-
The 21st century is characterized by the dual challenge of pursuing sustainable development while mitigating environmental degradation[1]. This has spurred an unprecedented global pursuit of transitioning from a fossil-based economy to a circular bio-economy, where renewable resources form the backbone of industrial processes and material production[2]. In this context, biomass, as the most abundant and renewable carbon source on Earth, presents a compelling alternative to fossil fuels and petrochemical-derived materials. The annual global production of biomass, primarily from agricultural, forestry, and municipal waste streams is enormous[3]. However, a significant portion of this biomass is underutilized, often disposed of through burning or landfilling, leading to greenhouse gas emissions, air pollution, and a missed opportunity for value creation[4]. The conversion of this low-value, heterogeneous biomass into high-value, functional materials is a cornerstone of green chemistry and sustainable engineering. Among the various thermochemical conversion technologies, torrefaction has emerged as a critically important pretreatment and primary conversion step[5]. Torrefaction is a thermochemical process conducted typically at 200–300 °C in an inert or oxygen-limited atmosphere[6]. Its significance lies not necessarily in producing a final, application-ready material in a single step, but in transforming raw biomass into a superior intermediate, or "precursor", with tailored properties that are ideal for subsequent functionalization into advanced carbon materials[7].
The inherent limitations of raw biomass for advanced applications are manifold. Its high moisture content leads to logistical challenges and high energy costs for transportation and processing[8]. Its heterogeneous and fibrous nature, comprising cellulose, hemicellulose, and lignin, results in inconsistent properties. A high atomic O/C ratio and an abundance of hydrophilic functional groups (e.g., hydroxyl and carboxyl) render it highly reactive, hygroscopic, and thermally unstable[9]. More critically, upon direct high-temperature processing like activation, the violent release of volatiles can cause structural collapse, yielding carbon materials with poorly developed porosity and unsatisfactory performance. The core chemical transformations during torrefaction are deoxygenation and aromatization[10]. Through reactions like dehydration, decarboxylation, and decarbonylation, torrefaction efficiently removes elemental O in the form of H2O, CO2, CO, and volatile organic compounds. This dramatically reduces the atomic O/C and H/C ratios, bringing the material's properties closer to those of coal or even low-grade biochar[11]. Concurrently, the biopolymer chains break down and reconfigures into more stable, cross-linked aromatic structures, which is a process known as aromatization.
Torrefaction pretreatment is efficient in improving biochar grindability and homogeneity. The torrefied biomass is brittle and much easier to pulverize into a fine, consistent powder, which is crucial for uniform subsequent processing and product quality[12]. Moreover, the removal of hydrophilic groups makes the material water-resistant, enabling its storage properties and stability. Most importantly for material science, the torrefied biochar possesses a stabilized carbon matrix that is primed for further functionalization[13]. This stable structure can withstand the aggressive conditions of chemical activation, leading to the creation of robust, high-surface-area porous biochar. Furthermore, the specific torrefaction condition can be fine-tuned to "program" the precursor for a final application scenario, such as supercapacitors, adsorbents, catalysts, and biomedical devices[14]. Although torrefaction technology has demonstrated great potential as a platform for precursor engineering of high-performance carbon materials in laboratory research, its industrial-scale application still faces numerous challenges. Currently, the development of continuous reactors (e.g., screw-type, moving bed) aims to improve processing efficiency and product uniformity. However, further research is needed in areas such as optimizing energy efficiency, expanding feedstock adaptability, enhancing process economics, and assessing environmental footprints. Overcoming these engineering and cost bottlenecks is crucial for realizing the large-scale sustainable application of torrefied biochar in energy, environmental, and biomedical fields.
The objective of this review is to provide a comprehensive and detailed analysis of the role of biomass torrefaction in the synthesis of functional carbon materials. The fundamental mechanisms of torrefaction are analyzed here, and its application in preparing advanced materials for energy storage (supercapacitors), environmental remediation (adsorption and catalysis), and biomedical engineering is also systematically explored[15]. A dedicated part will highlight the cutting-edge trend of multifunctional composite materials derived from torrefied biomass. Finally, the current state of the art is summarized, and the perspective on future research directions and challenges is also provided. By critically evaluating the extensive body of research in relevant fields, this review aims to underscore the immense potential of torrefaction as a keystone technology for the development of a sustainable and circular carbon-based materials industry. This review pioneers the systematic framing of biomass torrefaction as a versatile "precursor engineering" platform for non-fuel, high-value functional carbon materials, moving beyond its traditional role as a fuel pretreatment.
-
The efficiency and product characteristics of torrefaction, a thermochemical process, are predominantly governed by three interdependent parameters: temperature, residence time, and the carrier gas composition[16]. Temperature is undeniably the most influential factor, typically categorized into light (200–235 °C), mild (235–275 °C), and severe (275–300 °C) regimes. As the temperature gradually increases, the thermal degradation degree of biomass components intensifies[17]. Hemicelluloses, the most reactive polymer, decomposed between 200–300 °C, while cellulose and lignin undergo progressive depolymerization and cross-linking at higher temperatures[18]. This leads to increased mass and volatile matter loss, a significant reduction in the atomic O/C and H/C ratios, and a more pronounced aromatization of the carbon skeleton, directly enhancing the solid product's energy density and grindability[19]. The key torrefaction process parameters and their influence on product characteristics and application potential are listed in Table 1.
Table 1. Key torrefaction process parameters and their influence on product characteristics and application potential
Parameter Typical range Impact on solid product properties Temperature Light (200–235 °C) 1. Initial deoxygenation and aromatization; significant decrease in atomic O/C ratio.
2. Selective degradation of hemicelluloses; initial pore formation.
3. Preliminary improvement in grindability; enhanced hydrophobicity.Mild (235–275 °C) 1. Intensified deoxygenation and aromatization; further reduction in atomic O/C ratio and increase in HHV.
2. Cellulose begins to react; enhanced stability of the carbon skeleton.
3. Markedly improved grindability, yielding finer, more uniform powder.Severe (275–300 °C) 1. Deep deoxygenation and aromatization; formation of a highly cross-linked, stable carbon structure; atomic O/C ratio reaches its lowest.
2. Energy density (HHV) peaks, properties closer to coal.
3. Optimal mechanical stability, suitable for subsequent aggressive activation.Residence time 0–60 min Mass yield: Lower at longer times/higher temperatures.
Reaction pathways: Short, intense treatments promote rapid devolatilization.
Volatile composition: Affects the balance of condensable vs non-condensable gases.Atmosphere Inert: N2, Ar
Reactive: CO2, steam, airCarbon content: CO2 can enhance decarboxylation, slightly increasing carbon content.
Reaction kinetics: Steam can act as a soft oxidant and sweeping gas, influencing reaction pathways and by-product composition.
