-
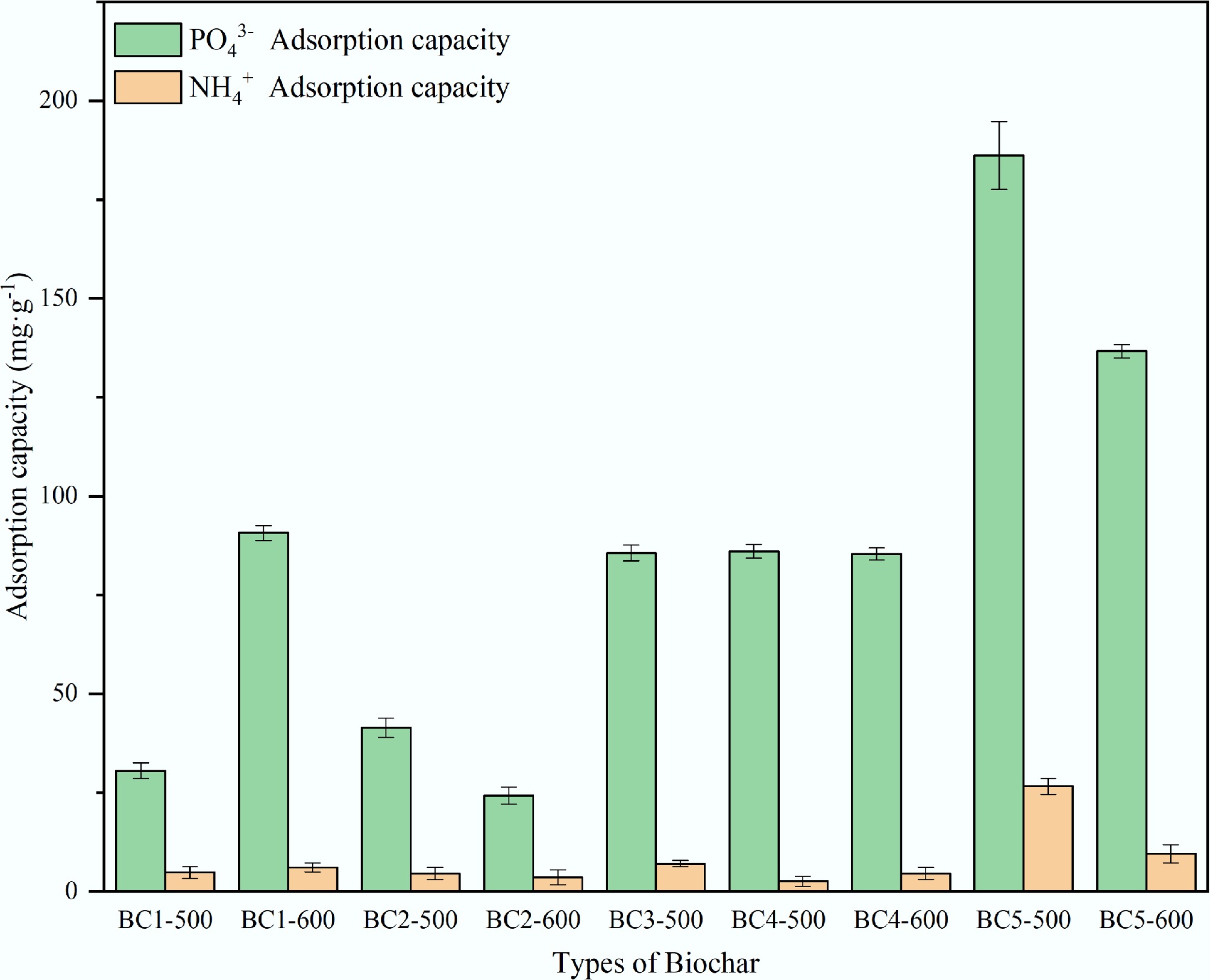
Figure 1.
Biochar screened for adsorption of NH4+ and PO43−.
-
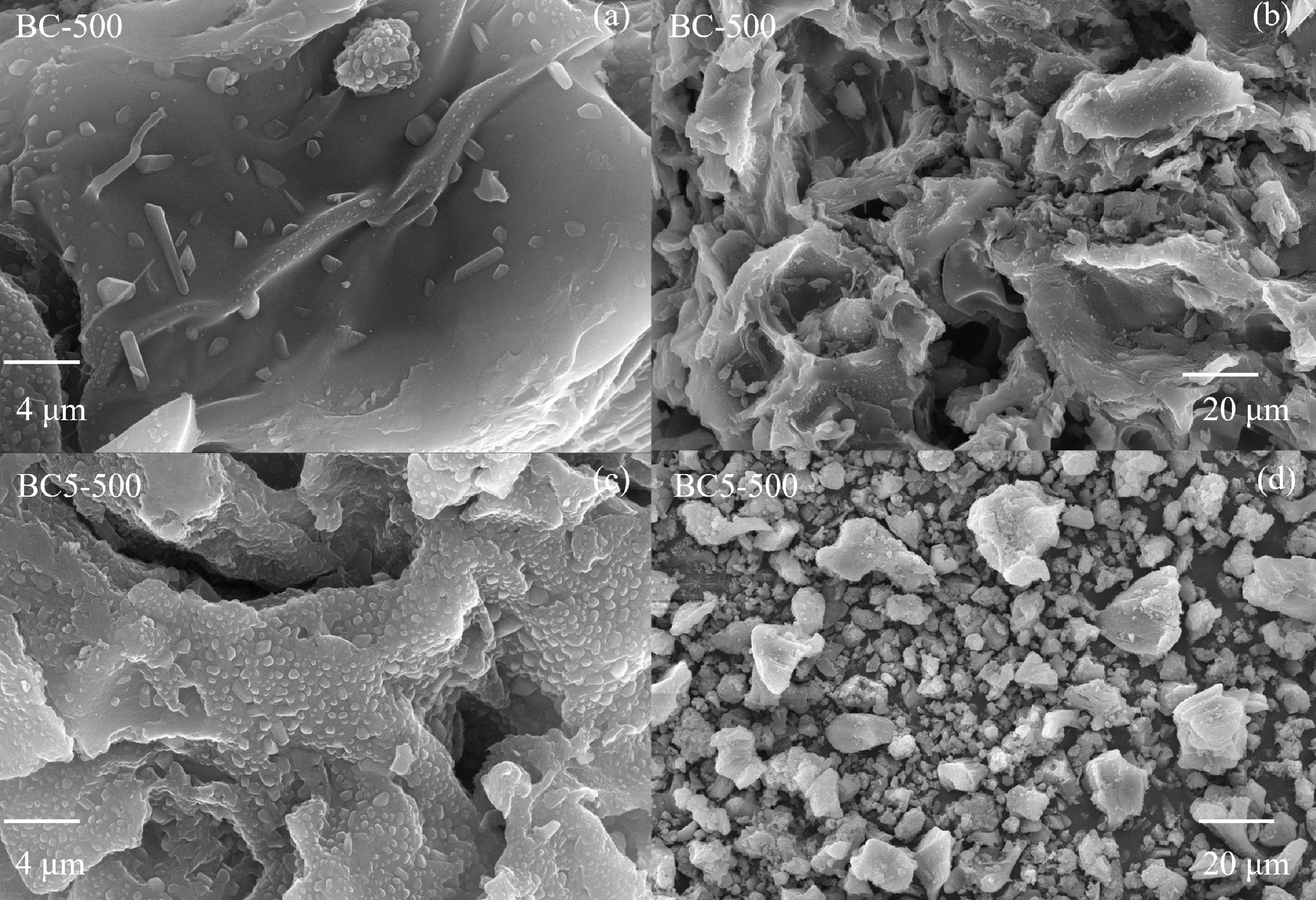
Figure 2.
