-
Ophthalmic complications, particularly leukemic retinopathy, affect up to 35% of acute leukemia patients, with acute myeloid leukemia (AML), posing a higher ocular risk than lymphoblastic subtypes[1]. Among these, subinternal limiting membrane (sub-ILM) hemorrhage is a sight-threatening complication, severely impairing visual development in children[2].
Traditional management with pars plana vitrectomy (PPV) under general anesthesia carries substantial risks for pediatric AML patients, including perioperative complications and systemic burdens[3]. Minimally invasive alternatives are urgently needed to address these limitations.
Tissue plasminogen activator (tPA), a fibrinolytic agent, has shown promise in resolving intraocular hemorrhages by dissolving fibrin clots[4]. Leveraging its mechanism, we explored injections of intravitreal tPA under topical anesthesia as a novel strategy for sub-ILM hemorrhage in pediatric AML. To our knowledge, this is the first report of this approach in this population, aiming to reduce systemic risks while achieving targeted hemorrhage clearance.
-
This retrospective case series included three pediatric patients with AML and sub-ILM hemorrhage treated at Hunan Children's Hospital between January and December 2024. All patients underwent a comprehensive ophthalmic evaluation, including best-corrected visual acuity (BCVA), intraocular pressure, a slit-lamp examination, spectral domain optical coherence tomography (SD-OCT), and fundus photography, to confirm sub-ILM hemorrhage. Under topical anesthesia (0.4% oxybuprocaine), each received 1–2 intravitreal injections of tPA (20 μg in 0.1 mL of a sterile solution[5]) following aseptic protocols. Postprocedure care included supine positioning for 6 hours and prophylactic moxifloxacin eye drops (3 days preinjection, 5 days postinjection). Outcomes were assessed via serial BCVA and SD-OCT to monitor resolution of the hemorrhage and safety.
The study was approved by the Institutional Ethics Committee of Hunan Children's Hospital (No. HCHLL-2024-204) on 28 May 2024, with informed consent waived because of its retrospective nature; the study is consistent with the principles of the Helsinki Declaration.
-
Three pediatric AML patients (two males, one female; mean age: 12 years) with four affected eyes diagnosed with sub-ILM hemorrhage were included. BCVA in all affected eyes was ≤ 20/200 (two eyes with finger counting, two eyes with hand motion), wherease the unaffected eyes maintained normal vision (20/20). Fundus examination and SD-OCT confirmed a preretinal hemorrhage with ILM elevation in all affected eyes (detailed clinical data are summarized in Table 1). All four eyes received one or two intravitreal tPA injections (20 μg in 0.1 mL of a sterile solution) under topical anesthesia. Within 1 month posttreatment, the BCVA of all treated eyes improved to ≥ 20/30: one patient (a 13-year-old male) had a recurrence of hemorrhage in the left eye at 8 weeks (BCVA declined to 20/400), which achieved sustained resolution (BCVA recovered to 20/20 at 10 weeks) after a second injection (Fig. 1); a 13-year-old female with bilateral sub-ILM hemorrhage and extensive leukemic retinopathy showed an improvement in BCVA to 20/30 (right eye) and 20/25 (left eye) at final follow-up (Fig. 2); a 10-year-old male with left eye hemorrhage had a stepwise improvement in BCVA (20/100 at 1 week, 20/25 at 3 months, and stable at 20/25 at 6 months) (Fig. 3). SD-OCT confirmed complete absorption of the sub-ILM hemorrhage in three eyes and near-complete resolution with restored foveal contour in the recurrent eye; three eyes showed transient sub-ILM cavity formation or ILM elevation, which did not affect visual recovery. During a median 5-month follow-up, no systemic adverse events or ophthalmic complications (e.g., endophthalmitis, retinal detachment, or intraocular pressure fluctuations) were observed, and all eyes maintained a sustained improvement in BCVA.
Table 1. Patient characteristics.
