-
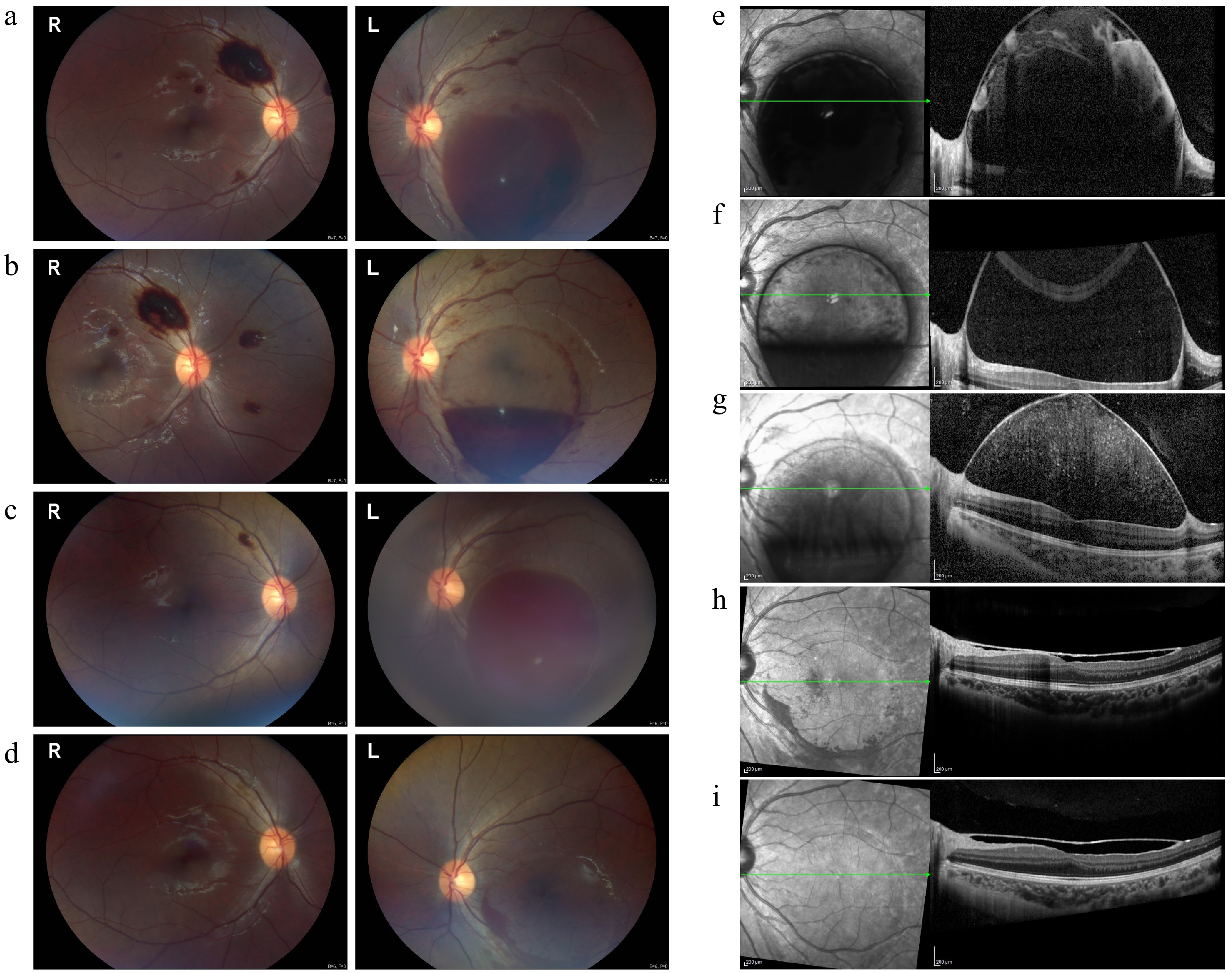
Figure 1.
Fundus and OCT images at various stages of treatment in Case 1. (a) Preinjection fundus image showing extensive preretinal hemorrhage in both eyes, with a larger sub-ILM hemorrhage affecting the macula in the left eye. (b) Two days after the first injection, partial absorption of the sub-ILM hemorrhage was observed in the left eye. (c) Eight weeks later, recurrent macular hemorrhage appeared in the left eye, while the right eye's hemorrhage resolved. (d) Two weeks after the second injection, near-complete resolution of the left eye's hemorrhage was observed, with a small residual hemorrhage remaining. (e)–(i) OCT images showing the progression of sub-ILM hemorrhage before and after the injections: (e) Sub-ILM fluid-filled cavity before the injections; (f) partial resolution two days after the first injection; (g) recurrence of hemorrhage at eight weeks; (h) significant absorption two weeks after the second injection; (i) complete resolution at two months, with restored macular foveal contour and elevated ILM.
