-
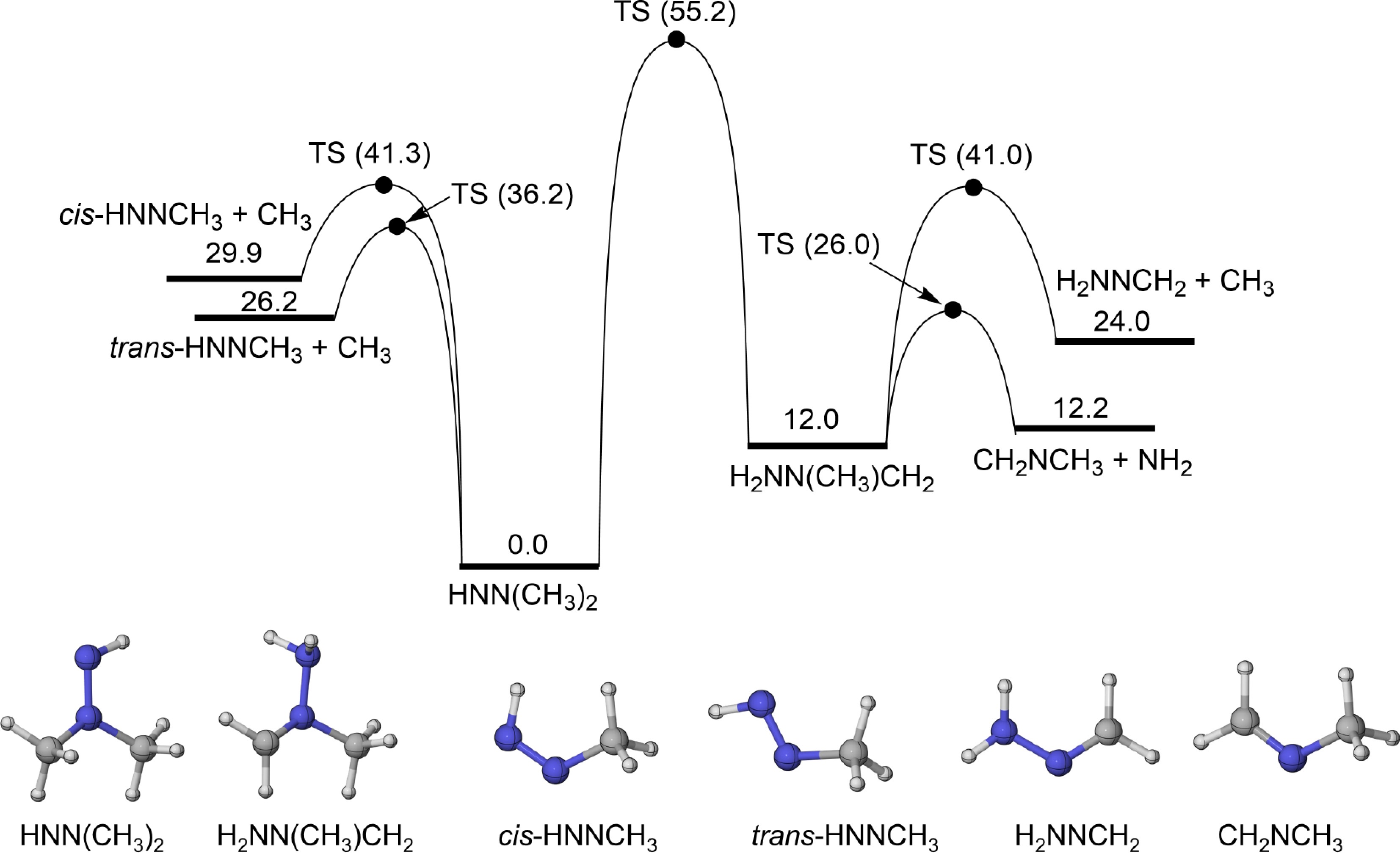
Figure 1.
PES for the isomerization and β-scission reactions of HNN(CH3)2 radical at CCSD(T)/CBS(D+T)//M06-2X/def2-TZVP level (unit: kcal/mol).
-
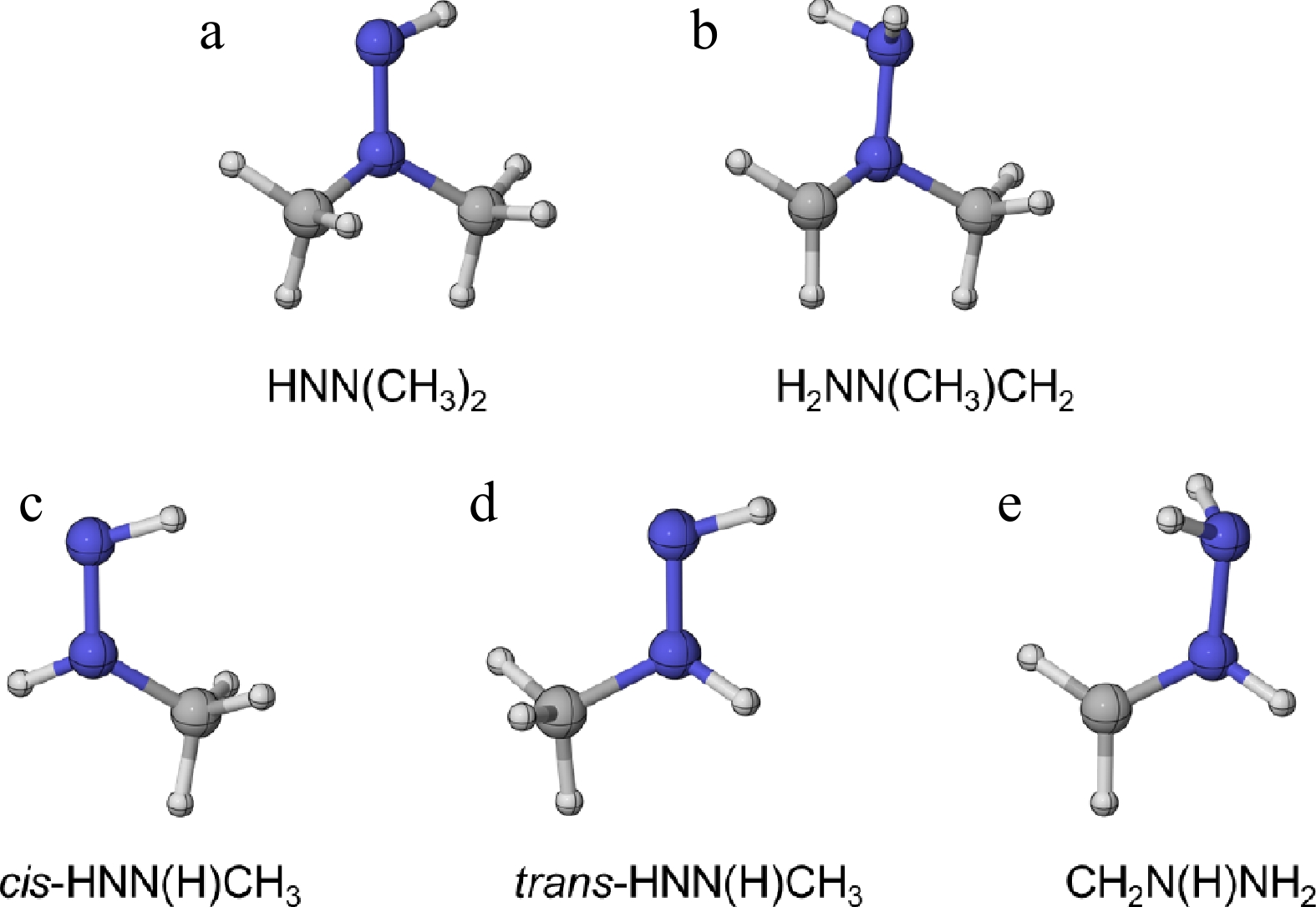
Figure 2.