Characteristic Case 1 Case 2 Case 3 Age (years) 13 13 10 Sex Male Female Male Diagnosis AML AML AML Affected eye Left eye Both eyes Both eyes Presentation Sudden vision decline Blurry vision Blurry vision BCVA at presentation (RE/LE) 20/20, hand movement 20/200, 20/400 20/20, finger counting Fundus findings Sub-ILM hemorrhage, diffuse hemorrhage Preretinal and intraretinal hemorrhages,
optic disc edema, cotton wool spots,
hard exudates, Roth spotsSub-ILM hemorrhage Initial IOP (RE/LE, mmHg) 12/11 14/13 16/16 Hemoglobin (g/L) 78, 77* 70 85 Leukocyte count (×109/L) 2.72, 2.51* 2.20 2.47 Platelet count (×109/L) 109, 121* 110 273 Treatment 2 tPA injections (LE) 1 tPA injection (BE) 1 tPA injection (LE) IOP the second day after injection (RE/LE, mmHg) 16/15, 17/16* 13/15 17/16 Follow-up period (months) 5 5 6 BCVA at the latest follow-up (RE/LE) 20/20, 20/20 20/30, 20/25 20/20, 20/25 Recurrence Yes No No Recurrence time 8 weeks – – Complications No No No AML, acute myelocytic leukemia; BCVA, best-corrected visual acuity; ILM, internal limiting membrane; IOP, intraocular pressure; LE, left eye; BE, both eyes; RE, right eye. *, relating to the second injection. 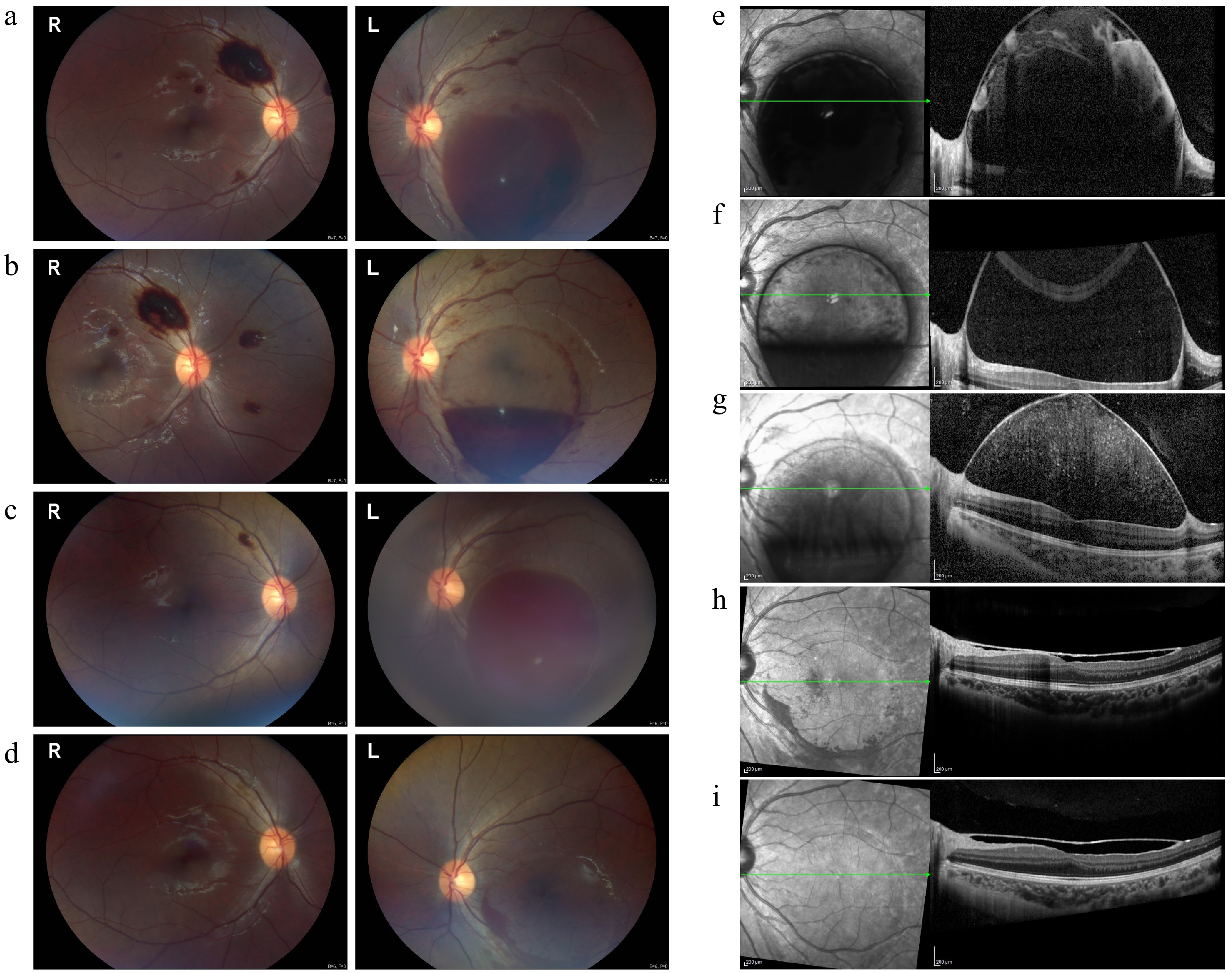
Figure 1.