Process direction: Prevents combustion; guides process toward controlled carbonization rather than gasification.Residence time, which is mainly in the range of 15–60 min, exhibits a complex interaction with temperature. Herein, a prolonged residence time at a lower temperature may achieve a mass yield similar to a shorter duration at a higher temperature. However, the reaction pathways and the resulting chemical composition of the torrefied biomass can differ substantially[20]. For instance, longer residence times at lower temperatures might favor dehydration reactions, while shorter, more intense treatments could promote more rapid devolatilization, affecting the balance of condensable and non-condensable gases produced[21]. While an inert environment (N2, Ar) is necessary to prevent combustion, the use of weakly reactive atmospheres like CO2 or steam is an insightful point for further study. Torrefaction in CO2 can enhance decarboxylation reactions, potentially leading to biochar with a slightly higher carbon content. Similarly, steam can act as a soft oxidizing agent and a sweeping gas, influencing the reaction kinetics and the composition of the liquid by-products[22]. The carrier gas composition fundamentally directs the process away from gasification and towards controlled solid carbonization.
The translation of these parameters from laboratory to industry is realized through reactor design, which is diverse and reflects the ongoing pursuit of scalability, energy efficiency, and product uniformity[23]. Batch reactors, such as simple tubular or fixed-bed furnaces, are invaluable for fundamental research and parameter optimization due to their simplicity and flexibility, allowing for precise control over individual test runs. For industrial-scale production, however, continuous reactors are essential for economic viability. Among these, screw-type reactors are prominent; an auger screw conveys and mixes the biomass through a heated barrel, offering excellent control over residence time. However, they can face challenges with abrasive feedstocks that cause wear on the screw and barrel[24]. Moving bed reactors, where biomass gravity-feeds through a vertical shaft, provide a plug-flow behavior, ensuring a narrow residence time distribution for a highly uniform product with minimal energy input, though they can be susceptible to bridging or channeling. Conversely, rotary drum reactors, where a slowly rotating inclined drum tumbles the biomass, excel in handling a wide variety of feedstock sizes and shapes. The tumbling action promotes highly uniform heat transfer, but achieving a consistent residence time can be more challenging compared to screw systems[20]. The choice of reactor is thus a critical compromise, balancing capital cost, operational complexity, feedstock adaptability, and the stringent specifications required for the final torrefied product.
Chemical and structural transformation mechanisms
-
The transformation of biomass torrefaction is a complex, multi-stage process driven by heat, which selectively targets its three main polymeric components through distinct but overlapping degradation pathways. This staged decomposition is key to understanding the resultant material's properties, including hemicellulose decomposition, cellulose stabilization, and lignin softening and partial decomposition[15].
As the most vulnerable polymer, hemicelluloses undergo extensive depolymerization within the 200–300 °C range, primarily through deacetylation and cleavage of glycosidic bonds. This breakdown is the dominant source of volatile release in the early stages of torrefaction. The process generates a significant portion of the condensable vapors, rich in acetic acid, formic acid, and furfural derivatives, which contribute to the corrosive nature of the bio-oil condensate. Simultaneously, non-condensable gases like CO and CO2 are liberated from decarboxylation and decarbonylation reactions[25]. This massive devolatilization from the hemicellulose matrix is a primary driver of mass loss, often accounting for 60%–80% of the initial volatile matter, and creates the initial, rudimentary pore network within the solid structure[26].
Cellulose stabilization is protected by its highly ordered crystal structure. Cellulose remains largely intact during the light torrefaction process, but becomes active in the mild to severe range (250–300 °C)[27]. Degradation initiates in the less-ordered amorphous regions, where dehydration reactions dominate, leading to the evolution of water vapor and the formation of anhydro-sugars like levoglucosan. This "stabilization" involves a gradual depolymerization and the removal of hydroxyl groups, resulting in a more thermally resilient and carbon-rich biochar[28]. The product is often referred to as "cellulose torrefaction", which serves as a crucial intermediate, initiating the formation of the nascent carbonaceous structure that will be further developed in subsequent processing.
Lignin, a complex, three-dimensional, cross-linked aromatic polymer, behaves differently. It does not melt but undergoes a glass transition and softening within the torrefaction temperature window[22]. Chemically, it is subject to limited breakdown through reactions such as demethoxylation (releasing methane and methanol) and the cleavage of weak β-O-4 aryl-ether linkages. However, its robust aromatic backbone remains largely unconsumed. Consequently, lignin is the major contributor to the solid yield, providing the structural integrity of the torrefied pellet, and acting as the primary source for the development of polycyclic aromatic char structure[28].
The net result of these parallel and sequential reactions is a solid product with fundamentally altered properties. Deoxygenation and aromatization are the core chemical phenomenon, with an atomic O/C ratio that can plummet from around 0.7 in raw biomass to below 0.3 in severely torrefied biochar[6]. This is physically evidenced by analytical techniques: Fourier Transform Infrared Spectroscopy (FT-IR) shows the dramatic disappearance of O-H and C=O bands, while X-ray diffraction (XRD) reveals the broadening and shifting of the (002) peak, indicating the formation of disordered, turbostratic carbon crystallites. Porosity development occurs as volatiles escape, but the resulting network is typically a combination of narrow micropores (from component decomposition), and macroporous channels inherited from the native biomass vasculature[29]. This leads to a modest specific surface area, usually below 300 m2/g, definitively underscoring the material's role as a precursor rather than a final porous product like activated carbon. Finally, the changes in elemental composition and energy content are direct consequences of deoxygenation[30]. The relative carbon content increases significantly (e.g., from around 50% to over 70%), while elemental H and O are removed. Since oxygen atoms contribute mass but little heating value, their removal leads to a marked increase in the higher heating value (HHV), from around 18 MJ/kg for raw biomass to over 25 MJ/kg for the torrefied biochar, thus enhancing its fuel quality and energy density[31].
