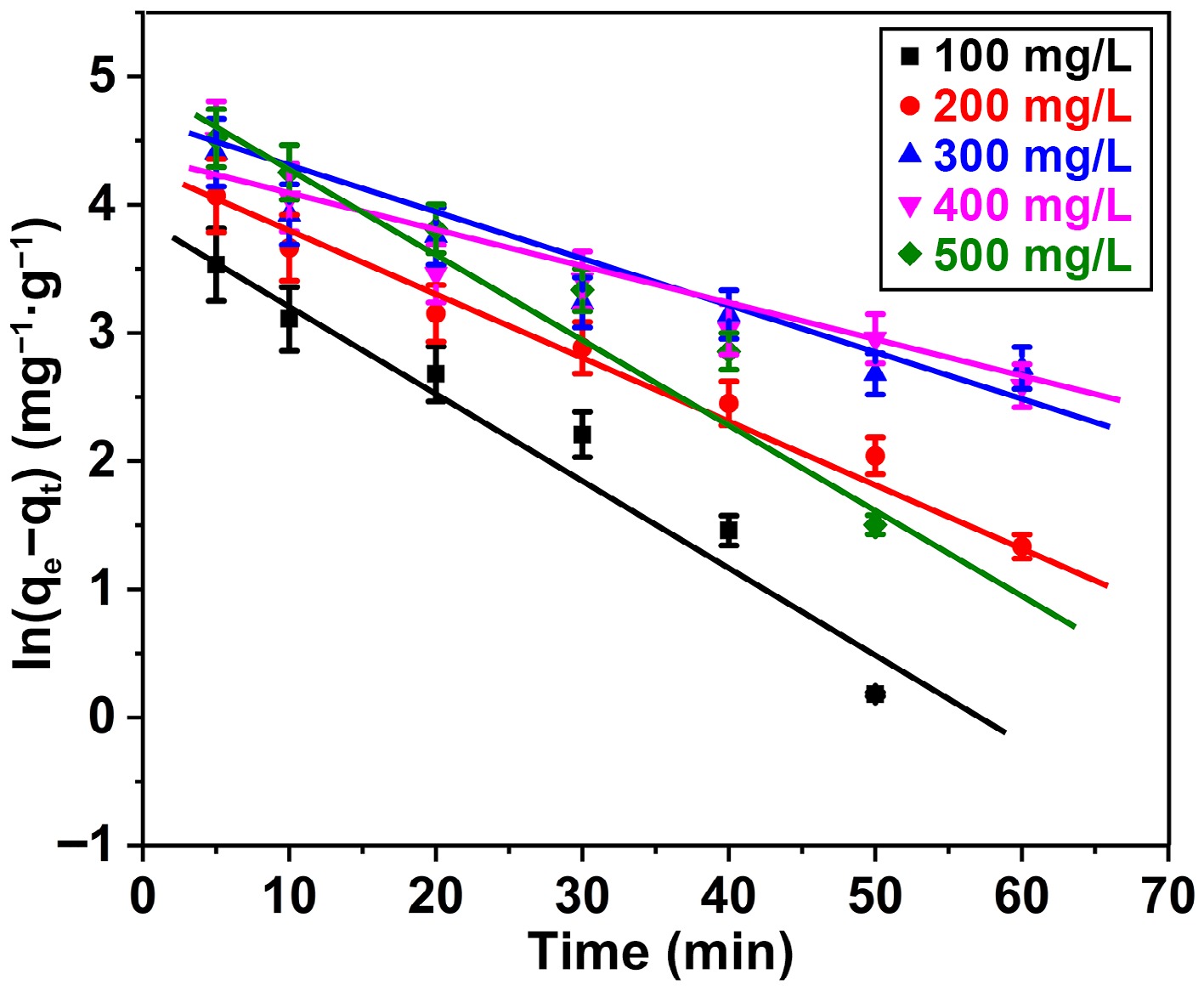
Figure 10.
Pseudo-first order plot for adsorption of BB3 ions on FNAC at FNAC dose = 0.10 g/100 mL, pH = 6.5 ± 0.1, temperature = 298 K, and contact time = 140 min.