Fundus and OCT images at various stages of treatment in Case 1. (a) Preinjection fundus image showing extensive preretinal hemorrhage in both eyes, with a larger sub-ILM hemorrhage affecting the macula in the left eye. (b) Two days after the first injection, partial absorption of the sub-ILM hemorrhage was observed in the left eye. (c) Eight weeks later, recurrent macular hemorrhage appeared in the left eye, while the right eye's hemorrhage resolved. (d) Two weeks after the second injection, near-complete resolution of the left eye's hemorrhage was observed, with a small residual hemorrhage remaining. (e)–(i) OCT images showing the progression of sub-ILM hemorrhage before and after the injections: (e) Sub-ILM fluid-filled cavity before the injections; (f) partial resolution two days after the first injection; (g) recurrence of hemorrhage at eight weeks; (h) significant absorption two weeks after the second injection; (i) complete resolution at two months, with restored macular foveal contour and elevated ILM.
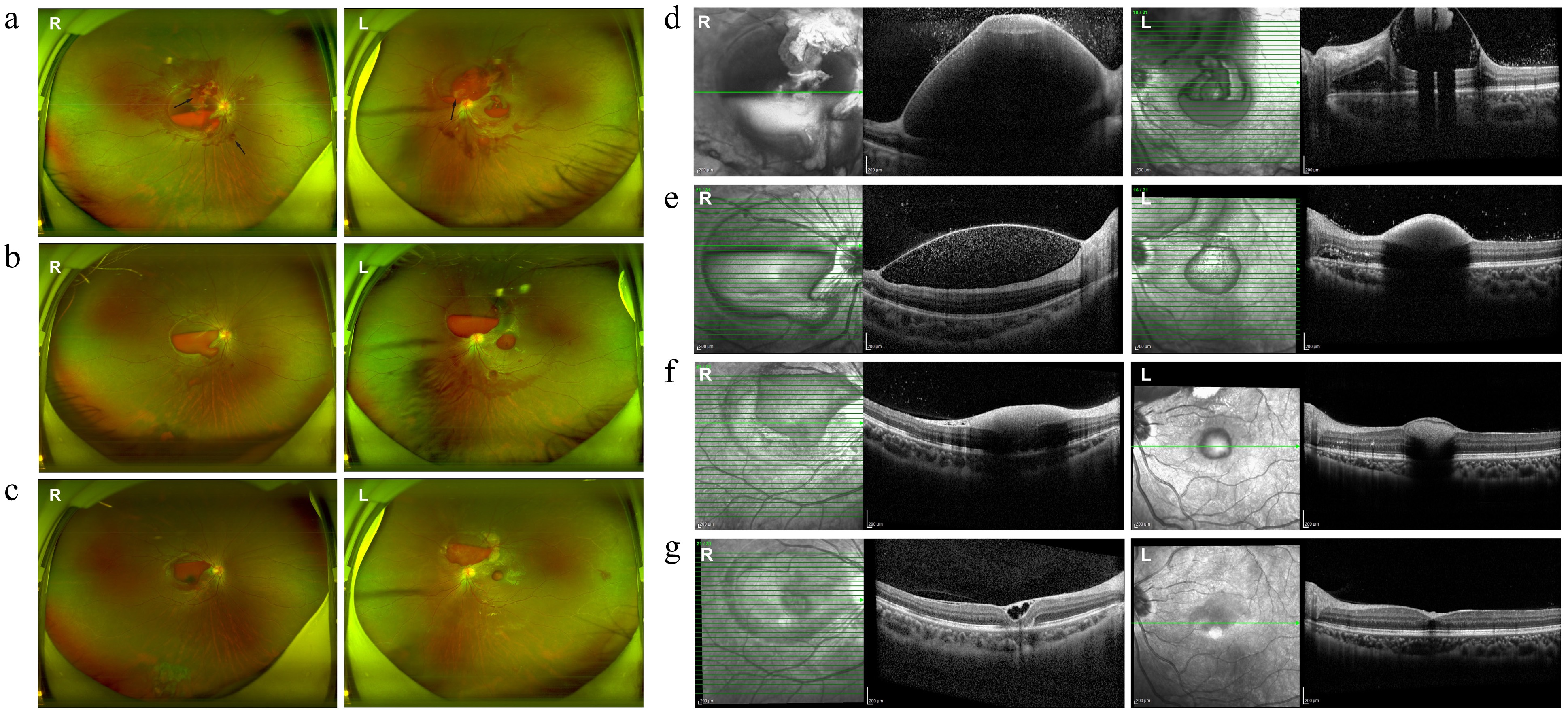
Figure 2.
Fundus and OCT images at various stages of treatment in Case 2. (a) Preinjection fundus image showing bilateral preretinal hemorrhage, hard exudates, blurred optic disc margins, diffuse vascular tortuosity, and Roth spots (black arrows). (b) One week post-injection, partial absorption of sub-ILM hemorrhage was noted, along with the resolution of hard exudates and improved optic disc clarity. (c) Three weeks postinjection, the sub-ILM hemorrhage became darker and more localized. (d)–(g) OCT