The "precursor engineering" paradigm
-
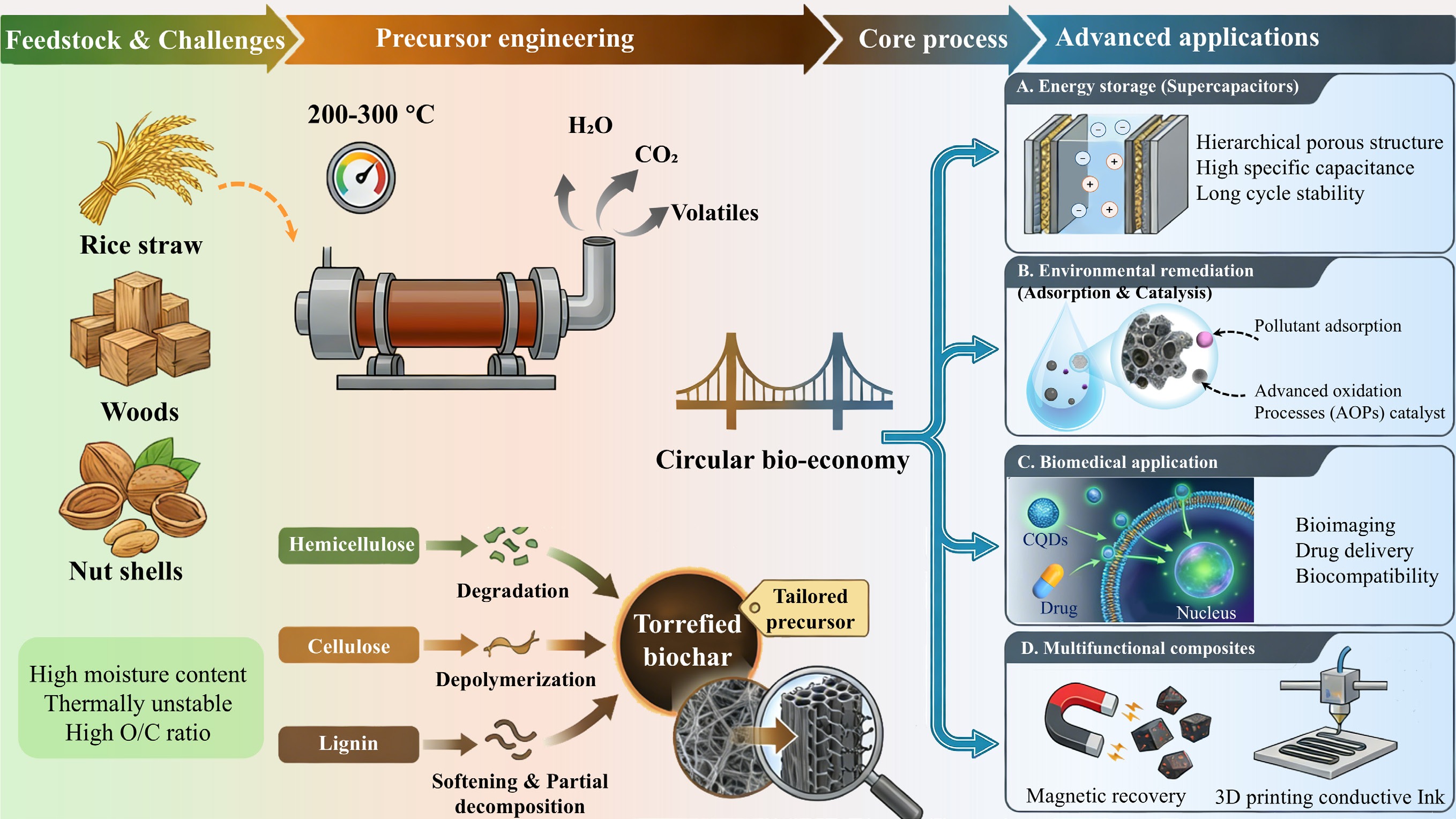
The true value of torrefaction in advanced materials science transcends mere biomass pretreatment, which lies in its foundational role in "precursor engineering". This paradigm suggests that the torrefied biomass is not a simple intermediate, but a strategically designed starting material whose initial properties dictate the performance ceiling of the final functional carbon material[6]. By meticulously selecting the biomass feedstock based on its inherent composition and precisely tuning the torrefaction parameters, one can deliberately "program" a precursor with a specific chemical composition, nano/micro-structure, and structural robustness. This strategic design is critical for optimizing downstream conversion processes like chemical activation or heteroatom doping[32]. The "Precursor engineering" paradigm of biomass torrefaction: a transformation pathway from feedstock to functional materials is shown in Fig. 1.
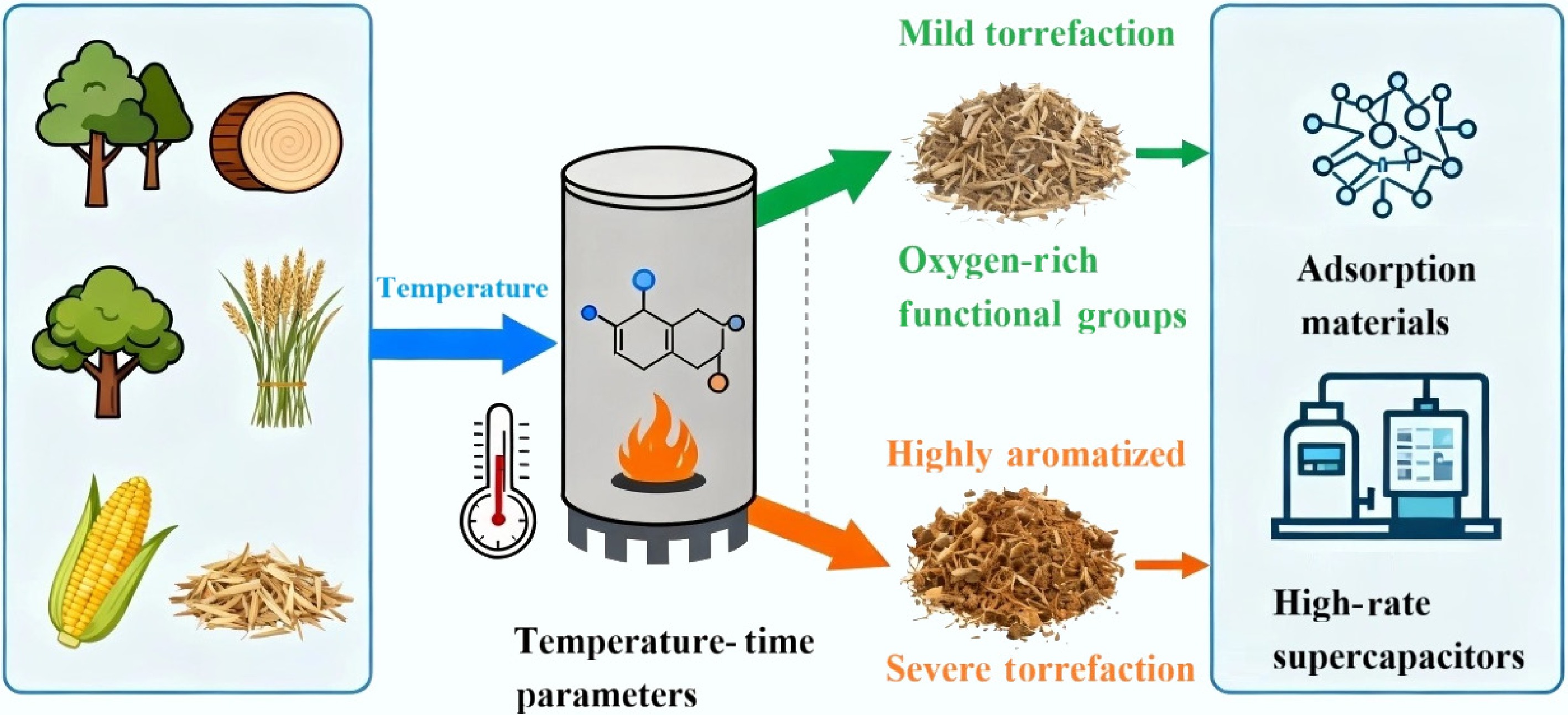
Figure 1.