-
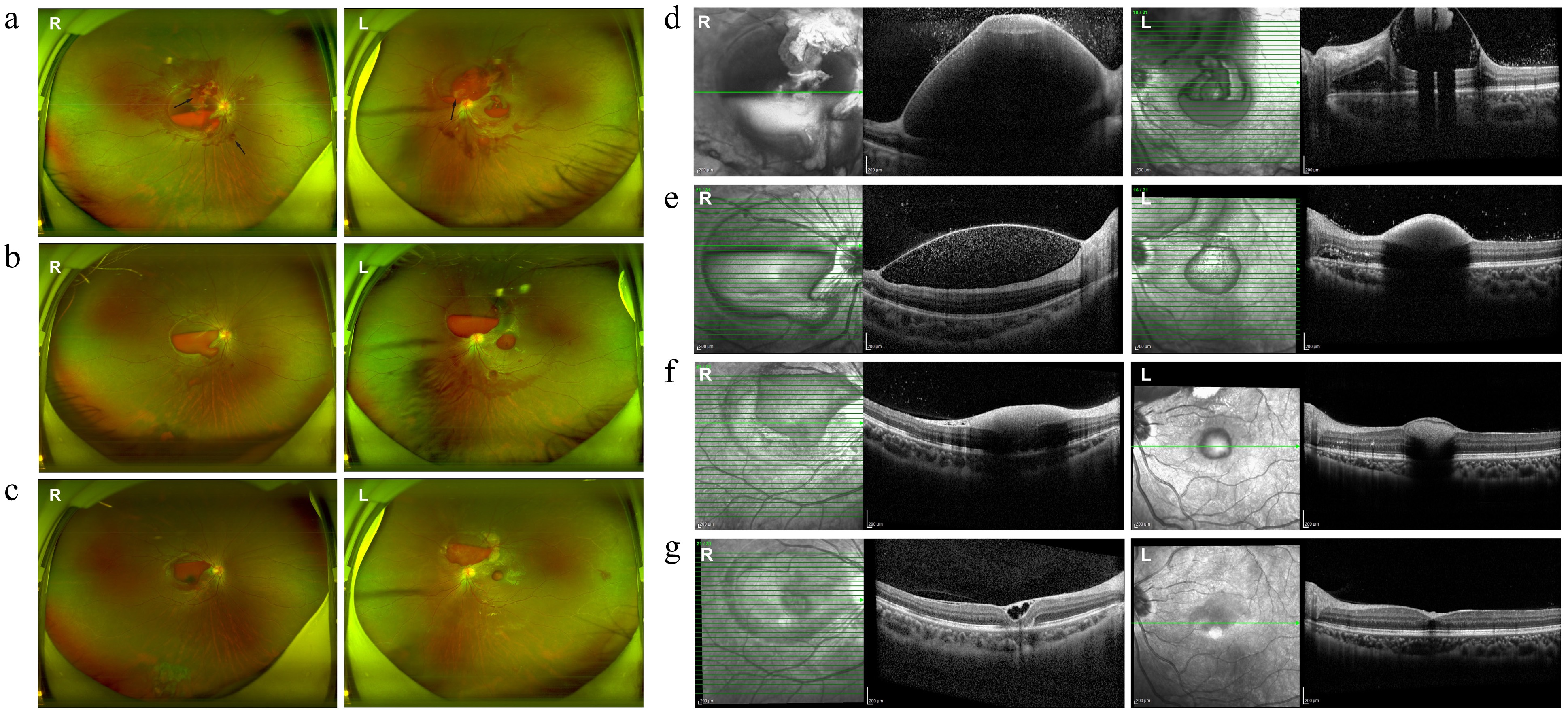
Figure 2.
Fundus and OCT images at various stages of treatment in Case 2. (a) Preinjection fundus image showing bilateral preretinal hemorrhage, hard exudates, blurred optic disc margins, diffuse vascular tortuosity, and Roth spots (black arrows). (b) One week post-injection, partial absorption of sub-ILM hemorrhage was noted, along with the resolution of hard exudates and improved optic disc clarity. (c) Three weeks postinjection, the sub-ILM hemorrhage became darker and more localized. (d)–(g) OCT images showing progression of the sub-ILM hemorrhage: (d) Extensive sub-ILM fluid-filled cavity before the injection obscuring retinal details; (e) significant absorption at 1 week postinjection; (f) reduced ILM elevation 3 weeks postinjection with residual medium to low reflective material; (g) at the latest follow-up, the right eye showed an epiretinal membrane and small sub-ILM cavities, while the left eye exhibited a nearly normal macular contour.