images showing progression of the sub-ILM hemorrhage: (d) Extensive sub-ILM fluid-filled cavity before the injection obscuring retinal details; (e) significant absorption at 1 week postinjection; (f) reduced ILM elevation 3 weeks postinjection with residual medium to low reflective material; (g) at the latest follow-up, the right eye showed an epiretinal membrane and small sub-ILM cavities, while the left eye exhibited a nearly normal macular contour.
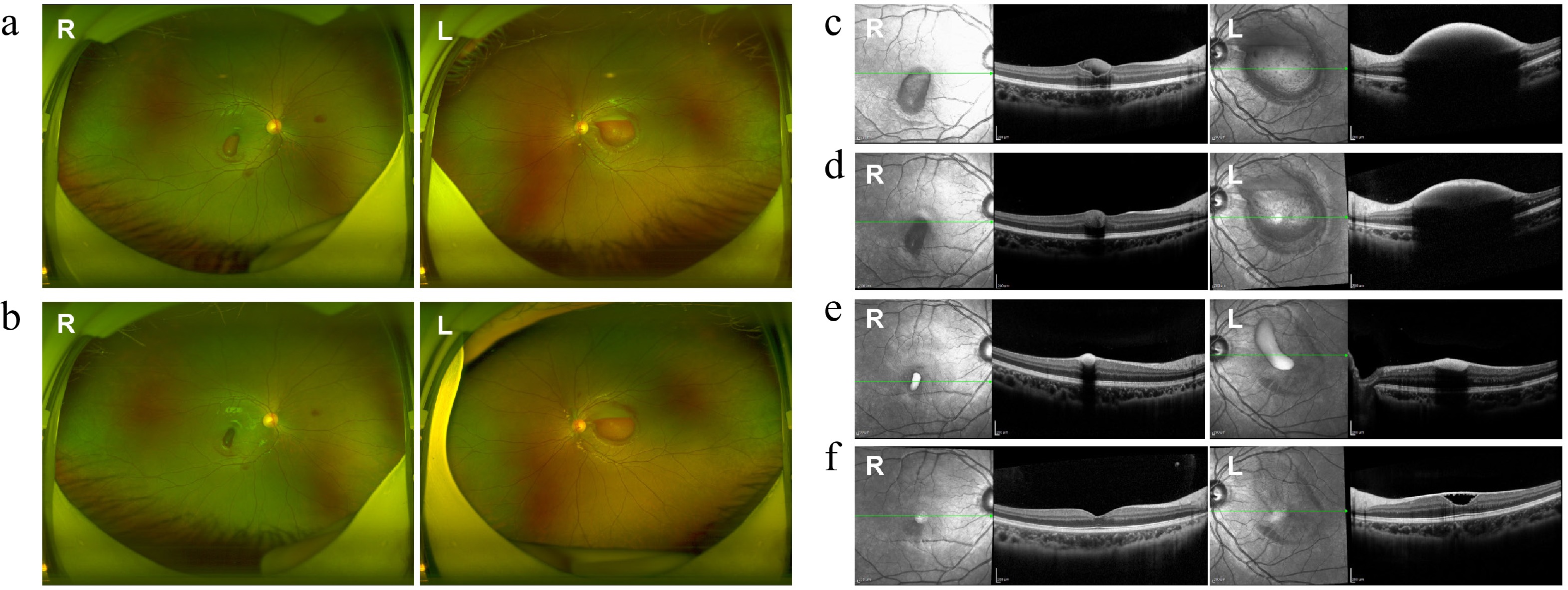
Figure 3.
Fundus and OCT images at various stages of treatment in Case 3. (a), (c) Pre-injection fundus and OCT images showed preretinal hemorrhage in both eyes. (b), (d) Fundus and OCT images three days postinjection showed significant absorption of hemorrhage in both eyes. (e), (f) OCT images taken 3 and 6 months after the injection showed gradual absorption of the sub-ILM hemorrhage. (f) A sub-ILM cavity was still observed in the left eye at the latest follow-up, while the macular structure of the right eye appeared normal.
-
Sub-ILM hemorrhage is a common yet challenging complication in pediatric patients with AML, which is characterized by slow clearance and a high risk of inducing macular damage[2]. If untreated, prolonged hemorrhage can result in permanent vision loss through mechanisms such as iron toxicity, photoreceptor disruption, and epiretinal membrane formation. Consequently, early surgical intervention is worth considering in all cases of sub-ILM hemorrhage, including Valsalva retinopathy and Terson syndrome[6]. Despite the availability of treatment options, current approaches are often invasive and require general anesthesia and stable systemic conditions for children. This study aimed to address this gap by exploring the feasibility and efficacy of intravitreal tPA injections as a minimally invasive alternative.
Severe preretinal macular hemorrhage is traditionally treated with PPV or neodymium-doped yttrium aluminum garnet (Nd : YAG) laser hyaloidotomy, either alone or in combination[3]. PPV is effective, but it requires general anesthesia, which poses significant risks in pediatric patients with AML. These risks include systemic complications and ocular issues such as cataracts, secondary glaucoma, and endophthalmitis[7]. Surgery is often delayed for several months until the patient is deemed to be fit for anesthesia, so retinal photoreceptor cell apoptosis may have already occurred[5]. Literature and clinical experience indicate that when PPV is performed at ≥ 4 months posthemorrhage, BCVA typically improves suboptimally (20/200)[8]. Nd:YAG laser hyaloidotomy, though less invasive, depends on the patient's cooperation, limiting its use in younger children, and carries risks such as epiretinal membrane formation and retinal detachment.