The optimized molecular configurations of (a), (b) UDMH radicals at the M06-2X/def2-TZVP level of theory, and (c)–(e) MMH radicals at the CASPT2/aug-cc-pVTZ level of theory[31].
-
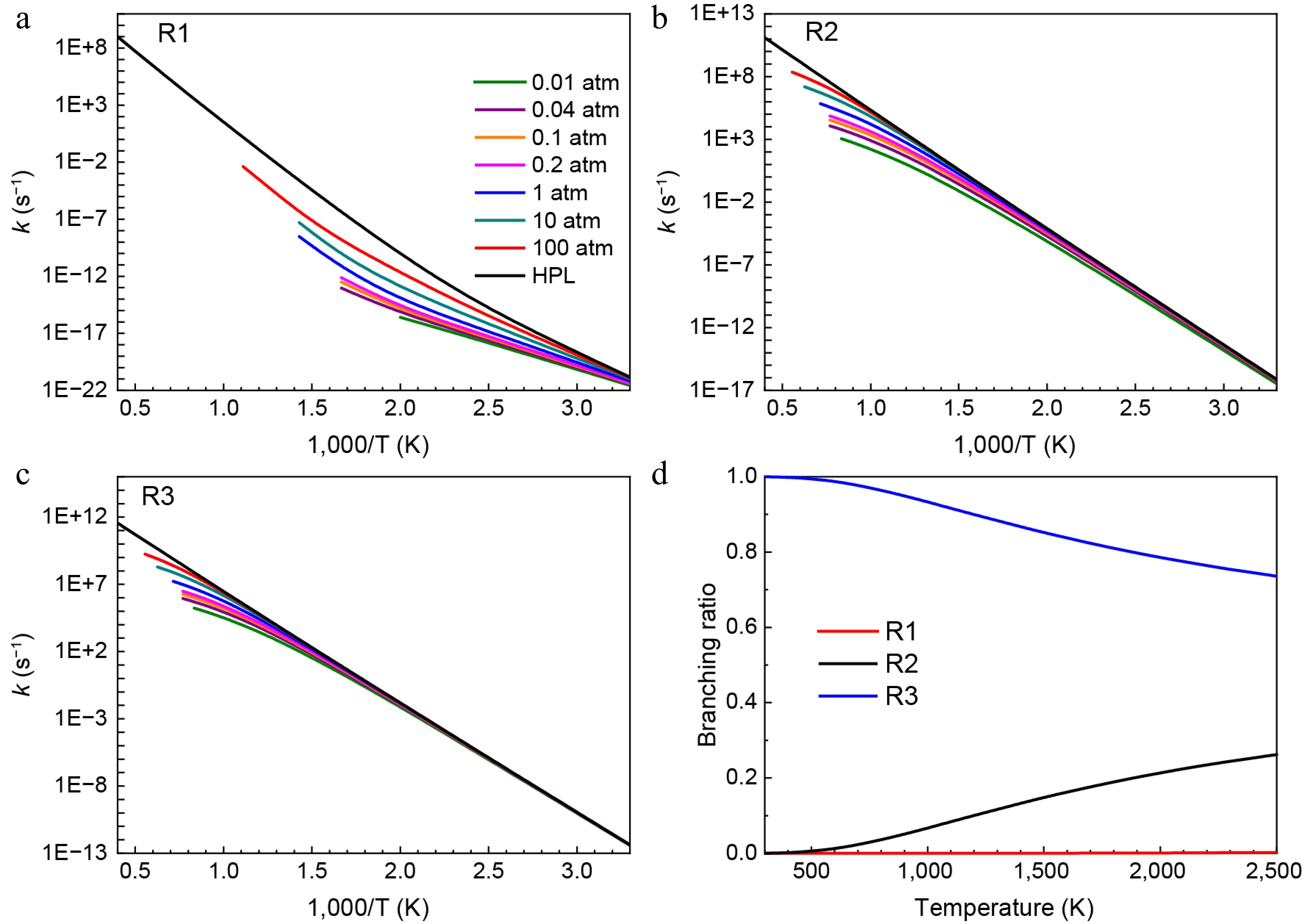
Figure 3.
Pressure-dependent rate constants for (a) R1, (b) R2, and (c) R3. (d) Branching ratios of all consumption reactions of HNN(CH3)2 radical at HPL.
