-
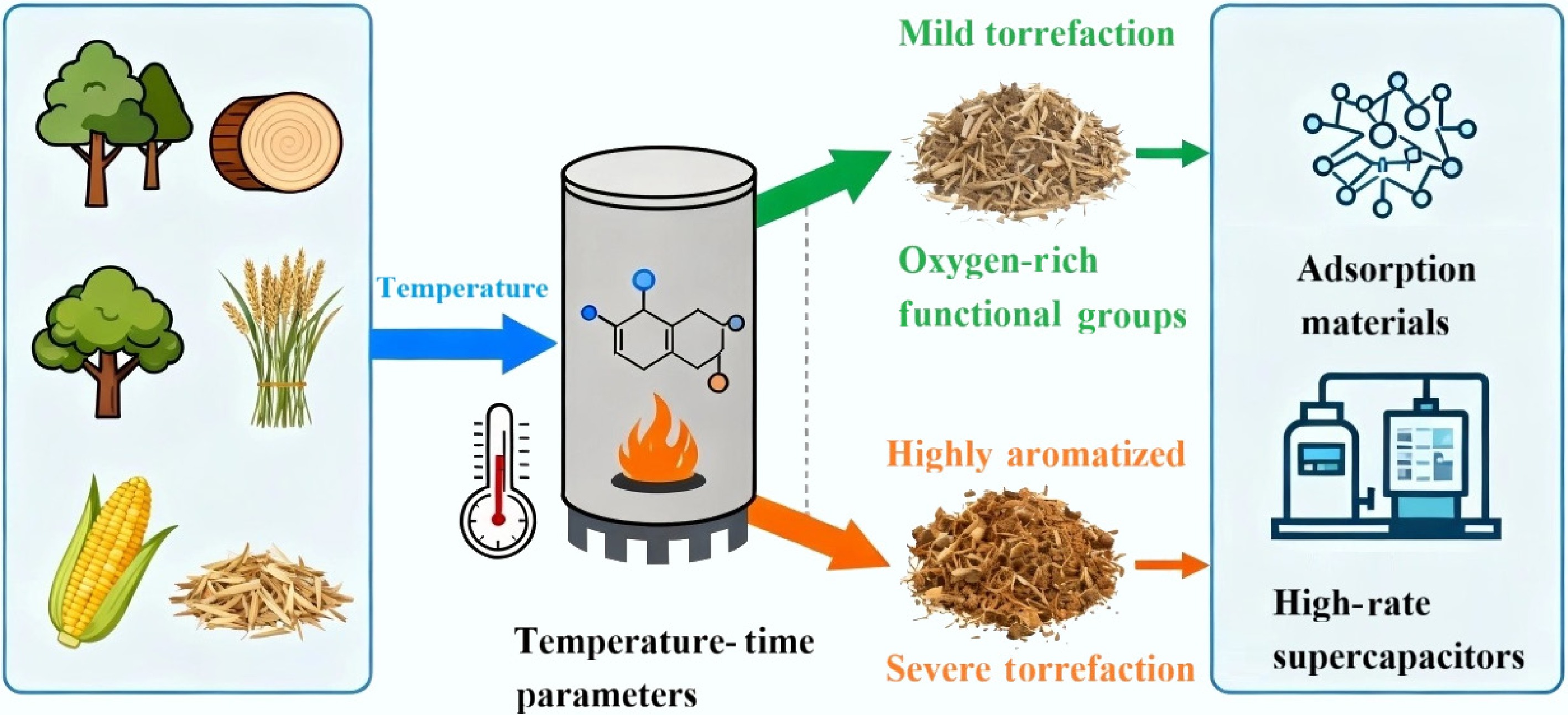
Figure 1.
The "precursor engineering" paradigm of biomass torrefaction: a transformation pathway from feedstock to functional materials.