Anti-vascular endothelial growth factor (VEGF) therapy has been explored in patients with myeloid leukemia for neovascular eye diseases, but its use is contraindicated in those with bleeding tendencies, rendering it unsuitable for the pediatric patients in this study. Leukemia-related retinal hemorrhage is a multifactorial process driven by thrombocytopenia, leukostasis, increased blood viscosity, disruption of the blood-retinal barrier, and systemic coagulation abnormalities (not solely VEGF-driven), so the primary treatment goal for these patients is to rapidly clear intraocular hemorrhage, restore visual function, and prevent long-term retinal toxicity and visual impairment[9].
In this context, intravitreal tPA offers a targeted and effective solution by dissolving fibrin clots and facilitating the rapid clearance of sub-ILM and subretinal hemorrhage. Compared to PPV or laser treatment, tPA reduces the risk of complications such as secondary glaucoma, cataract formation, and hyperopia, resulting in better long-term visual outcomes. Additionally, the use of topical anesthesia instead of general anesthesia is particularly advantageous for pediatric patients with systemic vulnerabilities, reducing systemic risks and alleviating financial and psychological burdens on families.
However, this study has limitations. First, larger sample sizes and long-term follow-up are needed to validate the efficacy and safety of tPA injection compared with PPV. To address this, we will strengthen collaboration with our hospital's Hematology-Oncology Department. A joint monitoring mechanism for pediatric AML patients will help proactively enroll those with sub-ILM hemorrhage, thereby improving case collection, standardizing treatment, and extending follow-up. Secondly, a sub-ILM cavity or elevated ILM was observed in three treated eyes. One possible explanation for this finding is the proliferation of cells on the retinal surface of the ILM. This is indicated by the presence of markers such as GFAP, cytokeratin 7, and CD688. Although no patients exhibited symptoms of metamorphopsia or vision decline, long-term follow-up remains essential due to the potential role of the ILM as a scaffold for epiretinal membrane formation[10]. Furthermore, the therapeutic potential of intravitreal tPA for sub-ILM hemorrhage due to other causes, such as Valsalva retinopathy and Terson syndrome, can be investigated to enhance the understanding of its potential applications.
-
In conclusion, intravitreal tPA injection can be a safe, effective, and minimally invasive treatment option for sub-ILM hemorrhage in pediatric patients with AML. This approach offers promising advantages, particularly in patients with compromised systemic health. Further research is needed to confirm its long-term efficacy and optimize the management of the associated complications.
This work was supported by the Natural Science Foundation of Hunan Province of China to Yu Tian (Grant No. 2022JJ80005) and the Hunan Disabled Rehabilitation Association Foundation of China to Yu Tian (Grant No. 2023XK0224).
-
This study was conducted in accordance with the principles of the Declaration of Helsinki. The research protocol was reviewed and approved by the Institutional Ethics Committee of Hunan Children's Hospital, Changsha, China (No. HCHLL-2024-204, approval date: 28 May 2024. Informed consent was waived for the following reasons: (1) The study utilized de-identified retrospective medical records, and no individual patient identifiers (e.g., names, medical record numbers, or facial features in images) were included; (2) the retrospective analysis posed no additional risks to patients; (3) waiving consent would not compromise the rights or welfare of the patients. This waiver was approved by the above ethics committee and is consistent with the principles of the Helsinki Declaration.
-
The authors confirm their contributions to the paper as follows: study conception and design: Tian Y, Wang A; data collection: Wang A, Qiao J; analysis and interpretation of results: Wang A, Li W, Wang C; draft manuscript preparation: Luo Y. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2026 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang A, Qiao J, Wang C, Li W, Luo Y, et al. 2026. Intravitreal tissue plasminogen activator for subinternal limiting membrane hemorrhage in pediatric acute myelocytic leukemia: a novel therapeutic strategy. Visual Neuroscience 43: e003 doi: 10.48130/vns-0025-0026
Intravitreal tissue plasminogen activator for subinternal limiting membrane hemorrhage in pediatric acute myelocytic leukemia: a novel therapeutic strategy
- Received: 22 June 2025
- Revised: 30 August 2025
- Accepted: 31 October 2025
- Published online: 23 January 2026