-
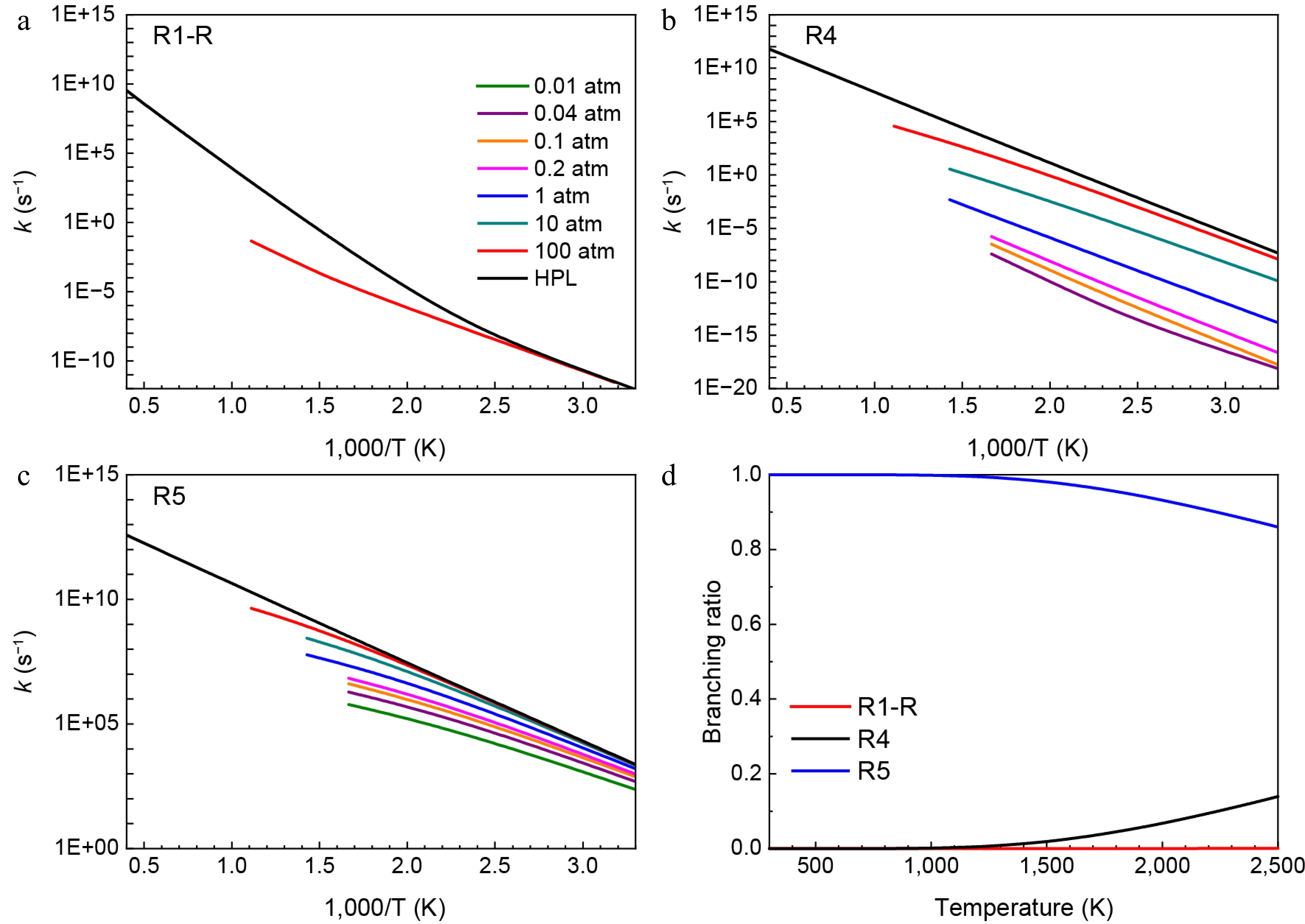
Figure 4.
Pressure-dependent rate constants for (a) R1-R, (b) R4, and (c) R5. (d) Branching ratios of all consumption reactions of H2NN(CH3)CH2 radical at HPL.
-
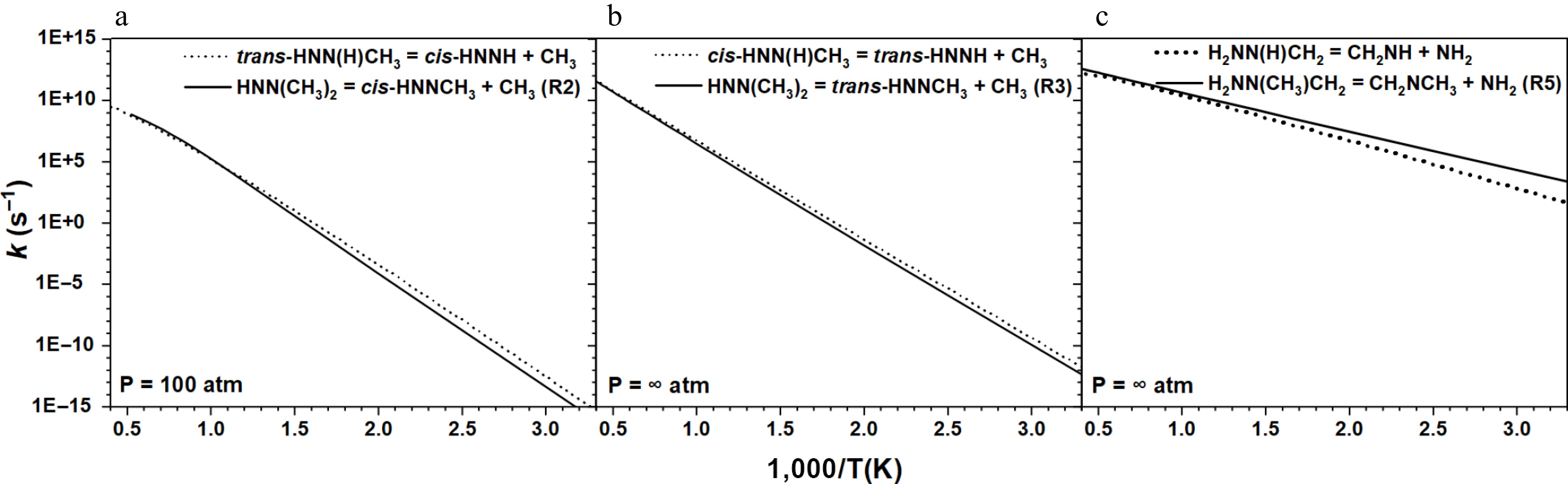
Figure 5.
Comparison of the rate constants for the β-scission reactions of (a), (b) HNN(CH3)2, and (c) H2NN(CH3)CH2 radicals with those of MMH radicals with similar configurational characteristics[31].