-
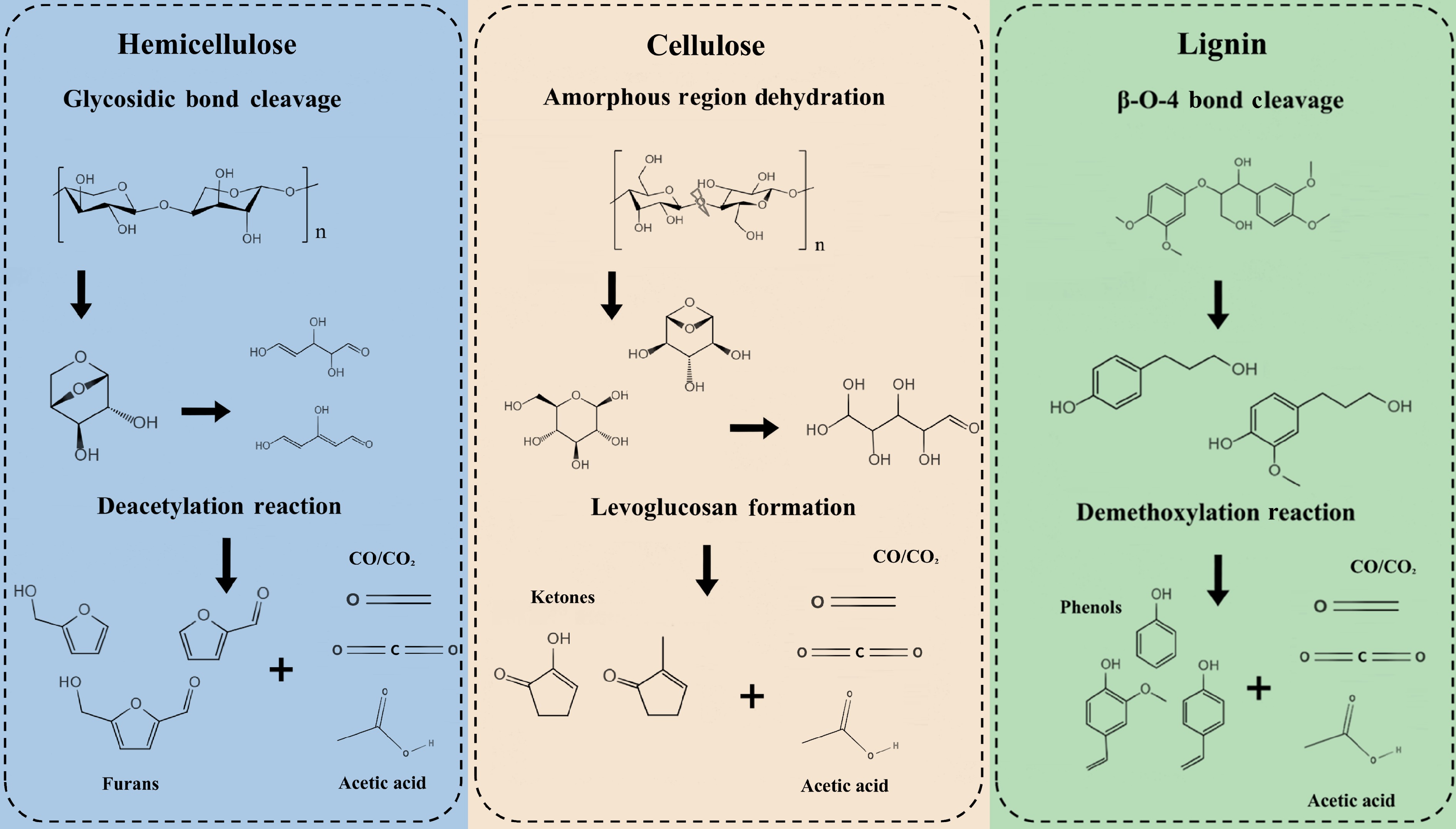
Figure 2.
The mechanisms of chemical and structural evolution in biomass components during torrefaction.