SEM images of (a), (b) the biochar (BC-500), and (c), (d) the Ca(OH)2-modified biochar (BC5-500).
-
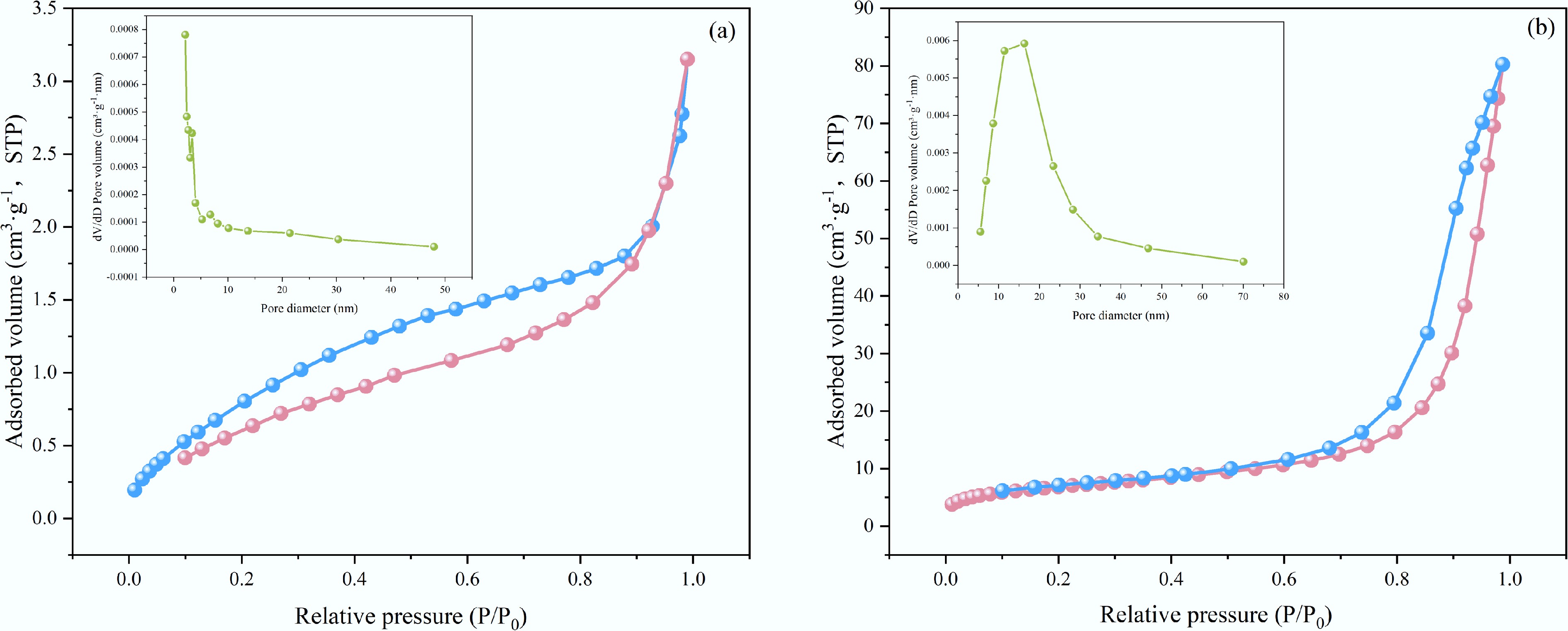
Figure 3.
Adsorption-desorption isotherms and pore size distribution of (a) BC-500, and (b) C5-500.