-
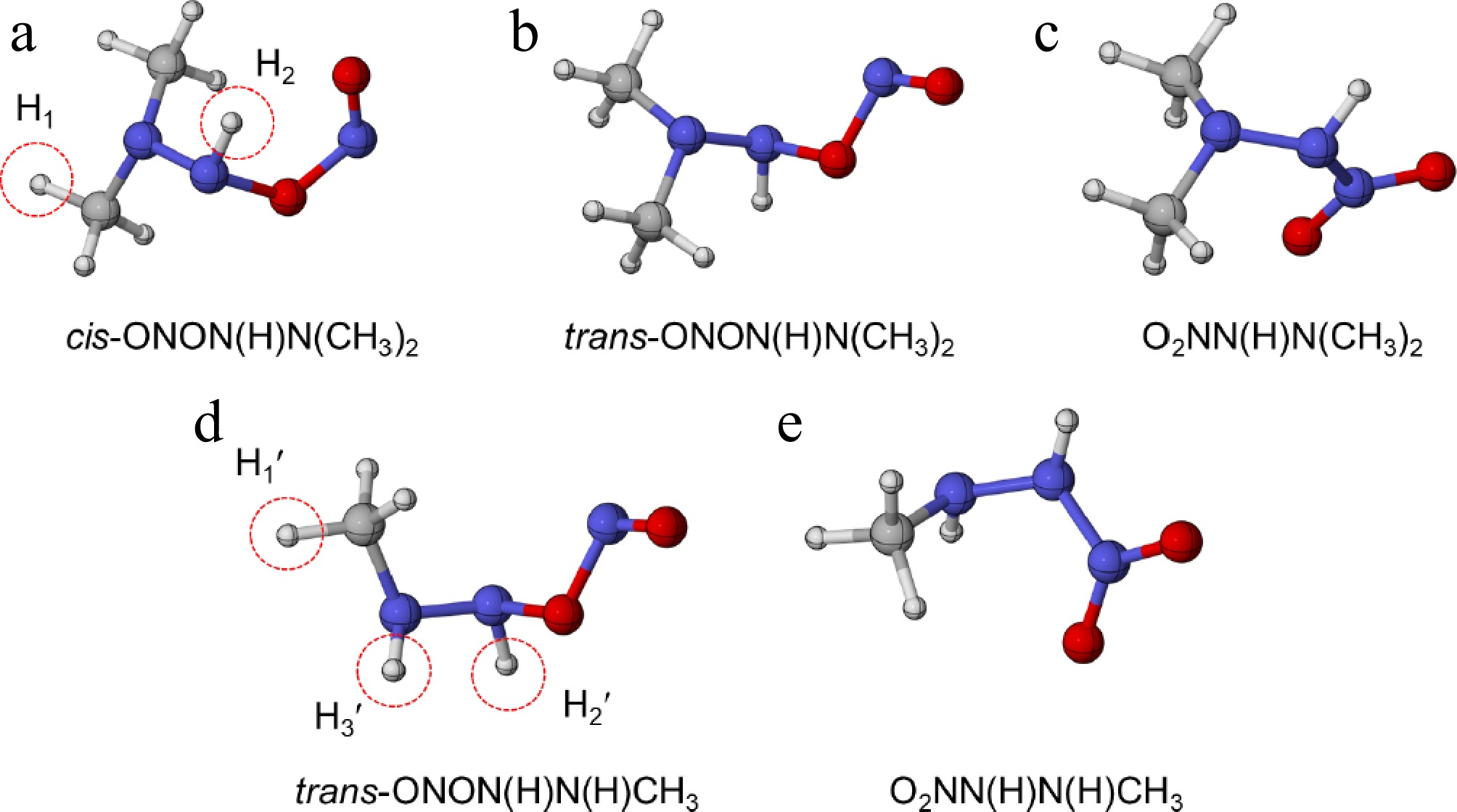
Figure 6.
The optimized molecular configurations of (a)–(c) HNN(CH3)2 + NO2 at the M06-2X/def2-TZVP level of theory, and (d) NHN(H)CH3 + NO2 at the ωB97X-D/6-311++G(d,p) level of theory[12].
-
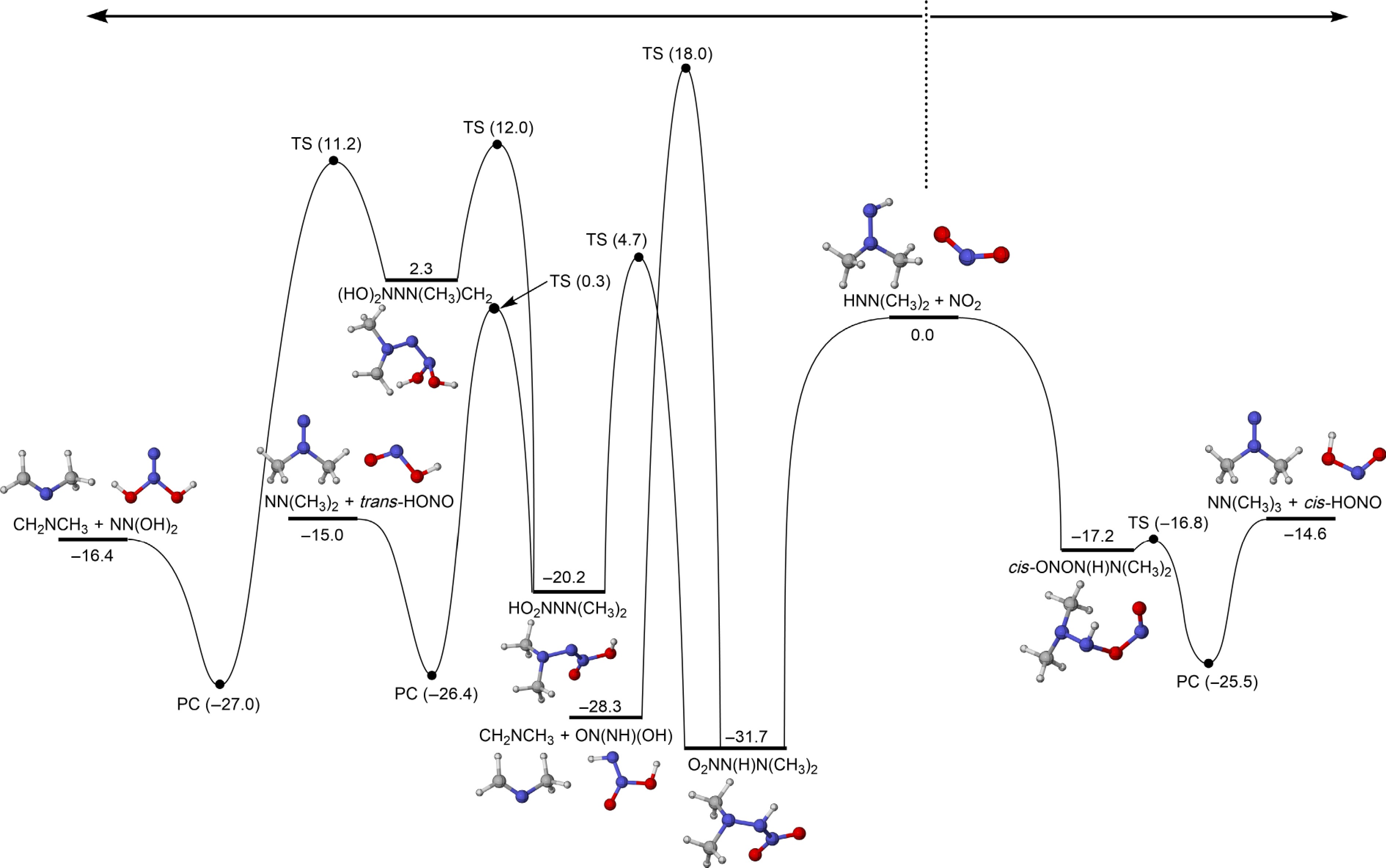
Figure 7.