-
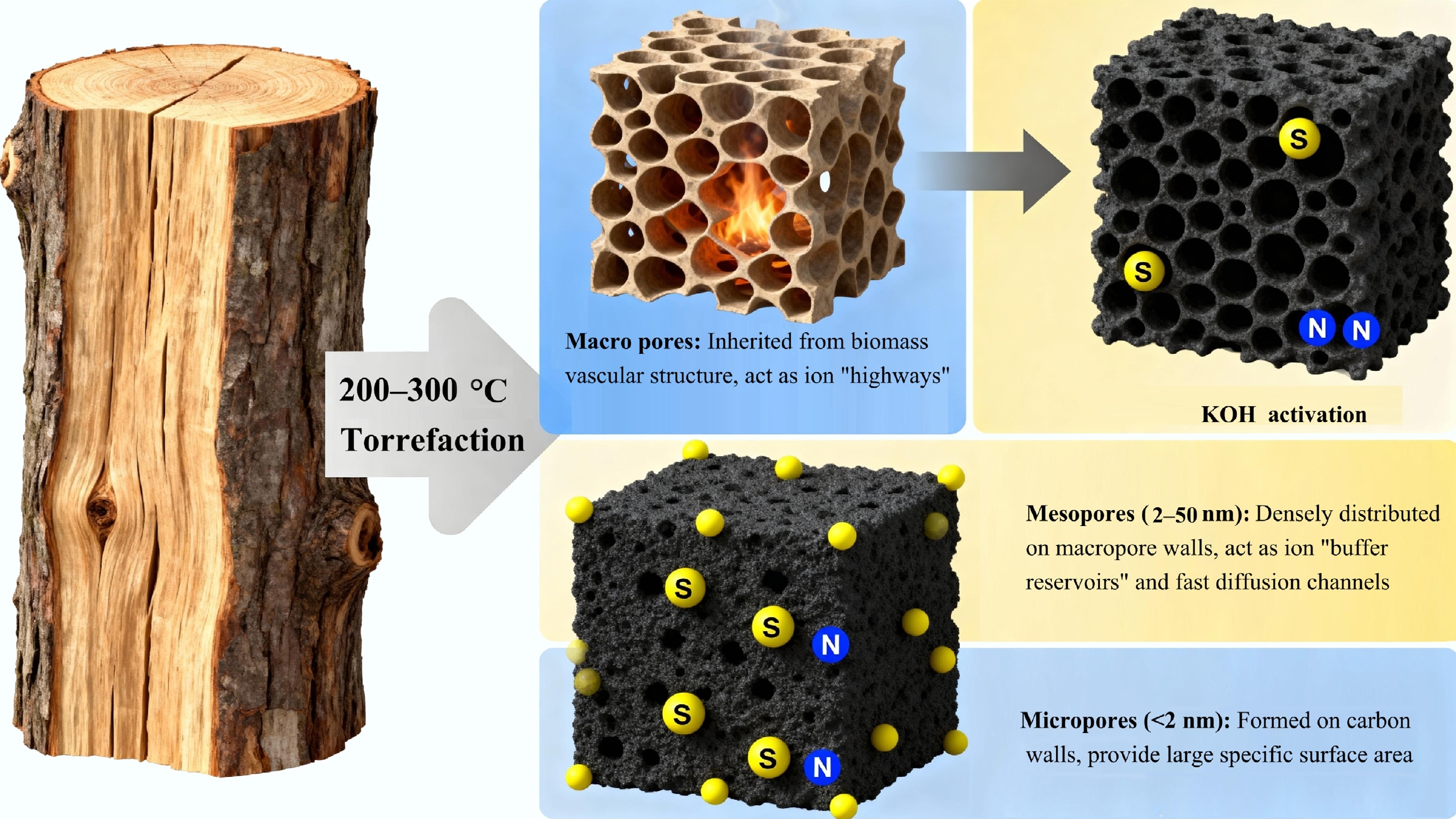
Figure 3.
The role of torrefaction-derived porous carbon in enhancing supercapacitor electrochemical performance.