-
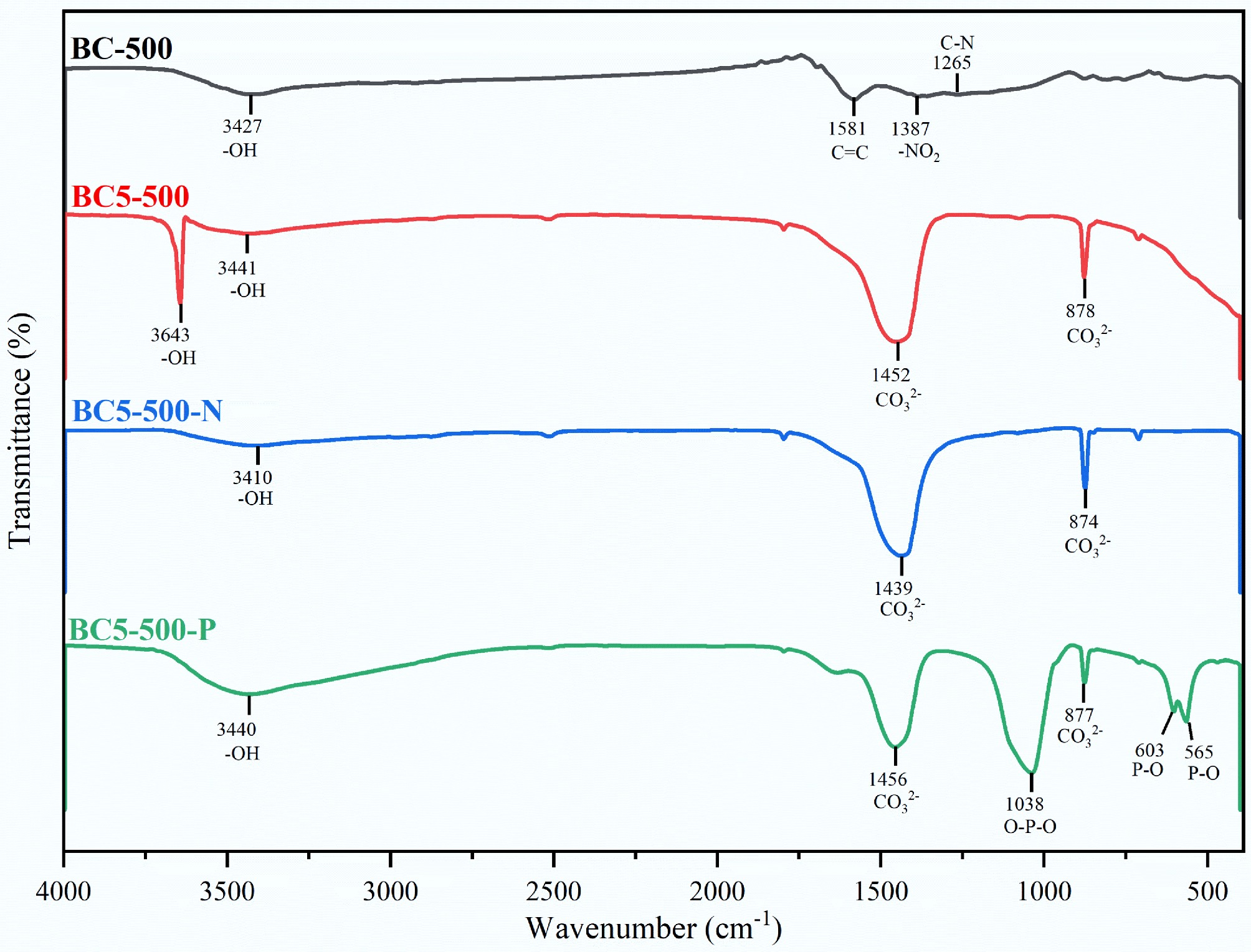
Figure 4.
FT-IR spectra of BC-500, BC5-500, and their derivatives after adsorption of NH4+ (BC5-500-N) and PO43− (BC5-500-P).
-
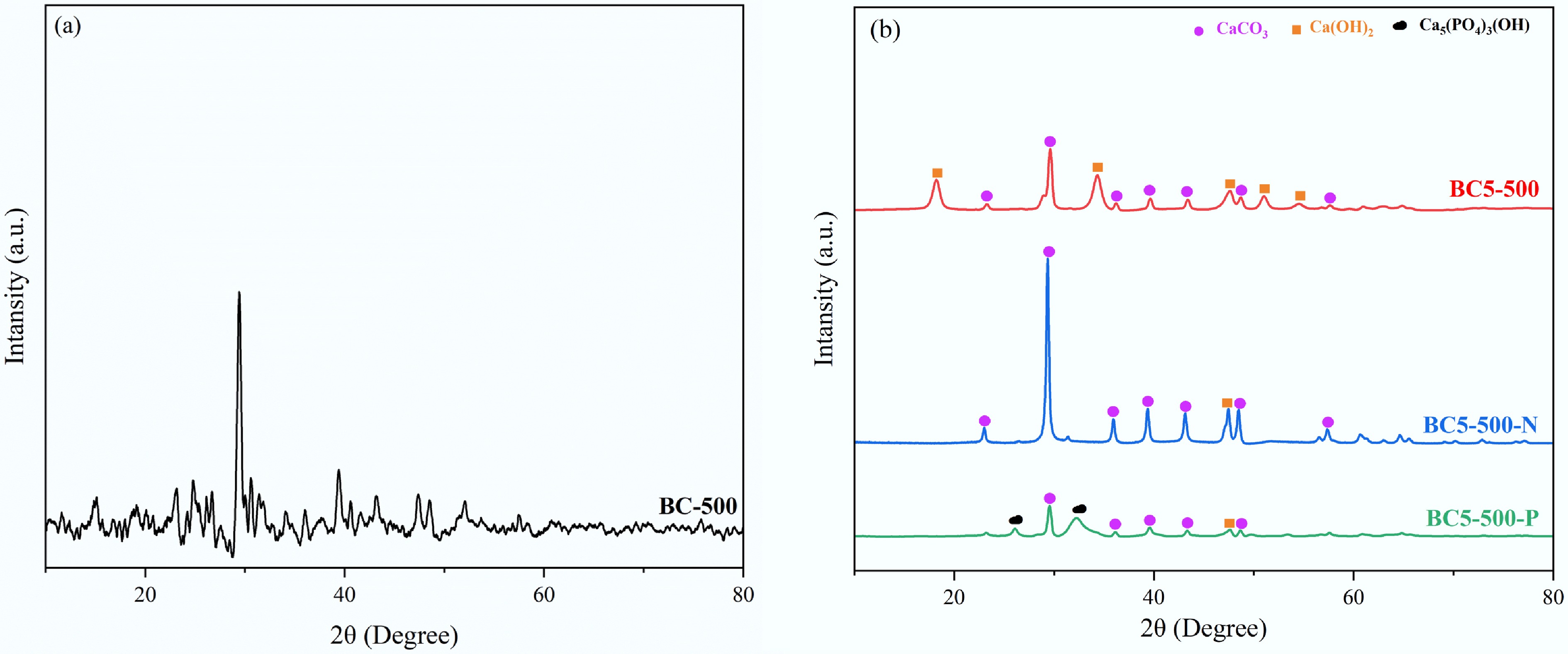
Figure 5.
XRD patterns of (a) BC-500, and (b) BC5-500 before and after adsorption (BC5-500, BC5-500-N, and BC5-500-P).