PESs for the addition-dissociation reactions of HNN(CH3)2 with NO2 at CCSD(T)/CBS(D+T)//M06-2X/def2-TZVP level of theory (unit: kcal/mol).
-
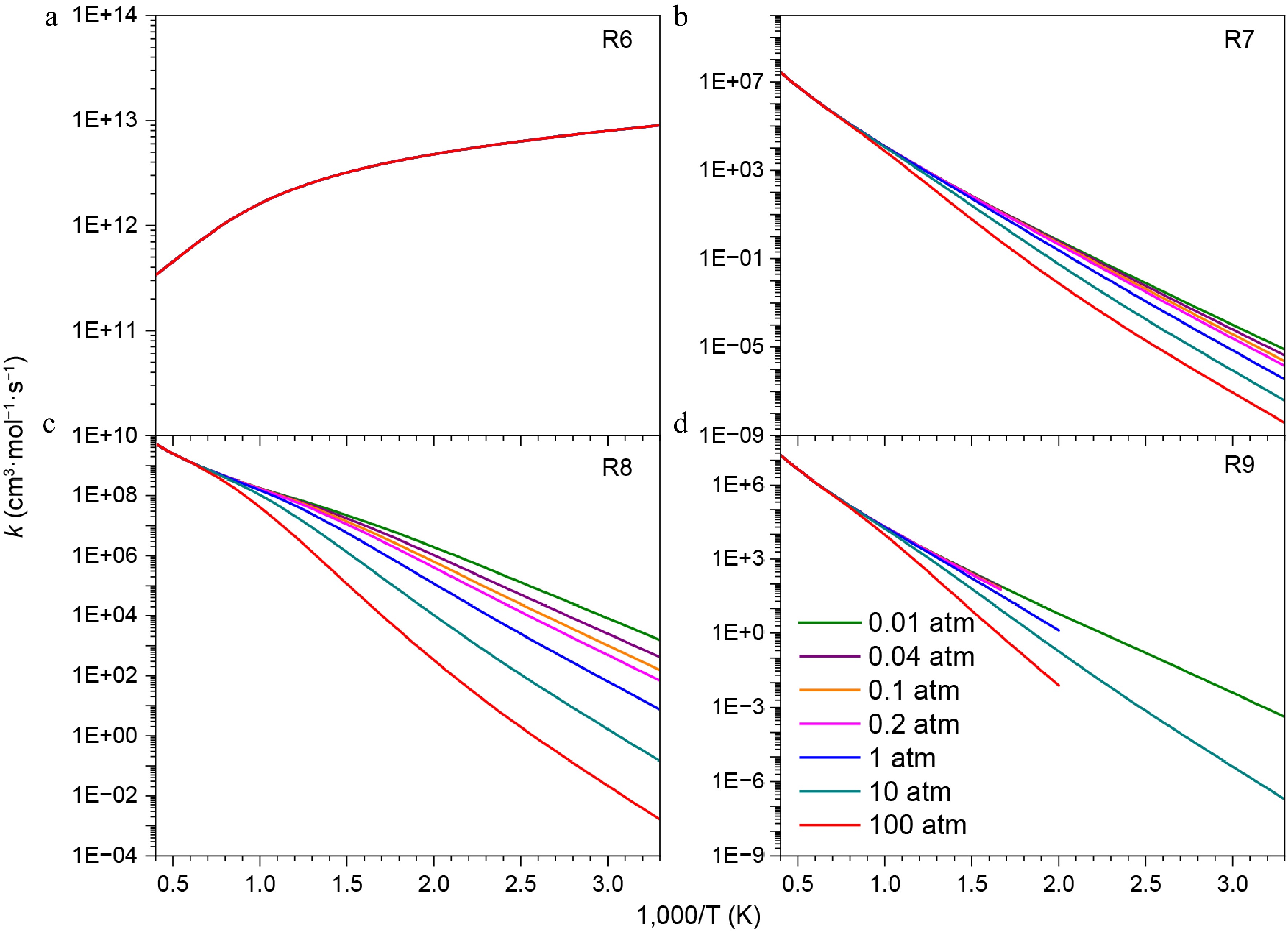
Figure 8.
Pressure-dependent rate constants for the addition-dissociation reactions of HNN(CH3)2 radical with NO2.