-
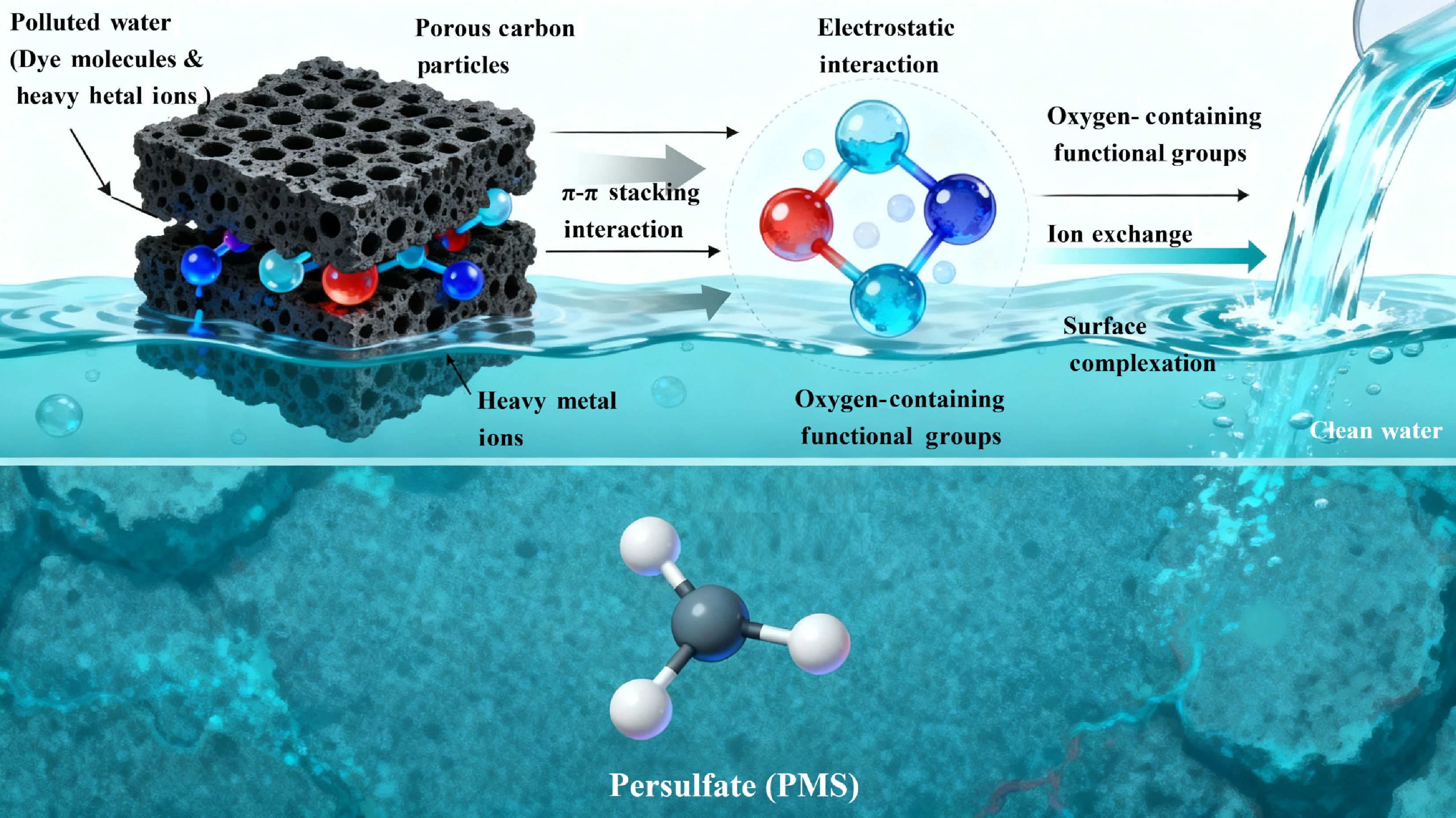
Figure 4.
The application performance of torrefaction-derived carbon materials in aquatic environmental remediation.