-
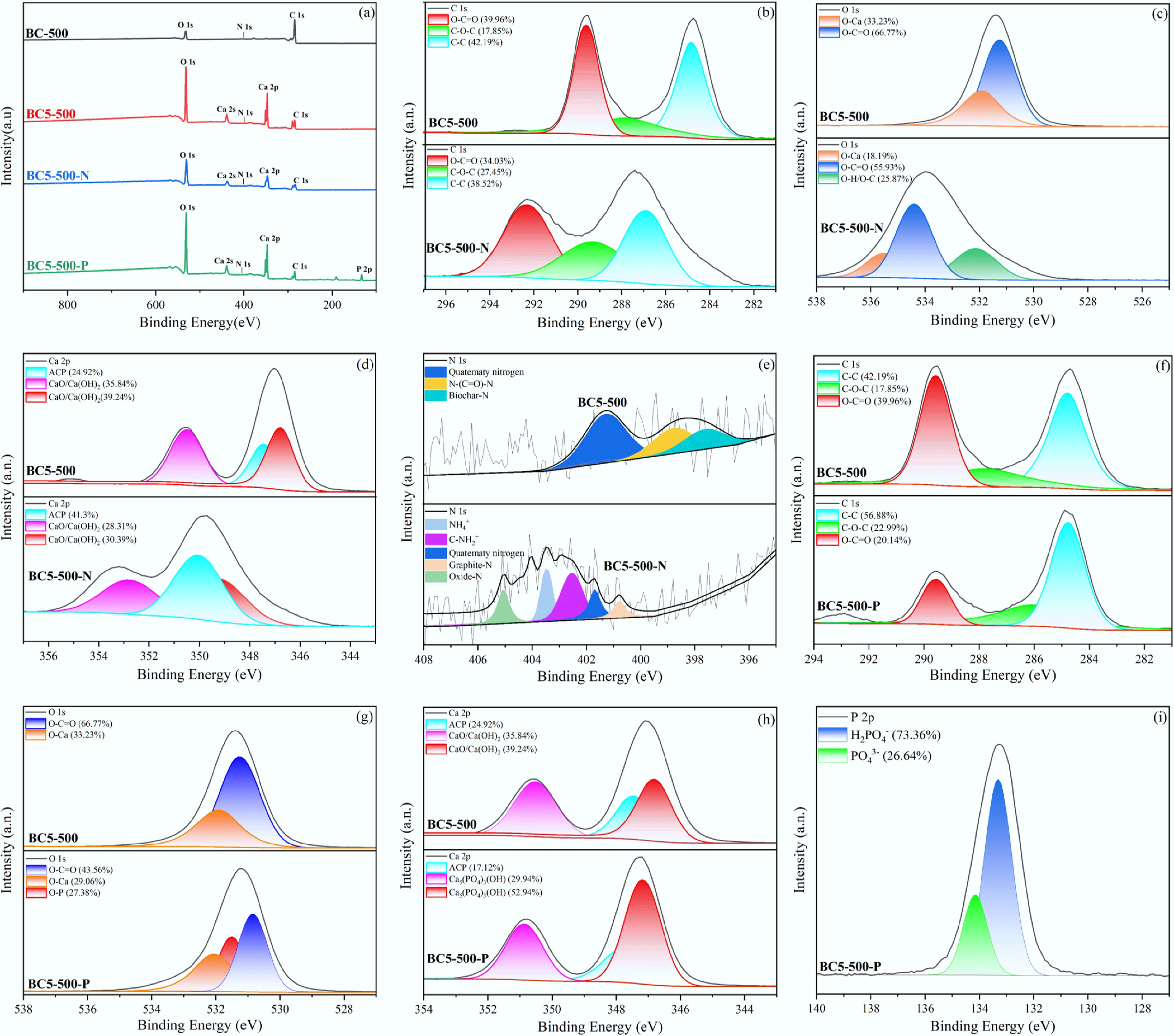
Figure 6.
XPS analysis of BC-500 and BC5-500 before and after adsorption: (a) survey spectra, (b)–(e) high-resolution C 1s, O 1s, Ca 2p, and N 1s spectra of BC5-500 and BC5-500-N, (f)–(h) high-resolution C 1s, O 1s, and Ca 2p spectra of BC5-500 and BC5-500-P, and (i) high-resolution P 2p spectrum of BC5-500-P.
-
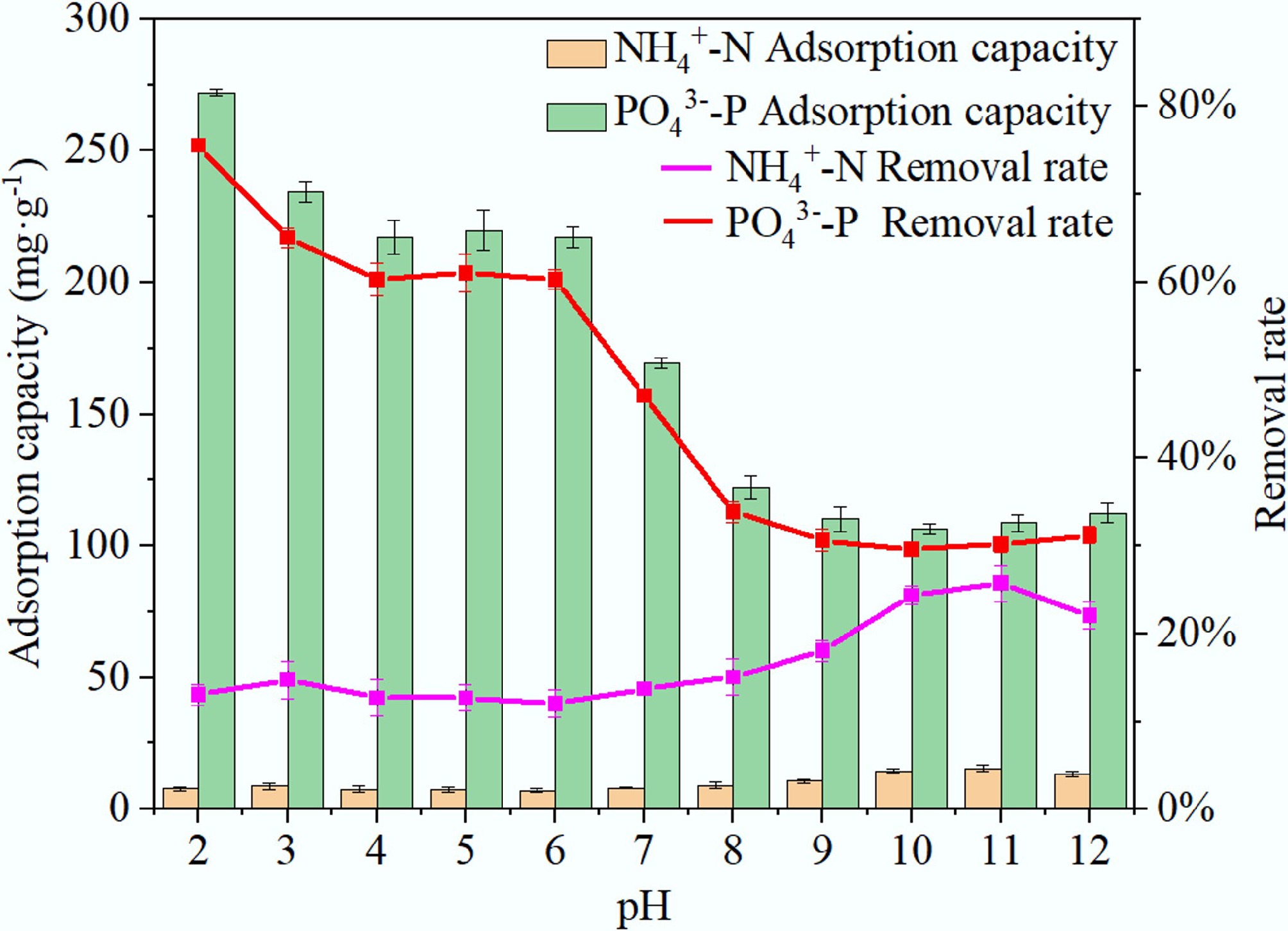
Figure 7.