-
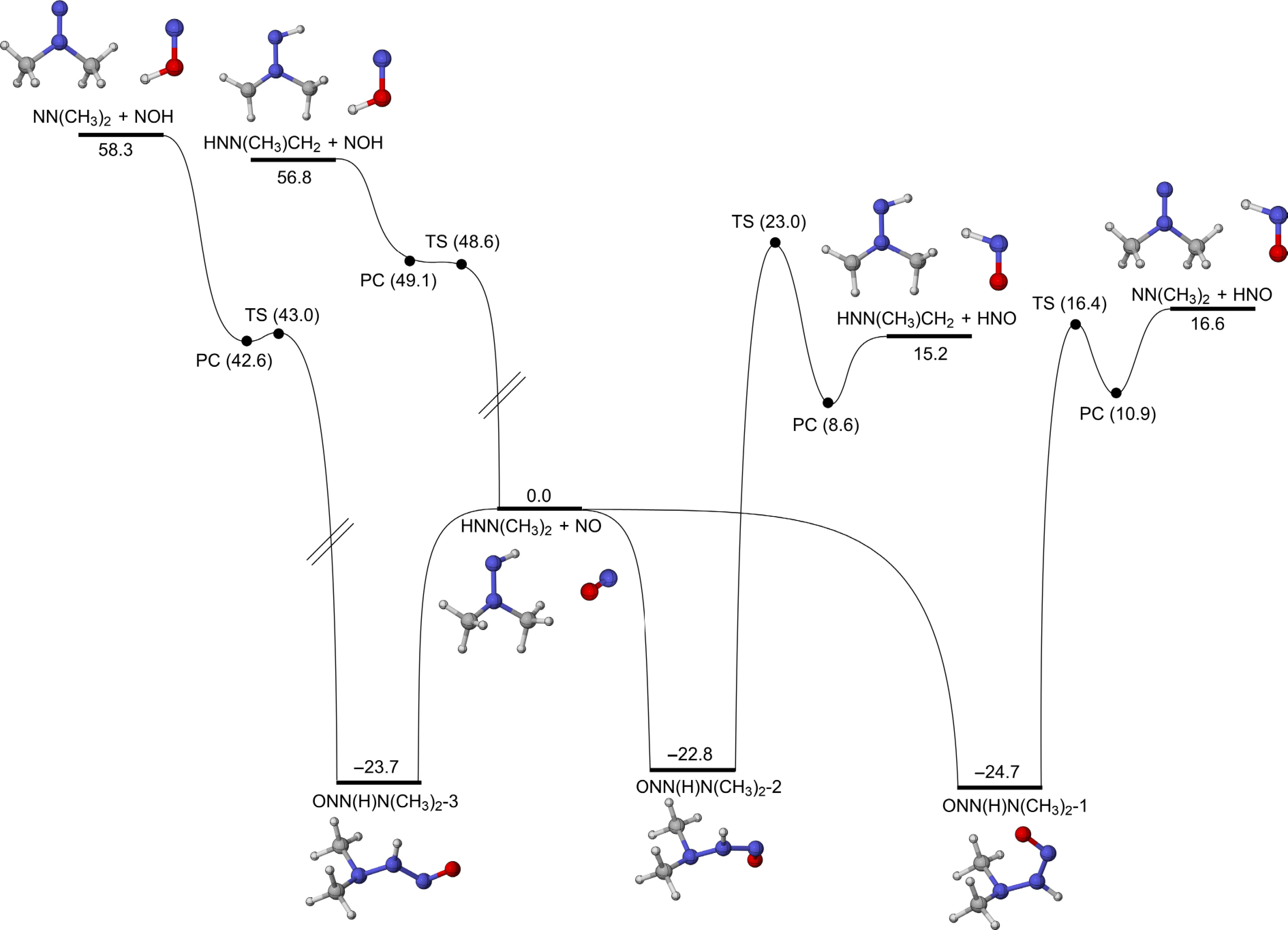
Figure 9.
PESs for the addition-dissociation reactions of HNN(CH3)2 with NO at CCSD(T)/CBS(D+T)//M06-2X/def2-TZVP level of theory (unit: kcal/mol).
-
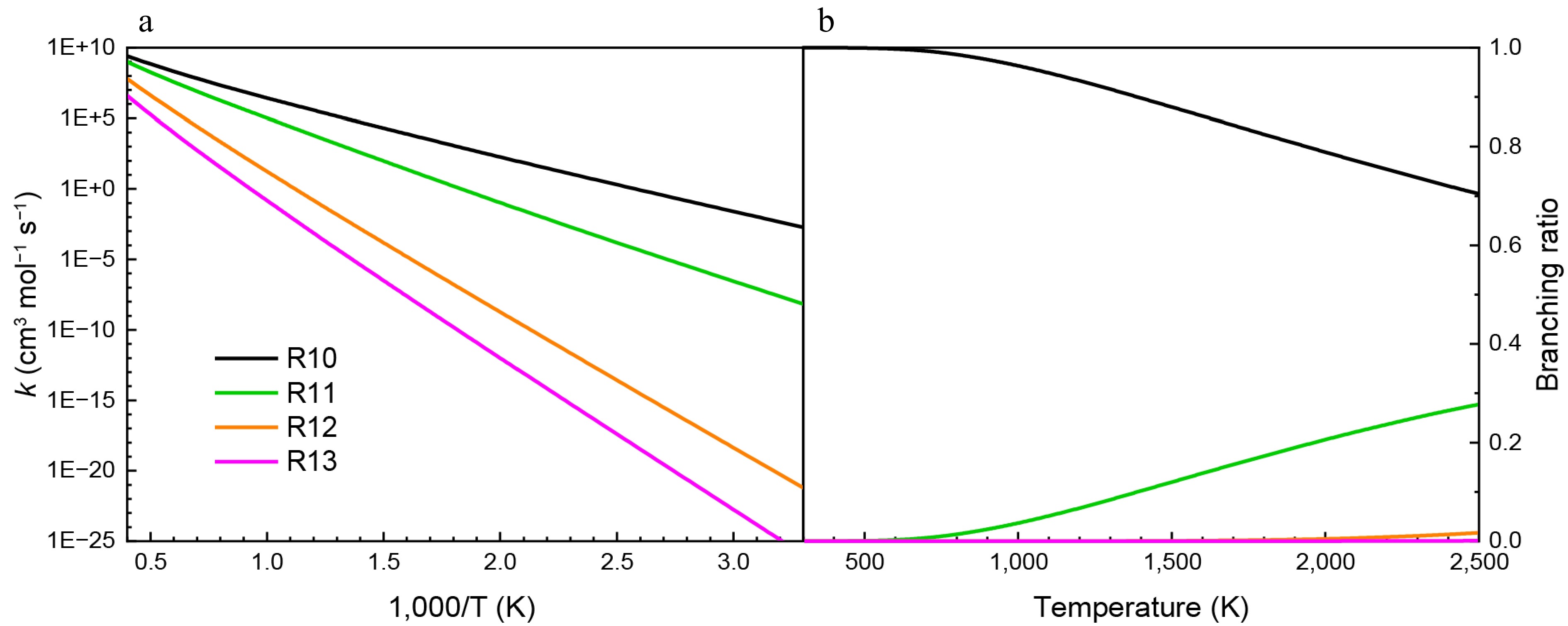
Figure 10.