The "precursor engineering" paradigm of biomass torrefaction: a transformation pathway from feedstock to functional materials.
The power of this approach is illustrated by comparing different torrefaction severities. A mild torrefaction (e.g., 235–250 °C) selectively degrades the most volatile components like hemicelluloses, while deliberately preserving a significant portion of the inherent oxygen-containing functional groups (e.g., carboxyl, hydroxyl) on the cellulose and lignin backbone. This outcome is not a shortcoming but a desired feature for specific applications, as an oxygen-rich precursor is highly beneficial when the final application demands high surface reactivity[33]. For instance, these preserved functional groups can serve as anchoring sites for metal ions in catalyst synthesis, or facilitate favorable interactions with specific pollutant molecules in adsorption processes. Conversely, a severe torrefaction (e.g., 275–300 °C) drives extensive deoxygenation and aromatization, creating a highly condensed, cross-linked carbon structure with a significantly enhanced mechanical stability[34]. This robust, low atomic O/C ratio architecture is ideally suited to withstand the aggressive chemical etching of subsequent activation, particularly with agents like KOH. The stable carbon matrix allows for controlled and extensive pore development without catastrophic structural collapse, enabling the creation of ultra-high-surface-area carbons with hierarchical porosity essential for high-performance supercapacitor electrodes[12]. The mechanisms of chemical and structural evolution in biomass components during torrefaction are shown in Fig. 2.
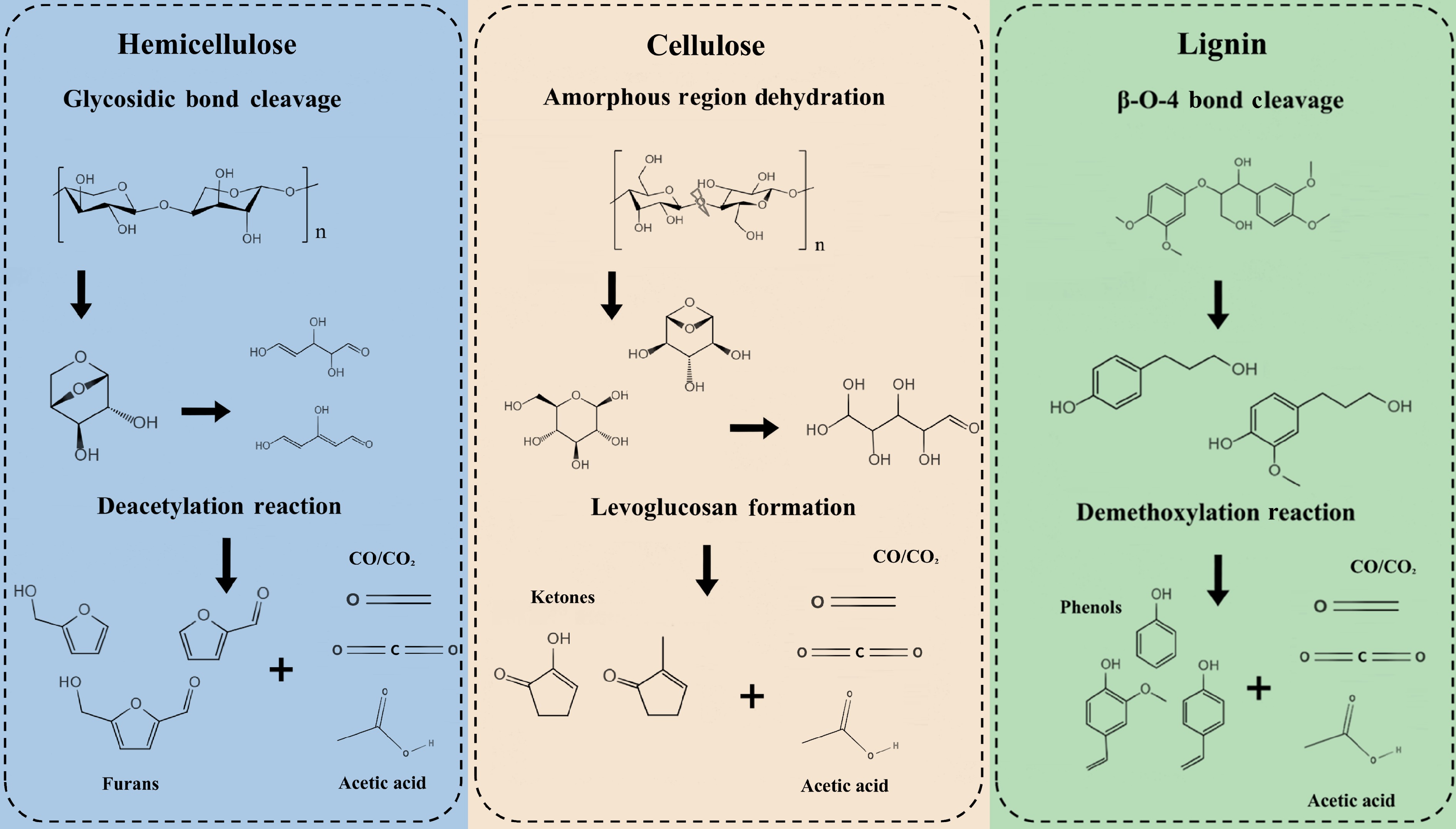
Figure 2.
The mechanisms of chemical and structural evolution in biomass components during torrefaction.
This tailored approach extends to incorporating functionality during torrefaction itself, such as through catalytic torrefaction or co-torrefaction with nitrogen-rich compounds, which can implant specific heteroatoms directly into the carbon matrix at this early stage[35]. Ultimately, the ability to fine-tune the precursor's properties makes torrefaction an exceptionally powerful and versatile first step[36]. It provides a scalable and sustainable platform for the rational synthesis of a wide array of functional carbon materials, moving beyond trial-and-error towards a more predictive and application-targeted design philosophy.