-
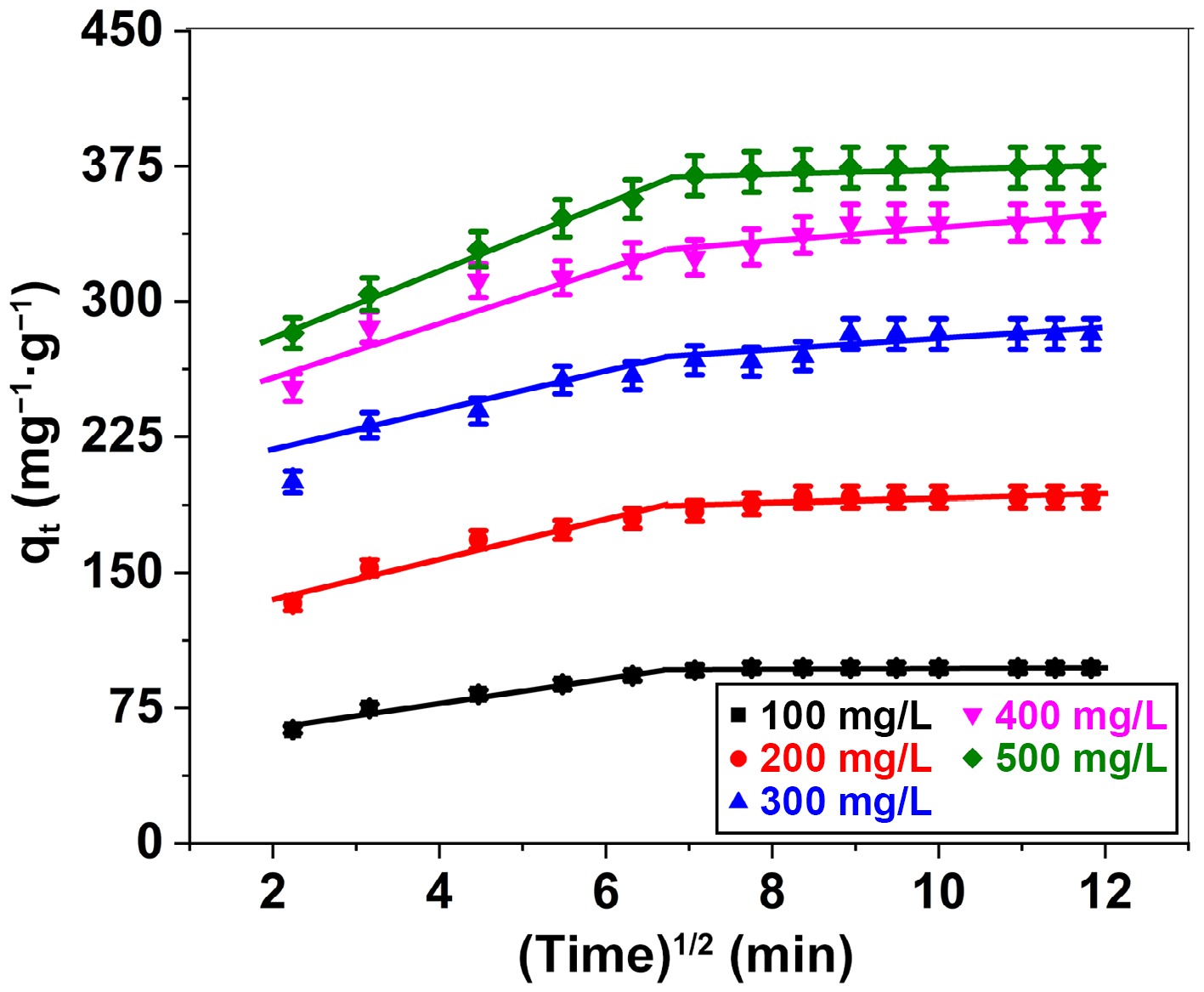
Figure 11.
Intraparticle diffusion plot for adsorption of BB3 ions on FNAC at FNAC dose = 0.10 g/100 mL, pH = 6.5 ± 0.1, temperature = 298 K, and contact time = 140 min.
-

Figure 12.
Mechanism of adsorption of BB3 dye on the FNAC surface.