(a) Rate constants, and (b) branching ratios for the addition-dissociation reactions of HNN(CH3)2 with NO at HPL.
-
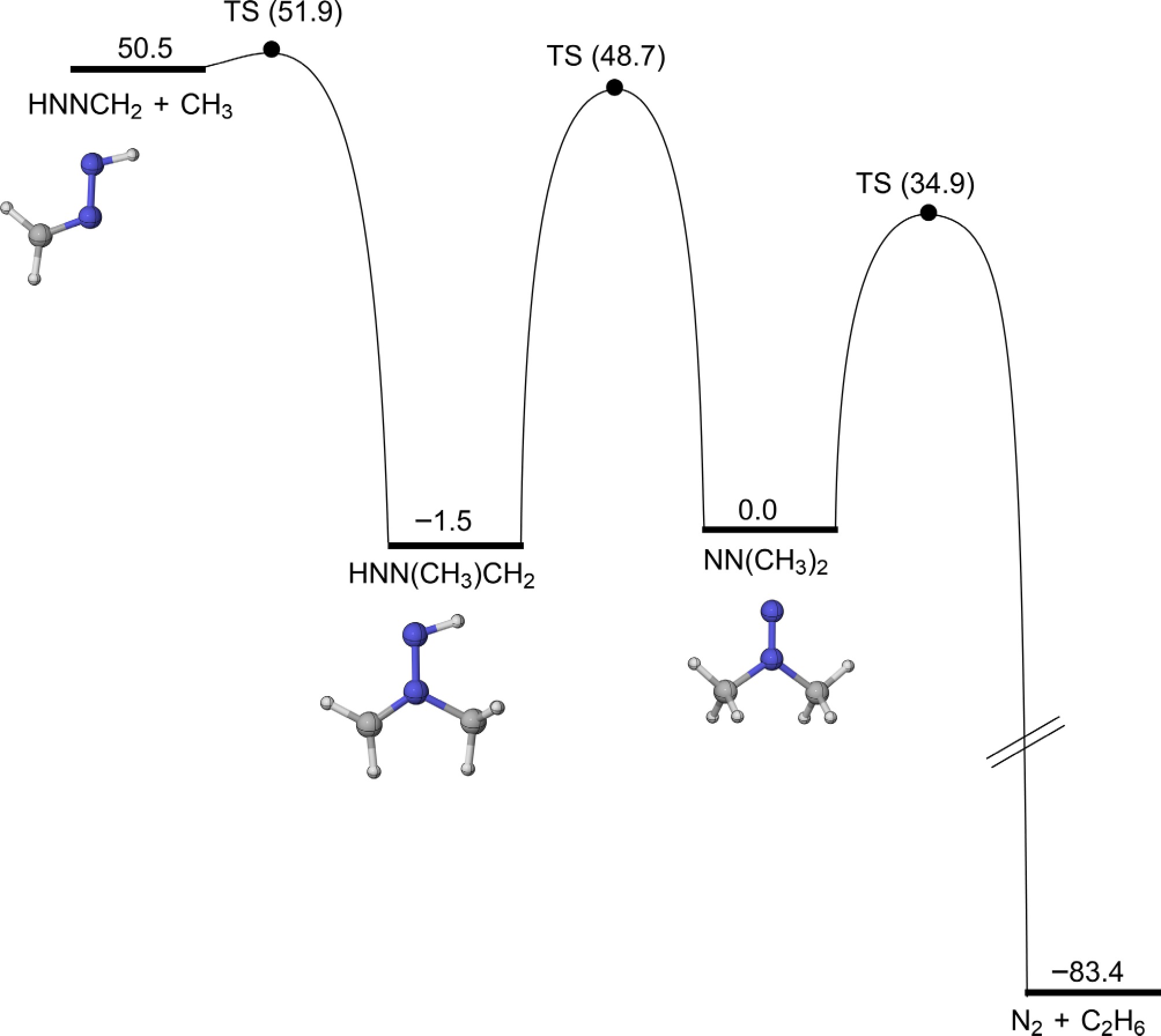
Figure 11.
PESs for the isomerizartion and β-scission reactions of NN(CH3)2 at CCSD(T)/CBS(D+T)//M06-2X/def2-TZVP level of theory (unit: kcal/mol).