The efficacy of this "precursor engineering" approach is highlighted by comparing with the direct activation of raw biomass. Under similar chemical activation conditions (e.g., with KOH), carbon materials derived from torrefied biomass typically demonstrate significant advantages in structural stability, pore tunability, and ultimate performance. For instance, for supercapacitor electrodes, the reinforced aromatized carbon skeleton provided by the torrefaction step, enables the creation of a robust hierarchical porous network during aggressive activation, rather than structural collapse. This not only achieves high specific surface areas (often exceeding 2,500 m2/g) but, more importantly, ensures an excellent rate capability and long-term cycling stability (capacitance retention > 95% over tens of thousands of cycles), metrics that often surpass those of materials prepared from the same, but non-torrefied raw biomass via direct activation. In the realms of adsorption and catalysis, materials based on torrefied precursors also exhibit superior and reproducible performance in terms of adsorption capacity for dyes, heavy metals, and organic pollutants, as well as catalytic degradation efficiency, owing to their tunable surface chemistry and optimized pore hierarchy. In contrast, the unstable structure and hydrophilicity of non-torrefied feedstocks makes precise control over pore structure and surface chemistry during activation challenging, leading to inconsistent or inferior performance. Therefore, torrefaction, as a critical "precursor engineering" step, is not a mere additional procedure, but fundamentally unlocks the performance ceiling of subsequent functionalization processes by upgrading the precursor quality, making it an indispensable link in the synthesis of high-performance functional carbon materials.
-
The performance of supercapacitors, particularly electrical double-layer capacitors (EDLCs), is intrinsically determined by the physicochemical properties of the electrode material[11]. Three parameters are paramount: a high specific surface area (SSA) to accommodate a vast electrical double-layer, optimal electrical conductivity to ensure efficient charge transport, and a well-tuned pore size distribution that matches the electrolyte ion dimensions, to facilitate rapid ion accessibility and diffusion[37]. Biomass-derived carbon materials, especially those utilizing torrefaction as a pre-treatment process, have emerged as a premier candidate to meet these demanding criteria sustainably and cost-effectively. Concerning the synthesis pathway for electrode preparation, the most prevalent and highly effective synthesis route is firstly biomass torrefaction, and then chemical activation (KOH, H3PO4, ZnCl2), thus for porous biochar structure acquisition[38]. While chemical activation is the critical step for porosity generation, the torrefaction step is the unsung hero that dictates the ultimate quality and performance of the final electrode. The performance of torrefaction-derived porous carbons as supercapacitor electrodes are listed in Table 2, and the role of torrefaction-derived porous carbon in enhancing supercapacitor electrochemical performance is shown in Fig. 3.
Table 2. The performance of torrefaction-derived porous carbons as supercapacitor electrodes
Biomass feedstock Torrefaction conditions (°C) Activation conditions Specific surface area (m²/g) Specific capacitance (F/g) Ref. Basswood 260 KOH 579 434 [90] Wooden chopsticks 300 KOH 62 74.77 [91] Sterculia foetida 250 KOH 713 387 [92] Food waste 260 KOH 734.4 189.7 [93] Corncob 300 KOH 1,722 382.6 [94] Syzygium oleana 250 KOH 1,218 188 [95] Sugarcane bagasse 300 KOH 791.2 314 [96] 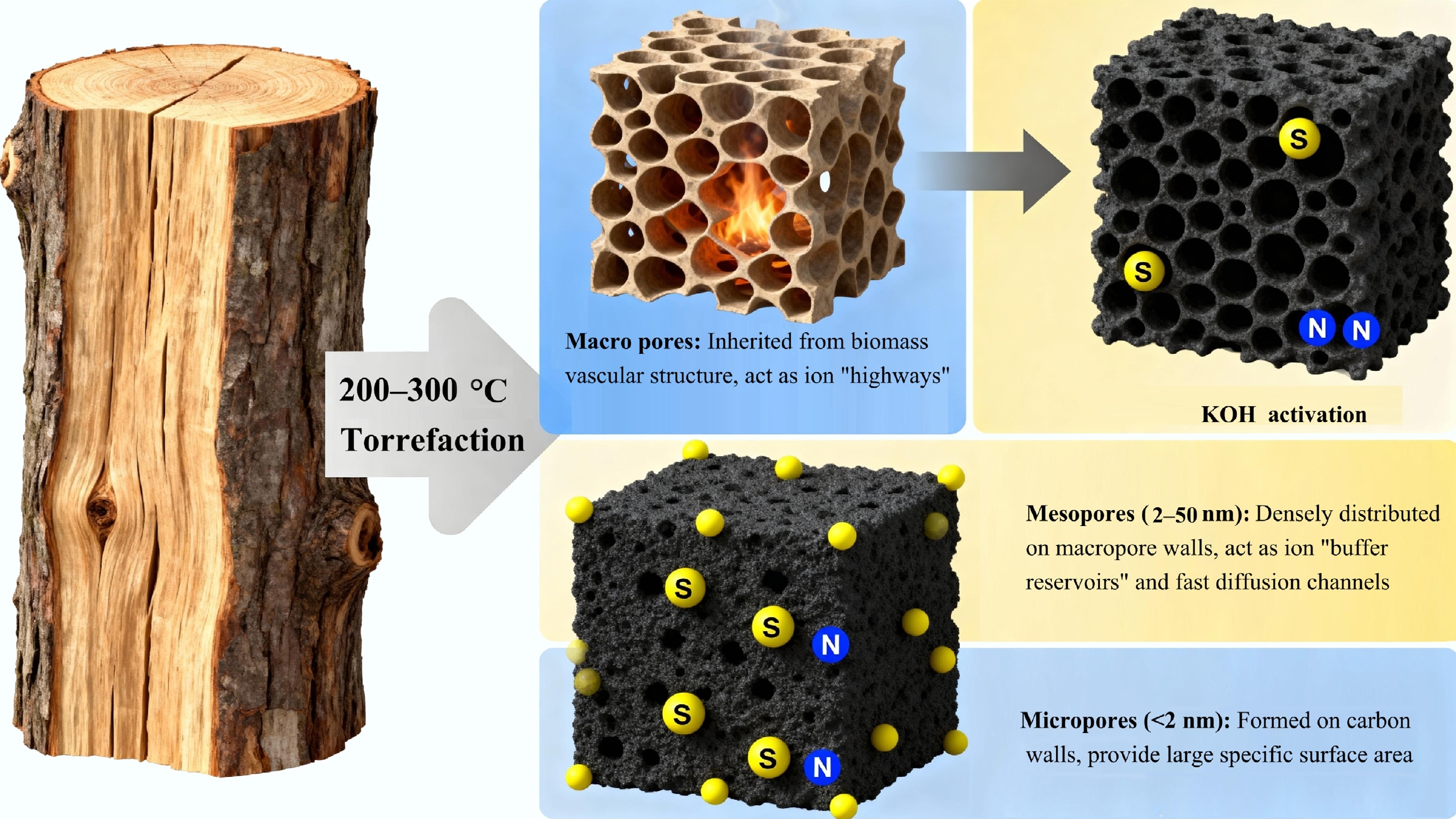
Figure 3.