-
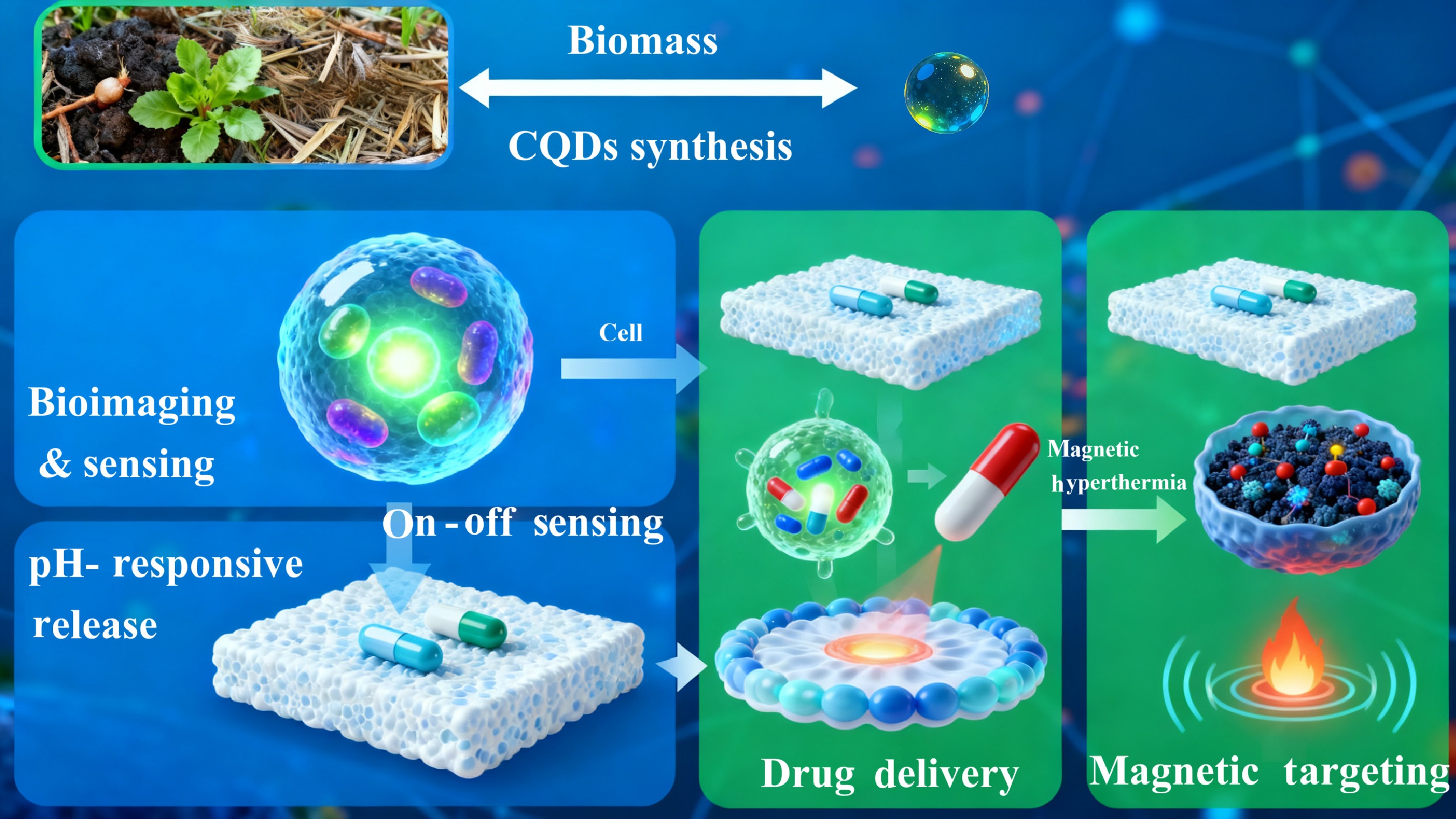
Figure 5.
The schematic illustration of biomedical applications for torrefaction-derived carbon quantum dots (CQDs).