-
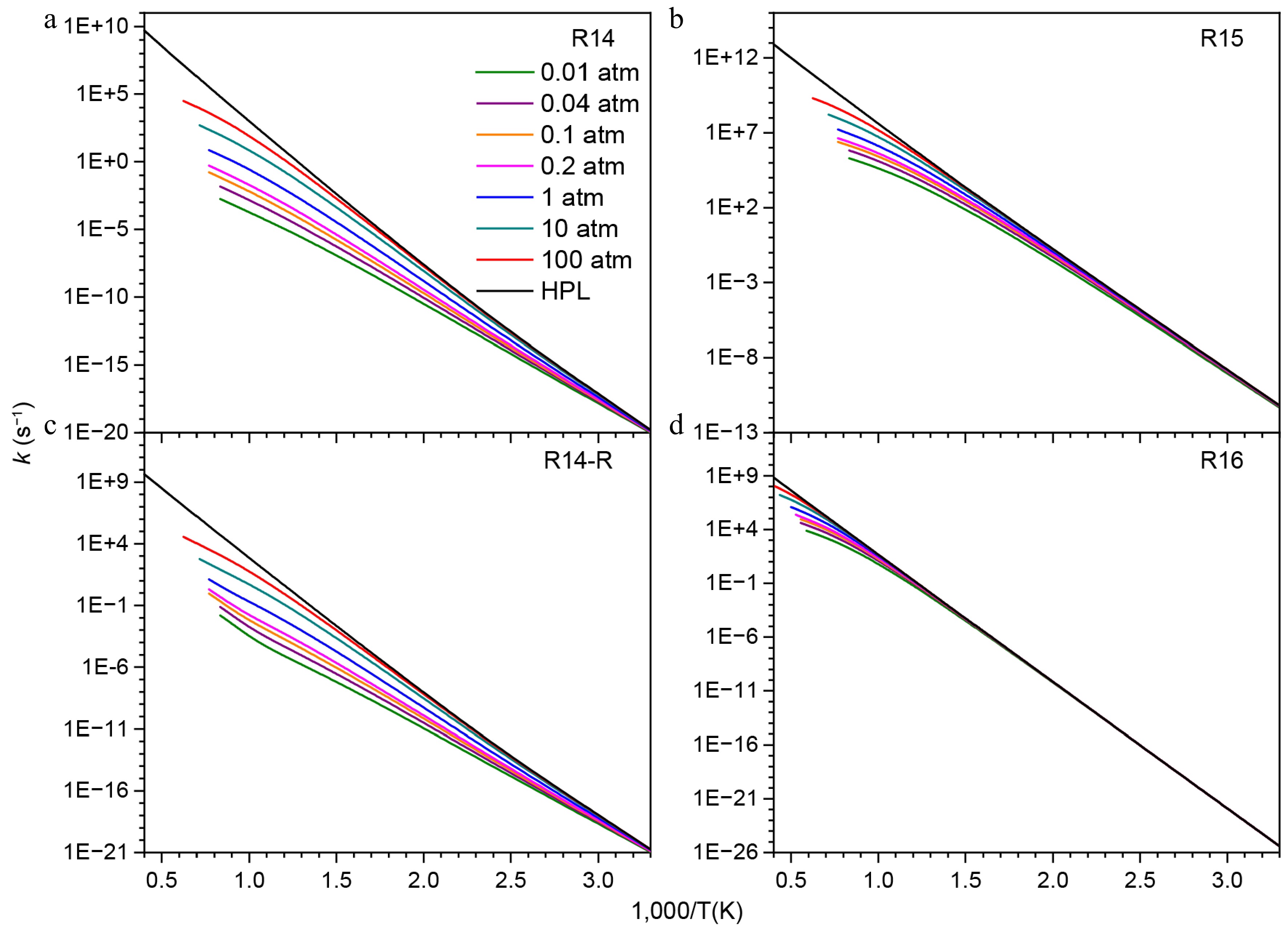
Figure 12.
Pressure-dependent rate constants for the consumption reactions of NN(CH3)2.
-
No. Reactions P (atm) A n Ea (cal/mol) 1 HNN(CH3)2 = H2NN(CH3)CH2 HPL 1.2258E−15 8.1005 37,245 2 HNN(CH3)2 = cis-HNNCH3 + CH3 HPL 1.3900E+12 0.78176 41,951 3 HNN(CH3)2 = trans-HNNCH3 + CH3 HPL 2.1770E+12 0.72387 36,806 4 H2NN(CH3)CH2 = H2NNCH2 + CH3 HPL 8.4385E+12 0.43102 29,575 5 H2NN(CH3)CH2 = CH2NCH3 + NH2 HPL 1.6031E+13 0.18499 14,279 6 HNN(CH3)2 + NO2 = NN(CH3)2 + cis-HONO 100 7.3949E+20 −2.7812 1,607.6 4.2465E+04 1.8100 −4,564.2 7 HNN(CH3)2 + NO2 = CH2NCH3 + ON(NH)(OH) 100 2.3499E+02 2.0956 22,579 8 HNN(CH3)2 + NO2 = NN(CH3)2 + trans-HONO 100 1.0116E+45 −9.5853 39,906 5.7045E+10 0.19569 20,318 9 HNN(CH3)2 + NO2 = CH2NCH3 + NN(OH)2 100 3.9191E+03 1.6501 21,623 10 HNN(CH3)2 + NO = NN(CH3)2 + HNO 100 1.7593E+03 2.2232 15,951 11 HNN(CH3)2 + NO = HNN(CH3)CH2 + HNO 100 5.1298E+03 2.1753 24,033 12 HNN(CH3)2 + NO = NN(CH3)2 + NOH 100 2.7881E+02 2.6552 41,967 13 HNN(CH3)2 + NO = HNN(CH3)CH2 + NOH 100 2.4904E−01 3.3194 46,582 14 NN(CH3)2 = HNN(CH3)CH2 HPL 3.7076E+10 0.99227 48,700 15 NN(CH3)2 = N2 + C2H6 HPL 6.2976E+10 1.5546 36,030 16 HNN(CH3)CH2 = HNNCH2 + CH3 HPL 3.5255E+12 0.29155 53,875 Table 1.
List of the Arrhenius parameters for the rate constants of isomerization and β-scission reactions of UDMH radicals, as well as the H-abstraction reactions of the HNN(CH3)2 radical and subsequent decomposition reactions at HPL or 100 atm conditions.
-
Reactions Energy barrier (kcal/mol) Ref. trans-HNN(H)CH3 = H2NN(H)CH2 49.3 (52.98) This work ([31]a) HNN(CH3)2 = H2NN(CH3)CH2 (R1) 55.2 This work trans-HNN(H)CH3 = cis-HNNH + CH3 42.2 (39.84) This work ([31]a) HNN(CH3)2 = cis-HNNCH3 + CH3 (R2) 41.3 This work cis-HNN(H)CH3 = trans-HNNH + CH3 37.2 (35.19) This work ([31]a) HNN(CH3)2 = trans-HNNCH3 + CH3 (R3) 36.2 This work H2NN(H)CH2 = CH2NH + NH2 13.5 (13.75) This work ([31]b) H2NN(CH3)CH2 = CH2NCH3 + NH2 (R5) 14.0 This work a Results were obtained at the theory level of QCISD(T)/cc-pV∞Z//B3LYP/6-311++G(d,p); b Results were obtained at the theory level of QCISD(T)/cc-pV∞Z//CASPT2/aug-cc-pVTZ. Table 2.
Comparison of the energy barrier for the isomerization and β-scission reactions of UDMH radicals with those of MMH radicals with similar configurational characteristics.
-
Table 3.
Relative energies of HNN(CH3)2 + NO2 at the CCSD(T)/CBS(D+T)//M062X/def2-TZVP level of theory and NHNHCH3 + NO2 at the RHF-UCCSD(T)-F12//ωB97X-D/6-311++G(d,p) level of theory[12].
Figures
(12)
Tables
(3)