-
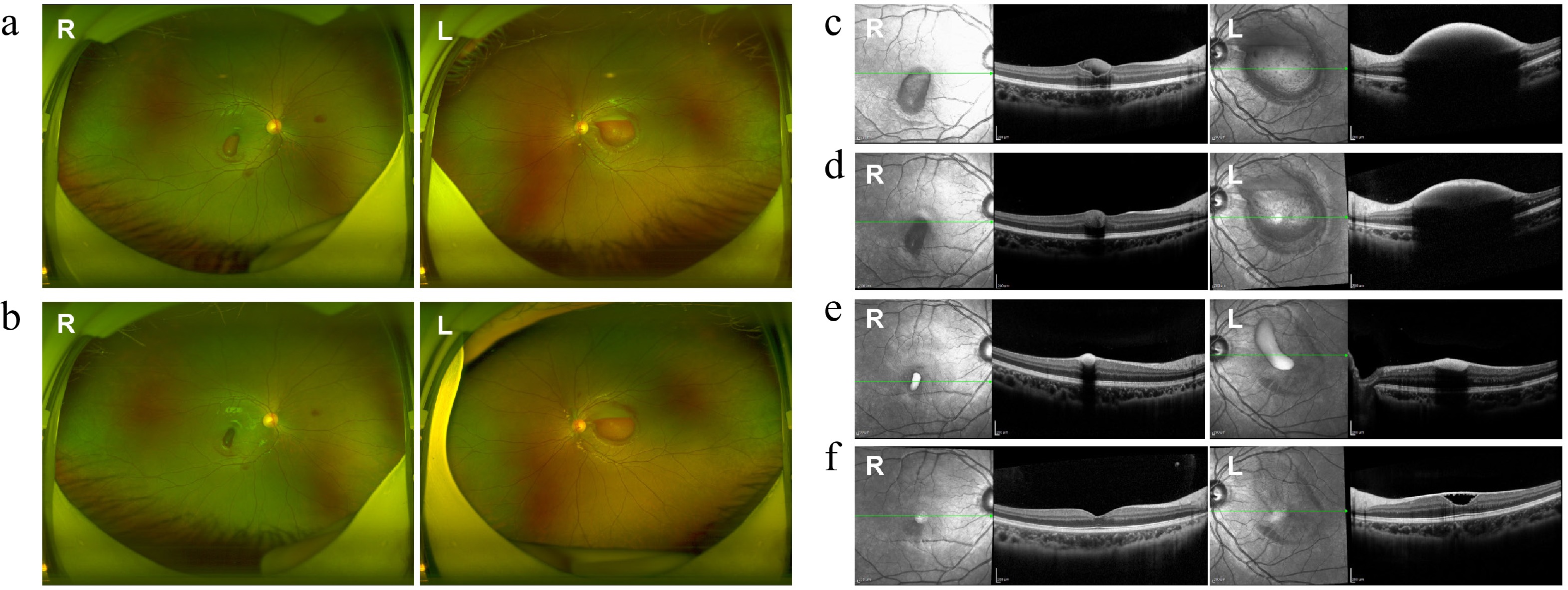
Figure 3.
Fundus and OCT images at various stages of treatment in Case 3. (a), (c) Pre-injection fundus and OCT images showed preretinal hemorrhage in both eyes. (b), (d) Fundus and OCT images three days postinjection showed significant absorption of hemorrhage in both eyes. (e), (f) OCT images taken 3 and 6 months after the injection showed gradual absorption of the sub-ILM hemorrhage. (f) A sub-ILM cavity was still observed in the left eye at the latest follow-up, while the macular structure of the right eye appeared normal.
-
Characteristic Case 1 Case 2 Case 3 Age (years) 13 13 10 Sex Male Female Male Diagnosis AML AML AML Affected eye Left eye Both eyes Both eyes Presentation Sudden vision decline Blurry vision Blurry vision BCVA at presentation (RE/LE) 20/20, hand movement 20/200, 20/400 20/20, finger counting Fundus findings Sub-ILM hemorrhage, diffuse hemorrhage Preretinal and intraretinal hemorrhages,
optic disc edema, cotton wool spots,
hard exudates, Roth spotsSub-ILM hemorrhage Initial IOP (RE/LE, mmHg) 12/11 14/13 16/16 Hemoglobin (g/L) 78, 77* 70 85 Leukocyte count (×109/L) 2.72, 2.51* 2.20 2.47 Platelet count (×109/L) 109, 121* 110 273 Treatment 2 tPA injections (LE) 1 tPA injection (BE) 1 tPA injection (LE) IOP the second day after injection (RE/LE, mmHg) 16/15, 17/16* 13/15 17/16 Follow-up period (months) 5 5 6 BCVA at the latest follow-up (RE/LE) 20/20, 20/20 20/30, 20/25 20/20, 20/25 Recurrence Yes No No Recurrence time 8 weeks – – Complications No No No AML, acute myelocytic leukemia; BCVA, best-corrected visual acuity; ILM, internal limiting membrane; IOP, intraocular pressure; LE, left eye; BE, both eyes; RE, right eye. *, relating to the second injection. Table 1.
Patient characteristics.
Figures
(3)
Tables
(1)