The role of torrefaction-derived porous carbon in enhancing supercapacitor electrochemical performance.
Far from being a simple pre-drying step, torrefaction is foundational to engineering a high-performance electrode. Its role can be dissected into three key contributions:
(1) Structural reinforcement and hierarchical pore engineering: The aromatized carbon skeleton formed during torrefaction acts as a robust, cross-linked scaffold. During the subsequent harsh chemical activation, for instance, with KOH at 600–800 °C, selective and homogeneous etching of the stable scaffold is achieved[39]. The etching reactions create porosity, but the torrefied precursor's mechanical integrity is crucial. It prevents excessive or catastrophic structural collapse during the violent gas evolution and carbon consumption, allowing for the creation of a well-defined, hierarchical porous network. This hierarchy is essential for balanced performance: micropores (< 2 nm) provide the immense surface area necessary for high charge storage via the electrical double-layer, while mesopores (2–50 nm) serve as vital ion-buffering reservoirs and low-resistance pathways, enabling rapid ion transport to the interior surfaces[40]. This synergy is key to achieving both high energy density (from micropores) and high-power density (from mesopores).
(2) Heteroatom doping for enhanced pseudocapacitance: While torrefaction is a deoxygenation process, it does not create an inert carbon surface. It strategically leaves behind stable oxygen-containing functional groups (e.g., quinones, ethers), and can preserve inherent heteroatoms like nitrogen (in the form of pyrrolic or pyridinic-N) if the biomass feedstock contains them (e.g., from protein-rich waste)[41]. Furthermore, the chemically reactive surface of the torrefied char serves as an excellent substrate for intentional post-doping with elements such as N, S, B, or P. These heteroatoms introduce Faradaic pseudocapacitance by undergoing highly reversible redox reactions at or near the electrode surface. This mechanism stores additional charge beyond the physical electrostatic attraction of EDLCs, significantly boosting the overall specific capacitance[25]. For example, quinone/hydroquinone pairs or nitrogen-based functional groups can contribute substantial pseudocapacitance in acidic or basic electrolytes, respectively.
(3) Enhanced electrical conductivity: The devolatilization and aromatization processes during torrefaction increase the relative content of sp2-hybridized carbon, forming nascent graphitic-like domains. This development enhances the intrinsic electronic conductivity of the carbon framework[42]. In a supercapacitor electrode, this high conductivity reduces the equivalent series resistance (ESR), minimizes ohmic losses (I2R) during rapid charge/discharge, and facilitates ultra-high power delivery. A low ESR also translates into better rate capability, meaning the capacitor can maintain a higher percentage of its capacitance even at very high current loads[10].
Exemplary performance and comparative advantage: Extensive research validates this integrated approach. KOH activation of torrefied rice husk, wood, or coconut shells routinely produces carbons with ultra-high SSAs, often exceeding 2,500 m2/g and sometimes approaching 3,000 m2/g. When fabricated into electrodes for testing in aqueous electrolytes, these torrefaction-derived porous carbons deliver impressive specific capacitances, typically ranging from 300 to 450 F/g at low current densities (e.g., 0.5 A/g)[36]. More importantly, they exhibit exceptional cycling stability, retaining over 95%–98% of their initial capacitance after 10,000 to 50,000 consecutive charge-discharge cycles[10]. This combination of high capacitance, excellent rate performance, and outstanding longevity often surpasses the performance of many more expensive carbon nanomaterials like graphene or carbon nanotubes, positioning torrefaction-derived carbons as a highly competitive and sustainable material for next-generation energy storage devices.
Environmental pollutant adsorption and catalytic degradation
-
The application of torrefaction-derived biochar in environmental remediation represents a paradigm shift towards sustainable and efficient pollution control technologies, effectively addressing both aqueous and gaseous contaminants through adsorption and advanced catalytic degradation.
Adsorption of pollutants (dyes, heavy metals, and VOCs)
-
Torrefaction is an efficient approach to improve biochar adsorption performance for environmental pollutants remediation. By creating a structurally robust and aromatized carbon matrix, it ensures that during the subsequent activation process, the development of porosity is controlled and extensive[43]. The stable torrefied matrix prevents pore wall collapse under aggressive chemical etching, allowing for the creation of a tailored pore architecture. For adsorption applications, achieving an ideal pore size distribution is even more critical than merely maximizing specific surface area[44]. The torrefaction-derived precursor enables the formation of a balanced network where micropores (< 2 nm) provide the foundational high surface area for capturing small molecules and gases, while a significant volume of mesopores (2–50 nm) is simultaneously created[45]. These mesopores are indispensable for the rapid diffusion and uptake of larger pollutant molecules, such as complex organic dyes and pharmaceuticals, which would otherwise experience kinetic limitations in purely microporous structures.
Torrefaction-derived biochar exhibits remarkable adsorption capacities for both cationic (e.g., Methylene Blue) and anionic (e.g., Methyl Orange) dyes, consistently reported in the range of 500 to 1,500 mg/g. The adsorption is a synergistic process involving pore filling within the hierarchical structure, π-π interactions between the dye's aromatic rings and the graphitic domains of the carbon, and electrostatic attractions modulated by the surface charge of the carbon, and the ionic nature of the dye[46]. Torrefaction-derived biochar is highly effective for heavy metal sequestration, with capacities frequently exceeding 200 mg/g. The mechanism is not purely physical but involves specific chemical interactions: ion exchange with protons from remaining carboxyl and phenolic hydroxyl groups, surface complexation forming stable chelates, and electrostatic precipitation onto the charged carbon surface[47]. Moreover, the inherent high microporosity, fine-tuned by the torrefaction-activation sequence, makes these carbons excellent for the physisorption of gaseous pollutants like benzene and toluene, with the narrow pores providing strong adsorption potentials for volatile organic compound (VOC) molecules. The application of torrefaction-derived carbon materials in environmental remediation is listed in Table 3, and the application performance of torrefaction-derived carbon materials in aquatic environmental remediation is shown in Fig. 4.