-
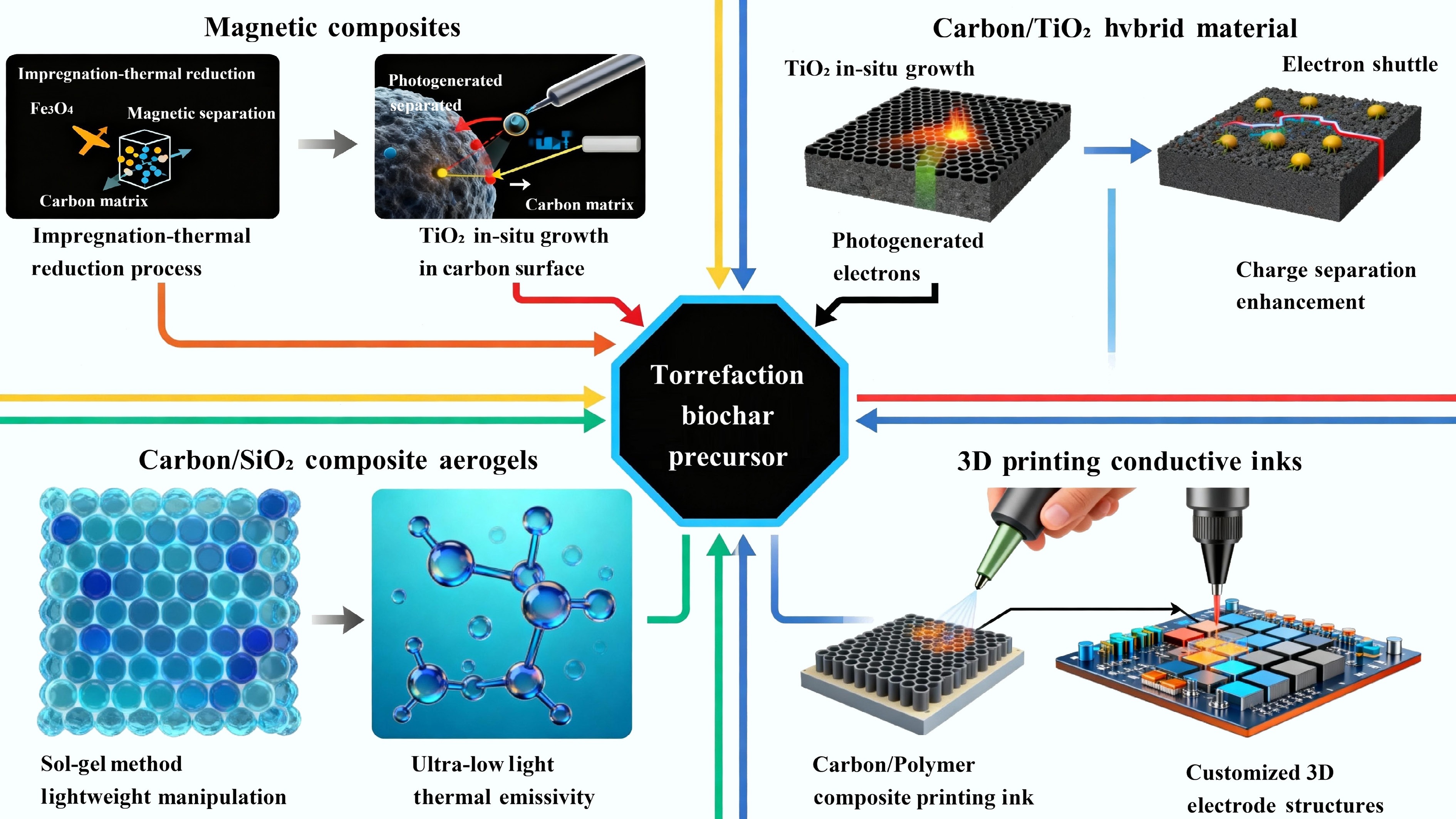
Figure 6.
The design strategies and applications of multifunctional composites based on torrefied biomass.
-
Parameter Typical range Impact on solid product properties Temperature Light (200–235 °C) 1. Initial deoxygenation and aromatization; significant decrease in atomic O/C ratio.
2. Selective degradation of hemicelluloses; initial pore formation.
3. Preliminary improvement in grindability; enhanced hydrophobicity.Mild (235–275 °C) 1. Intensified deoxygenation and aromatization; further reduction in atomic O/C ratio and increase in HHV.
2. Cellulose begins to react; enhanced stability of the carbon skeleton.
3. Markedly improved grindability, yielding finer, more uniform powder.Severe (275–300 °C) 1. Deep deoxygenation and aromatization; formation of a highly cross-linked, stable carbon structure; atomic O/C ratio reaches its lowest.
2. Energy density (HHV) peaks, properties closer to coal.
3. Optimal mechanical stability, suitable for subsequent aggressive activation.Residence time 0–60 min Mass yield: Lower at longer times/higher temperatures.
Reaction pathways: Short, intense treatments promote rapid devolatilization.
Volatile composition: Affects the balance of condensable vs non-condensable gases.Atmosphere Inert: N2, Ar
Reactive: CO2, steam, airCarbon content: CO2 can enhance decarboxylation, slightly increasing carbon content.
Reaction kinetics: Steam can act as a soft oxidant and sweeping gas, influencing reaction pathways and by-product composition.
Process direction: Prevents combustion; guides process toward controlled carbonization rather than gasification.Table 1.