-
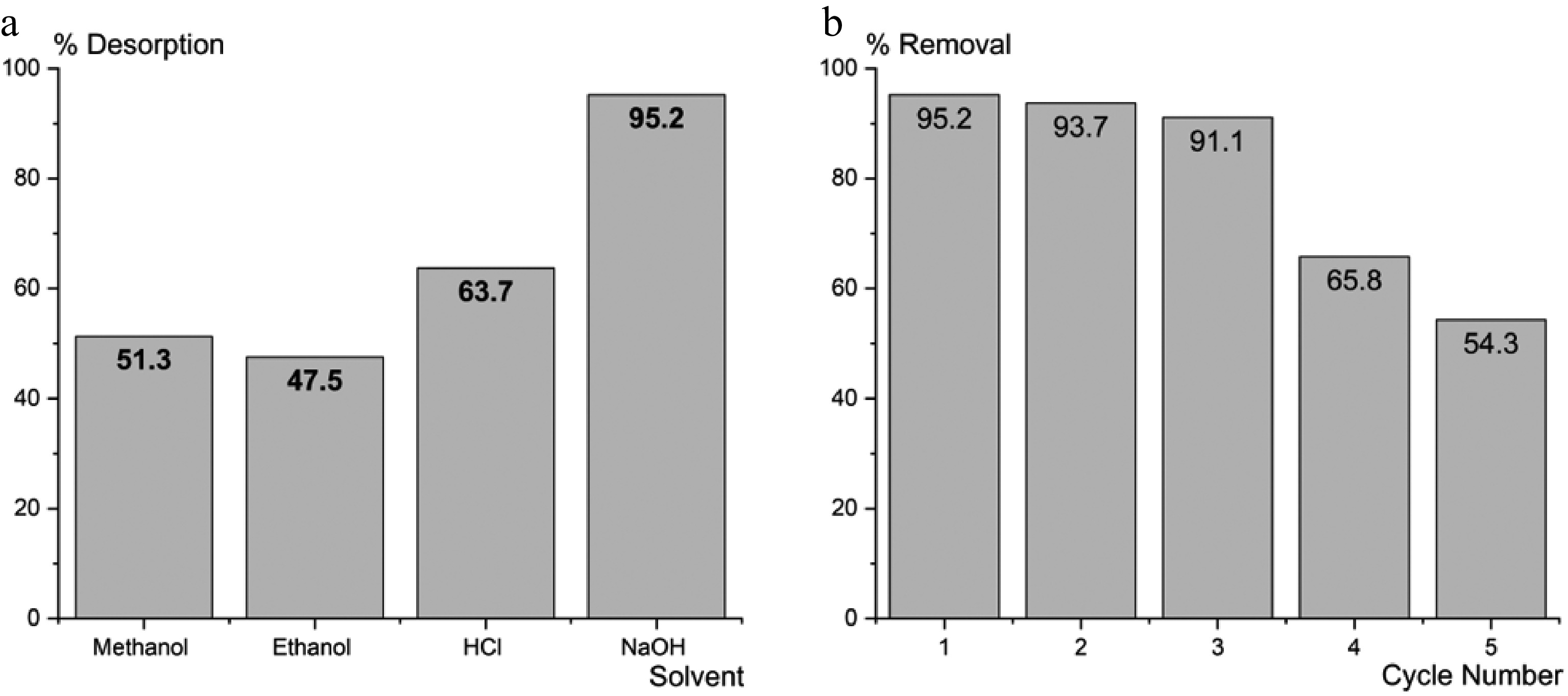
Figure 13.
(a) Effect of solvent on regeneration of FNAC. (b) FNAC reusability.
-
Isotherm Isotherm constant Constant values Langmuir qmax (mg/g) 389.1 R2 0.9986 RL 0.0169 Freundlich KF (mg/g) 84.63 1/n 0.3453 R2 0.8981 Table 1.
Langmuir and Freundlich isotherm constants for the BB3 adsorption onto FNAC.
-
Adsorbent Surface area (m2/g) qmax (mg/g)/
% removalRef. Heveabra siliensis seed coat 1,225 227.27 [27] Silybum marianum stem 122.56 36.8 [28] Waste ash 935 2.73 [29] Persea americana nuts 1,593 625 [30] Cedar sawdust 1.33 85.3 [31] Carbon-silica composite 297 1,295.9 [32] Sugar cane bagasse − 72.32% [33] Macadamia seed husks 99.0% [34] Fox nut shell 1,813.2 374.6 Current study Table 2.
Evaluative analyses of the adsorption capacity of BB3 across various adsorbent substrates.
-
Kinetic models Parameters Initial dye concentration (mg/L) 100 200 300 400 500 Pseudo-first order kinetic qe (mg/g) 52.71 66.91 72.85 85.72 155.94 k1 (min−1) 0.0685 0.0457 0.0278 0.0340 0.0660 R2 0.9575 0.9861 0.9357 0.9447 0.9613 Pseudo-second order kinetic qe (mg/g) 100.5 196.08 285.71 357.14 384.61 k2 [/g(mg·min)] 0.00337 0.00171 0.0010 0.0009 0.00112 R2 0.9994 0.9982 0.9953 0.9992 0.9968 qe (mg/g) [experimental] 97.2 191.5 281.7 343.6 374.6 Table 3.
Variables computed from the PFO and PSO kinetic model.
-
Temp (K) ∆S° [J(K·mol)] ∆H° (kJ/mol) ∆G° (kJ/mol) 298 −123.72 ± 5.19 −43.62 ± 2.07 −6.751 ± 0.39 303 −6.133 ± 0.42 308 −5.514 ± 0.33 Table 4.
Thermodynamic parameters concerning the adsorptive elimination of BB3 by FNAC.
Figures
(13)
Tables
(4)