Table 3. The application of torrefaction-derived carbon materials in environmental remediation
Target pollutant Material type and modification Performance metric Primary mechanism(s) Ref. Methylene blue Torrefied rice husk Adsorption capacity: 322–628 mg/g Pore filling (hierarchical porosity)
π-π interactions with graphitic domains
Electrostatic attraction with negatively charged surface[97] Lead (Pb2+) Torrefied coconut shell Adsorption capacity: 201.19 mg/g Ion exchange with H+ from -COOH and -OH groups
Surface complexation forming stable (COO)2 Pb precipitates[98] Tetracycline Torrefied rice husk Adsorption capacity: 68.97 mg/g Pore filling (hierarchical porosity)
π-π interactions with graphitic domains
Electrostatic attraction with negatively charged surface[99] 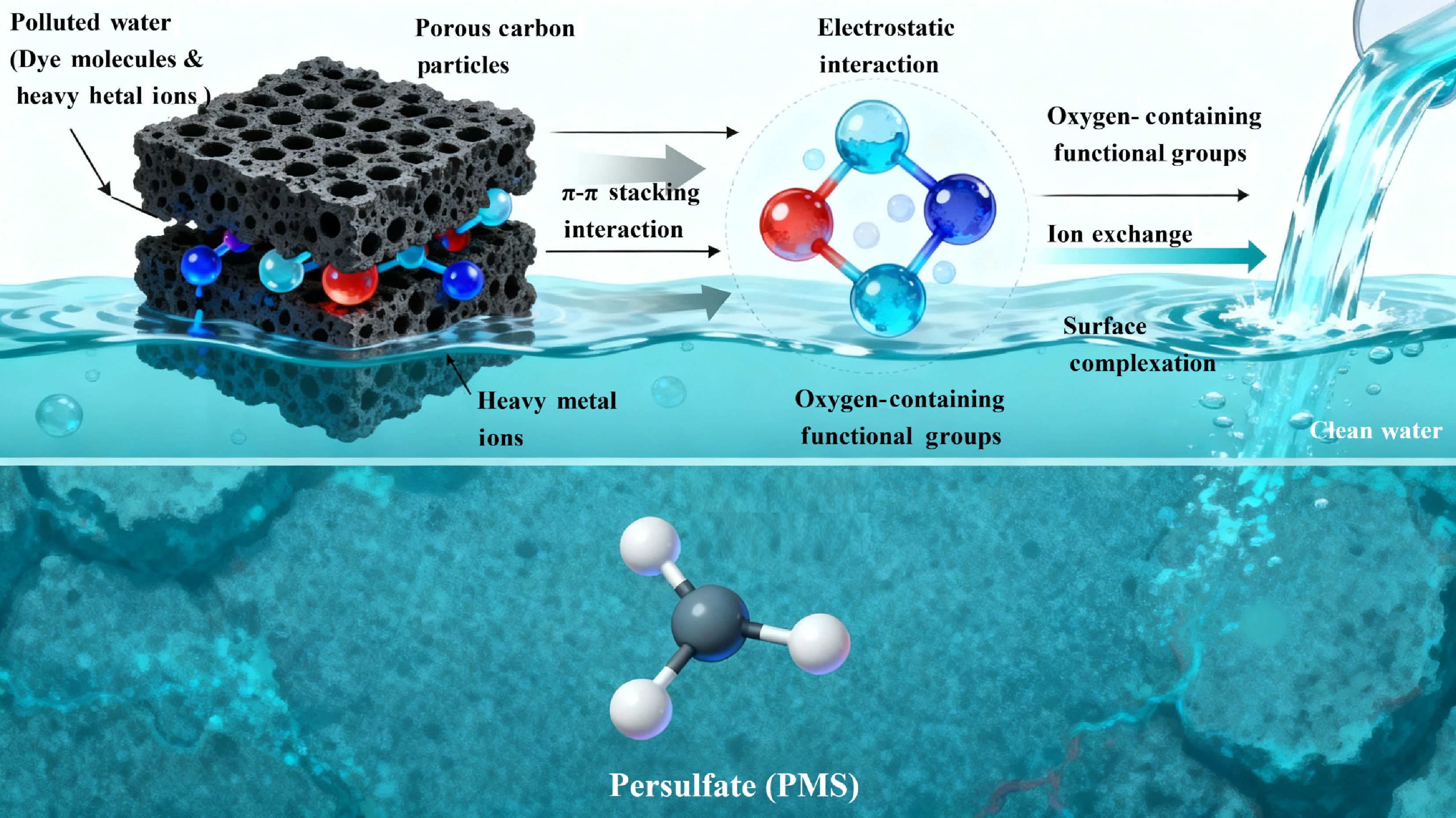
Figure 4.
The application performance of torrefaction-derived carbon materials in aquatic environmental remediation.
Catalytic degradation via advanced oxidation processes (AOPs)
-
Beyond adsorption, torrefaction-derived biochar is pivotal in catalytic AOPs for the destructive removal of organic pollutants. The role of torrefaction is critical in both routes. Firstly, it provides a stable and functional catalyst support. The high-surface-area, chemically inert, yet electrically conductive torrefied carbon matrix is ideal for anchoring and dispersing catalytic nanoparticles (e.g., zero-valent iron, Fe3O4, Co3O4). This dispersion prevents nanoparticle agglomeration during synthesis and operation, while the strong metal-support interaction minimizes leaching, thereby significantly enhancing the catalyst's longevity and reusability[48]. Secondly, torrefaction enables intrinsic catalytic activity in metal-free systems. When torrefaction-derived biochar is further doped with heteroatoms like nitrogen, it transforms into highly efficient metal-free catalysts for activating peroxymonosulfate (PMS) or peroxydisulfate (PDS)[49]. The incorporation of nitrogen, specifically graphitic N and pyridinic N, polarizes the adjacent carbon atoms in the sp2 lattice, creating positively charged sites that dramatically lower the energy barrier for cleaving the peroxide O-O bond. This activation generates a suite of powerful oxidizing species, including radical ones like sulfate radicals (SO4•−) and hydroxyl radicals (•OH), as well as non-radical pathways involving singlet oxygen (1O2) and direct electron transfer from the pollutant to the oxidant via the carbon catalyst.
A key advantage lies in the synergistic effects of hybrid systems. In metal-loaded catalysts, the conductive carbon support acts as an electron bridge, facilitating rapid electron transfer from the adsorbed pollutant molecule to the oxidant or the metal active site[50]. This synergy significantly accelerates the degradation kinetics compared to either component alone. The performance of these catalysts is formidable. They have been successfully deployed to degrade a wide spectrum of recalcitrant aquatic pollutants, including antibiotics (e.g., tetracycline, sulfamethoxazole), pesticides, and endocrine-disrupting chemicals, often achieving > 95% degradation within minutes under optimized conditions[51]. This efficacy, combined with the advantages of easy magnetic or gravitational separation and excellent reusability over multiple cycles, positions torrefaction-derived carbon catalysts as superior and sustainable alternatives to homogeneous catalysts.
Bio-medical functionalization
-
The application of torrefaction-derived carbon materials in the biomedical field represents a frontier where sustainability meets high technology, demanding exceptional material properties: superior biocompatibility, precisely controlled surface chemistry, and often, nanoscale dimensions for intracellular interaction[52]. Unlike bulkier applications, here the carbonization process is applied to well-defined, often molecular, biomass precursors to create highly uniform functional nanomaterials.