Key torrefaction process parameters and their influence on product characteristics and application potential
-
Biomass feedstock Torrefaction conditions (°C) Activation conditions Specific surface area (m²/g) Specific capacitance (F/g) Ref. Basswood 260 KOH 579 434 [90] Wooden chopsticks 300 KOH 62 74.77 [91] Sterculia foetida 250 KOH 713 387 [92] Food waste 260 KOH 734.4 189.7 [93] Corncob 300 KOH 1,722 382.6 [94] Syzygium oleana 250 KOH 1,218 188 [95] Sugarcane bagasse 300 KOH 791.2 314 [96] Table 2.
The performance of torrefaction-derived porous carbons as supercapacitor electrodes
-
Target pollutant Material type and modification Performance metric Primary mechanism(s) Ref. Methylene blue Torrefied rice husk Adsorption capacity: 322–628 mg/g Pore filling (hierarchical porosity)
π-π interactions with graphitic domains
Electrostatic attraction with negatively charged surface[97] Lead (Pb2+) Torrefied coconut shell Adsorption capacity: 201.19 mg/g Ion exchange with H+ from -COOH and -OH groups
Surface complexation forming stable (COO)2 Pb precipitates[98] Tetracycline Torrefied rice husk Adsorption capacity: 68.97 mg/g Pore filling (hierarchical porosity)
π-π interactions with graphitic domains
Electrostatic attraction with negatively charged surface[99] Table 3.
The application of torrefaction-derived carbon materials in environmental remediation
-
Composite type Primary synthesis strategies Key synergistic properties Representative applications Magnetic carbon 1. Pre-/post-torrefaction impregnation with metal salts (e.g., Fe3+, Co2+) followed by thermal reduction.
2. Co-precipitation of magnetic nanoparticles (e.g., Fe3O4) onto carbon surface.1. High adsorption/catalytic capacity of carbon matrix.
2. Carbon matrix prevents nanoparticle agglomeration, enhances electron transfer, and stabilizes nanoparticles against leaching.1. Magnetically recoverable adsorbents/catalysts for wastewater treatment.
2. Magnetically guided drug delivery and magnetic hyperthermia for cancer therapy.
3. Resource recovery (e.g., precious metals from e-waste).Carbon/TiO2 hybrids 1. Sol-gel method using precursors.
2. In-situ growth/deposition of TiO2 from solution.
3. Chemical vapor infiltration (CVI).1. Pollutant pre-concentration by carbon adsorbent
2. Carbon acts as an electron shuttle, suppressing charge carrier recombination in TiO2.
3. Carbon can act as a photosensitizer, extending light absorption into the visible range.1. Highly efficient photocatalytic degradation of pollutants in air and water.
2. Photocatalytic water splitting for H2 production.
3. CO2 photoreduction to solar fuels.Carbon/SiO2 composites Sol-gel synthesis using silica precursors (e.g., TEOS), followed by ambient (xerogel) or supercritical (aerogel) drying. 1. Nanocomposite reinforcement: Carbon scaffold imparts mechanical robustness to brittle silica.
2. Thermal superinsulation: Synergy between silica's nanoporosity and carbon's role as an infrared opacifier results in ultra-low thermal conductivity.
3. Unique electrothermal properties: Combination of thermal insulation and electrical conductivity.1. Next-generation thermal superinsulators for buildings, pipelines, and aerospace.
2. Lightweight, insulating substrates for electronics.
3. Electrothermal insulation and self-sensing structures.3D-printable conductive
inks1. Dispersion of fine torrefied carbon powder into a polymeric binder matrix (e.g., PDMS for flexibility, PVDF for stability, PLA for biodegradability).
2. Additives (surfactants, rheology modifiers) for optimal printability.1. Structural design freedom from 3D printing.
2. Shear-thinning rheology for extrusion-based printing and shape retention after deposition.
3. Multi-functionality: Can combine conductivity with flexibility, biodegradability, or high surface area.1. Custom-shaped, interdigitated micro-supercapacitors and battery electrodes.
2. Wearable and flexible strain sensors (electronic skin).
3. High-surface-area biosensors and conductive scaffolds for tissue engineering.Table 4.
Multifunctional composite systems based on torrefied biomass and their synergistic characteristics
Figures
(6)
Tables
(4)