Adsorption of NH4+ and PO43– on BC5-500 as a function of solution pH.
-
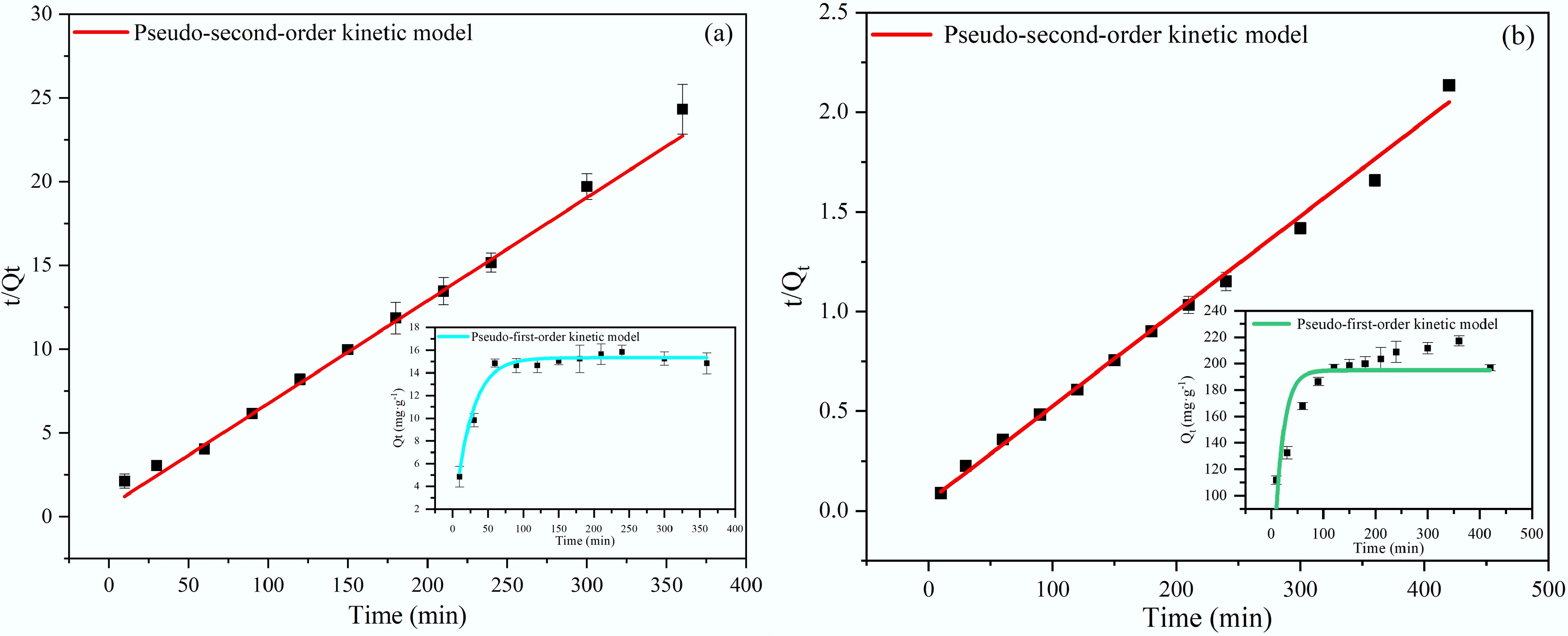
Figure 8.
(a) Adsorption kinetics of NH4+ on BC5-500. (b) Adsorption kinetics of PO43− on BC5-500.
-
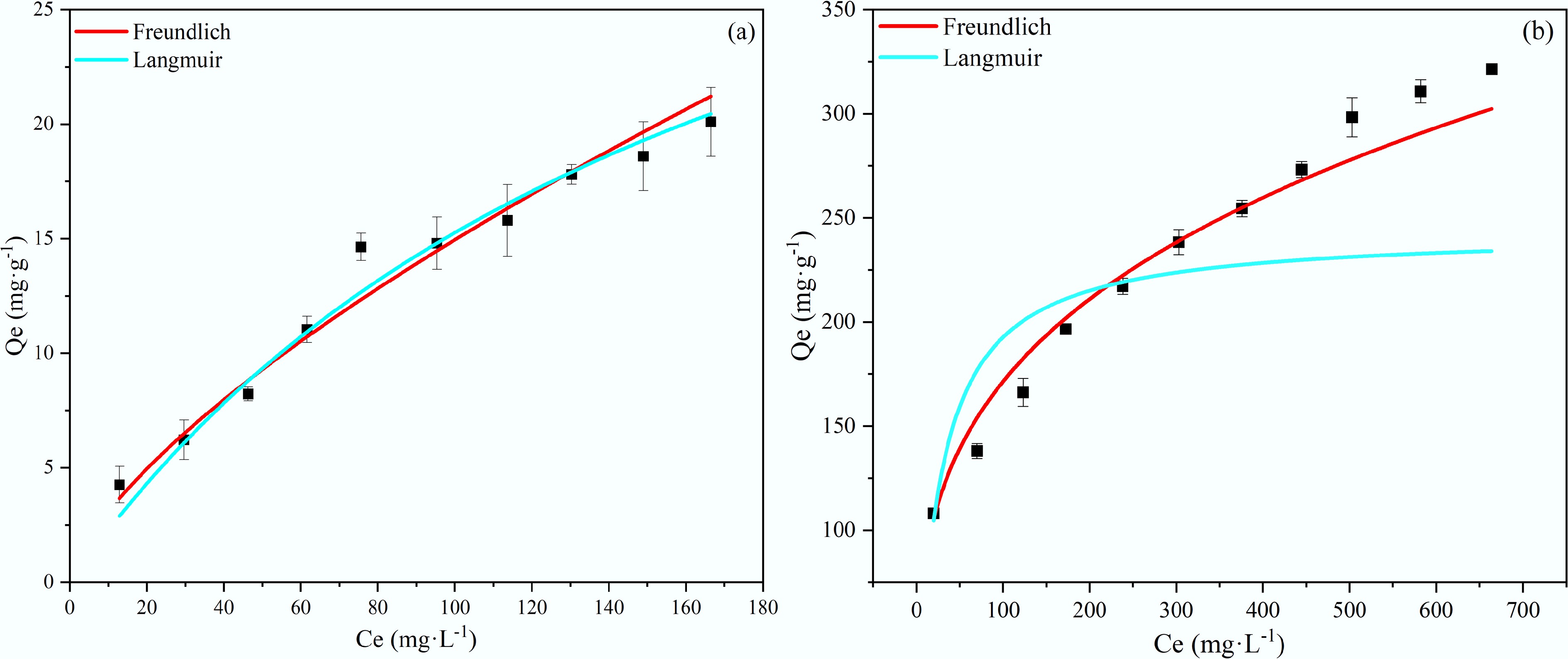
Figure 9.
Adsorption isotherms of (a) NH4+, and (b) PO43− on BC5-500.
-
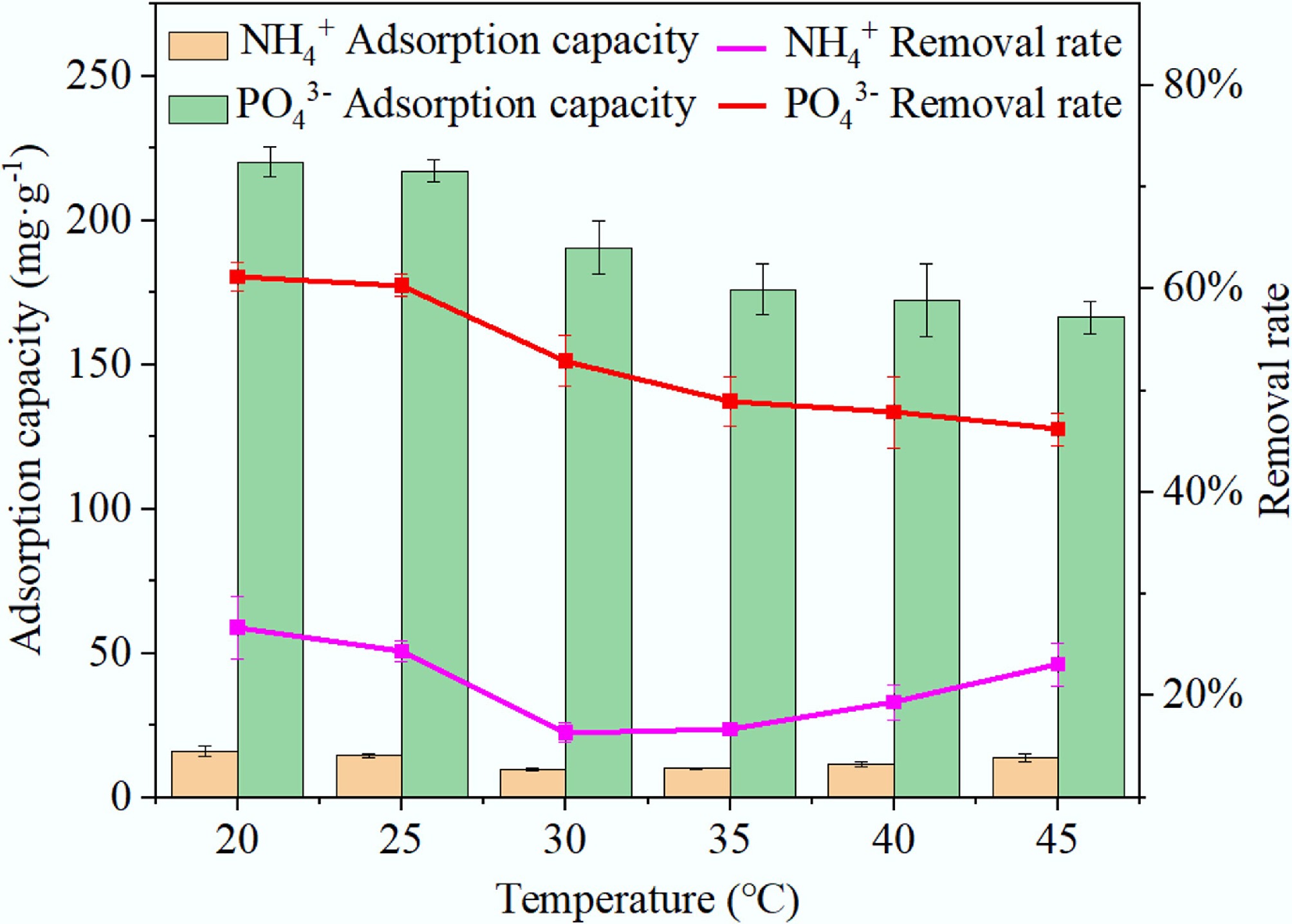
Figure 10.
Influence of reaction temperature on the adsorption capacity of BC5-500 for NH4+ and PO43–.
-
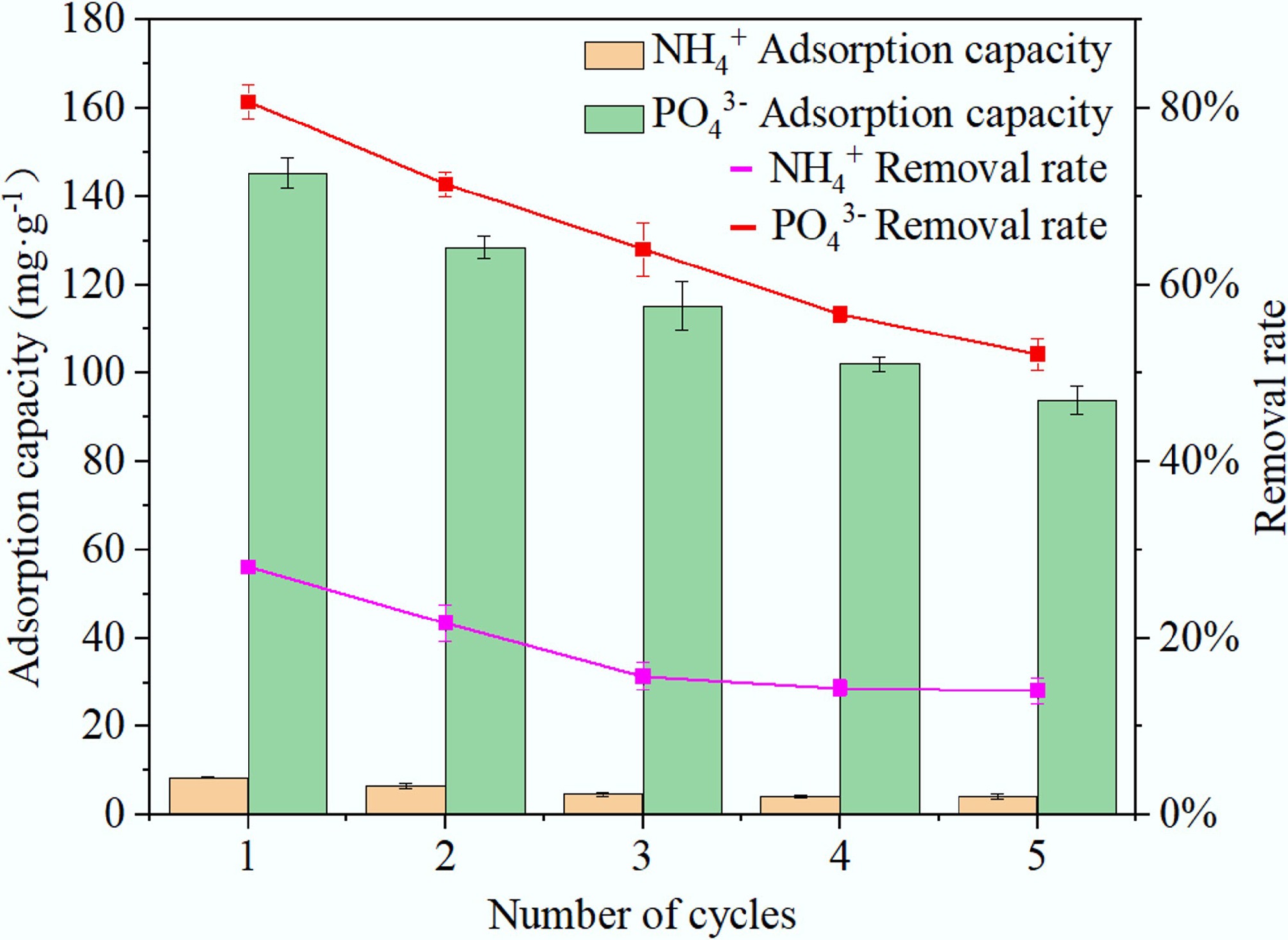
Figure 11.
Reusability of BC5-500 for the adsorption of NH4+ and PO43– over five consecutive cycles.
-
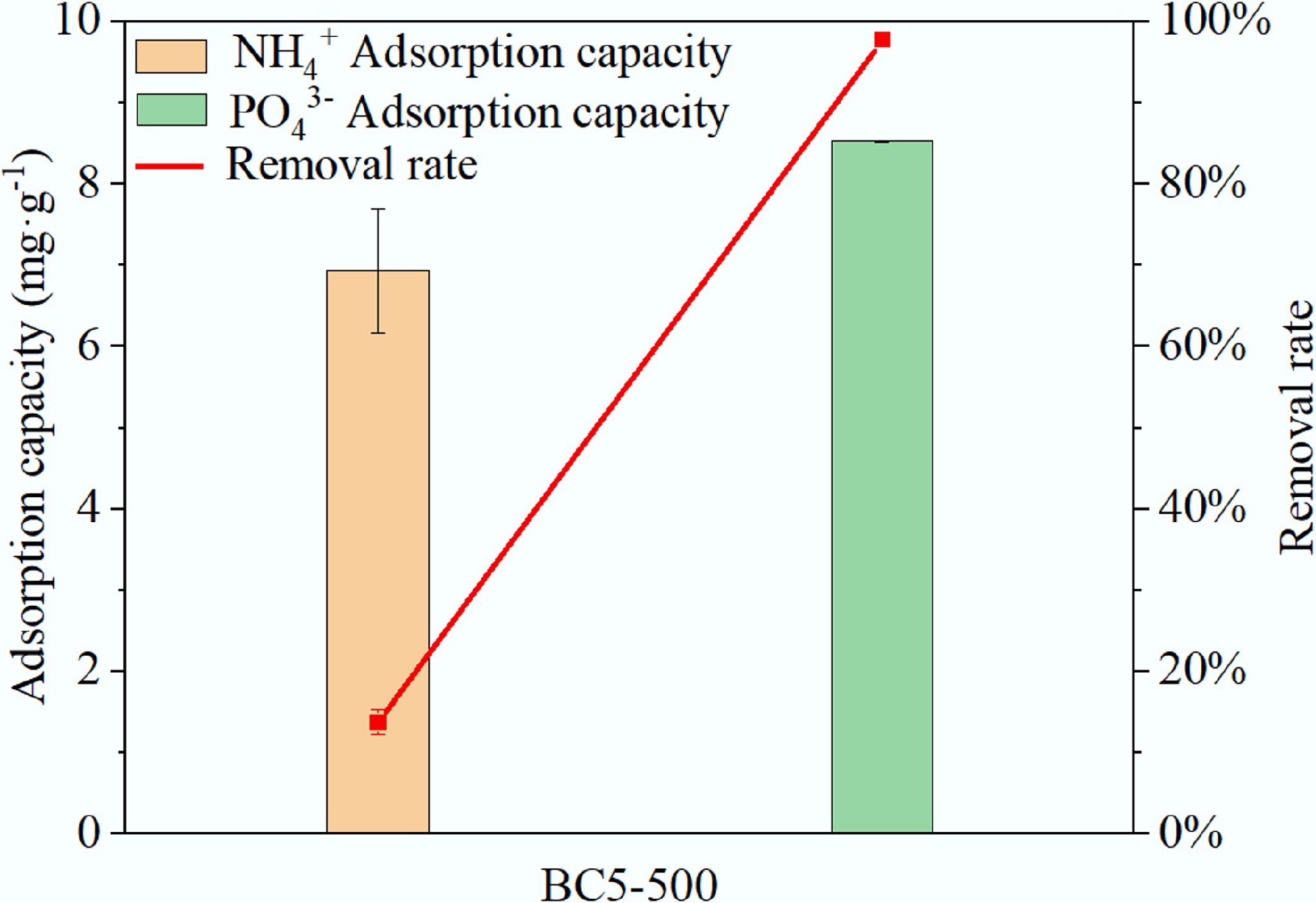
Figure 12.
Removal of NH4+ and PO43– from actual swine wastewater by BC5-500.
-
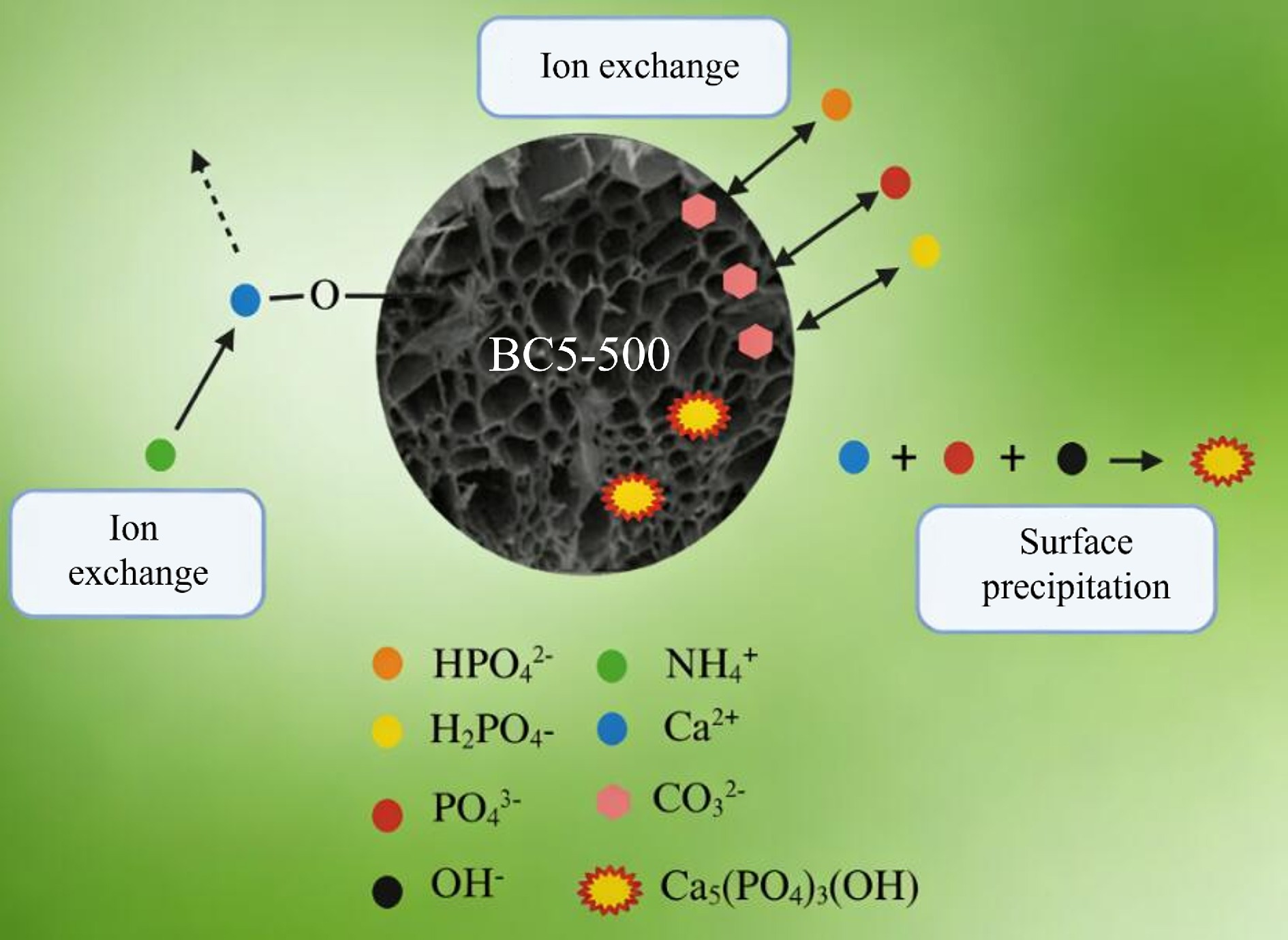
Figure 13.
Schematic diagram of the adsorption mechanism of NH4+ and PO43– on BC5-500.
-
Biochar name Adsorbate (mg·g−1) Reaction kinetics pH range Ref. NH4+ PO43− BS600 114.64 31.05 Pseudo-second-order kinetics 8.5–9.7 [22] MgB 15.22 − Pseudo-first-order kinetics 6.0–8.0 [23] SB > 28.2 > 120 Pseudo-second-order kinetics Unadjusted pH [24] MgB-A 37.72 73.29 Pseudo-second-order kinetic 4.0–8.0 [25] Table 1.
Comparison among modified biochars for NH4+ and PO43− adsorption
-
Biochar name SBET (m2·g−1) Total pore volume (cm3·g−1) Average pore size (nm) BC-500 3.71 0.0048 5.24 BC5-500 4.29 0.0079 20.01 Table 2.
Physical properties of BC-500 and BC5-500
-
Biochar name Adsorbate Pseudo-first-order kinetic model Pseudo-second-order kinetic model Qe K1 R2 Qe K2 R2 BC5-500 NH4+ 15.33 0.04148 0.9371 16.27 0.00012 0.9863 PO43– 194.94 0.06142 0.7500 209.65 0.00047 0.9962 Table 3.
Kinetic parameters for NH4+ and PO43− adsorption onto BC5-500
-
Biochar name Adsorbate Langmuir Freundlich Qm KL R2 n KF R2 1/n BC5-500 NH4+ 41.87 0.00574 0.967 1.145 0.632 0.962 0.873 PO43– 243.33 0.0380 0.868 3.335 43.077 0.990 0.300 Table 4.
Parameters of the adsorption isotherms for NH4+ and PO43– onto BC5-500
-
Biochar
nameAdsorbate Temperature
(°C)K0 ΔG0 ΔH0 ΔS0 BC5-500 NH4+ 293 0.219 3.700 45.645 167.890 298 0.195 4.067 303 0.118 5.390 308 0.121 5.417 313 0.144 5.036 318 0.180 4.535 PO43− 293 0.945 0.1367 –20.896 –71.714 298 0.912 0.228 303 0.675 0.99 308 0.575 1.416 313 0.551 1.551 318 0.517 1.751 Table 5.
Thermodynamic parameters for the adsorption of NH4+ and PO43– onto BC5-500
Figures
(13)
Tables
(5)