The nucleation and growth of carbon quantum dots (CQDs) are directly governed by the carbonization parameters. The temperature and duration precisely control the degree of carbonization, determining the size of the sp2-hybridized carbon core, and the nature of the sp3-hybridized defect sites within it[53]. Simultaneously, the chemical composition of the precursor and the reaction medium dictates the surface state, passivating the core with a rich array of functional groups such as carbonyls, carboxyls, hydroxyls, and amines[54]. The synergy between the carbon core and this surface state is the fundamental determinant of the CQDs photoluminescence (PL) properties. A larger sp2 domain and specific surface functional groups can redshift the fluorescence, enabling tunable emissions across the blue, green, and even red spectrum from a single precursor system by simply varying the synthesis temperature[55]. This inherent surface functionalization is a primary advantage. The abundant -COOH, -OH, and -NH2 groups are not passive, but are reactive handles for sophisticated bioconjugation. These groups allow for covalent attachment of targeting ligands (e.g., folic acid for cancer cells overexpressing folate receptors), recognition elements (e.g., antibodies for specific antigen binding), or therapeutic cargos (e.g., anti-cancer drugs like Doxorubicin), transforming the inert carbon nanomaterial into a biologically active agent[56]. A schematic illustration of biomedical applications for torrefaction-derived CQDs is shown as Fig. 5.
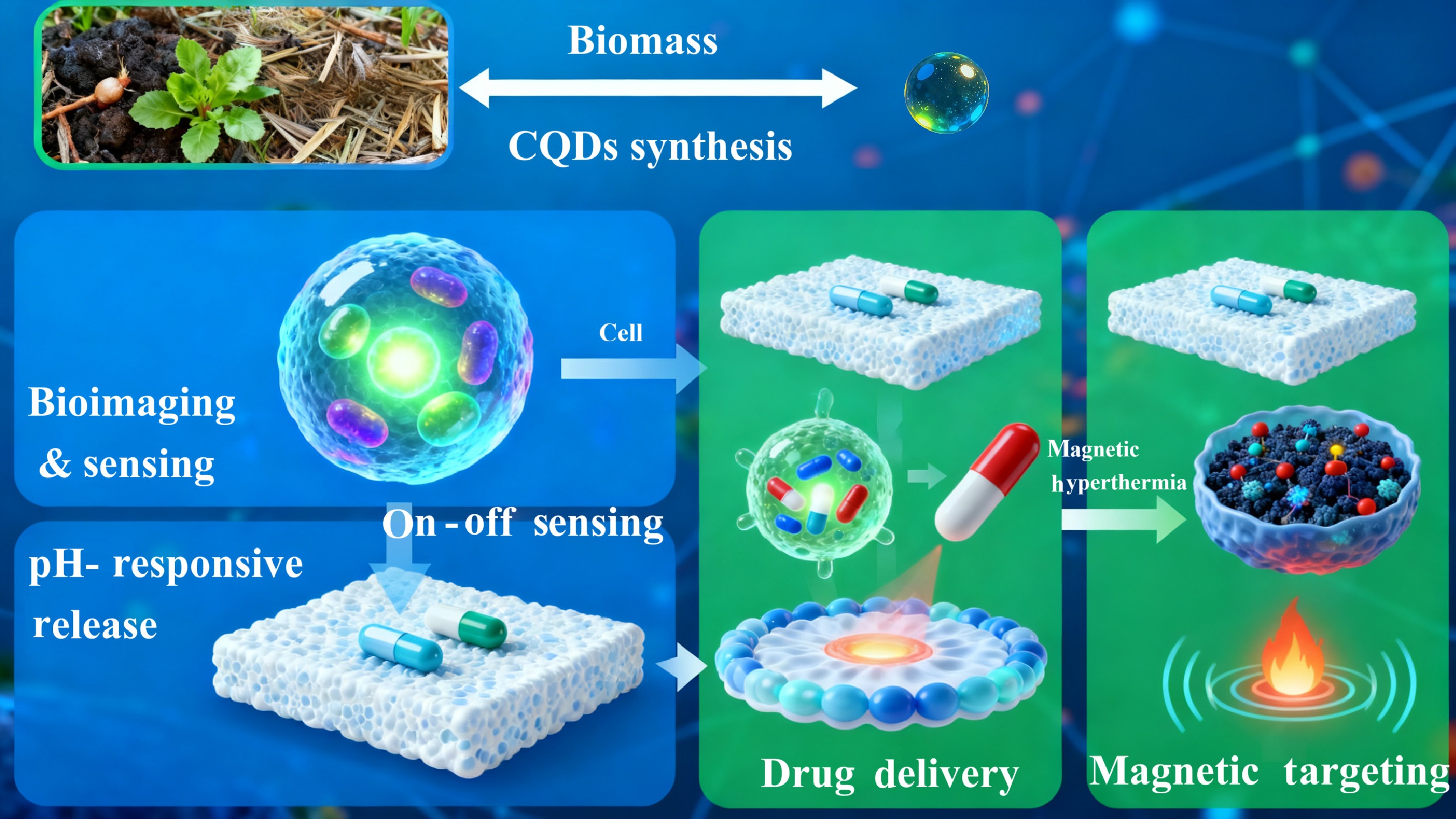
Figure 5.
The schematic illustration of biomedical applications for torrefaction-derived carbon quantum dots (CQDs).
Biomass-derived CQDs are heralded as biocompatible, low-toxicity alternatives to traditional semiconductor quantum dots, which are often composed of toxic heavy metals like cadmium. Their tunable, strong, and stable fluorescence make them excellent probes for high-contrast in vitro cellular imaging[57]. Furthermore, their small size and surface engineering facilitate their use in in vivo imaging, where they can act as contrast agents for mapping tumors and visualizing vascular structures, often with higher clearance rates and reduced long-term toxicity compared to inorganic counterparts. Moving beyond CQDs, porous carbon nanoparticles derived from torrefied precursors offer a robust platform for drug delivery[58]. Their mesoporous structures can be loaded with high doses of chemotherapeutic agents. The true sophistication lies in engineering 'gatekeepers' on the surface that are responsive to the unique microenvironment of a tumor, such as lower pH (acidosis) or higher glutathione levels. This enables a stimulus-responsive drug release, ensuring the cytotoxic payload is unleashed predominantly within the tumor tissue, thereby drastically minimizing off-target side effects and enhancing therapeutic efficacy.
The versatile photophysical properties of CQDs are exploited in highly sensitive biosensing platforms. Their fluorescence can be quenched through various mechanisms, such as Förster resonance energy transfer (FRET), photoinduced electron transfer (PET), or static quenching, when they specifically interact with target analytes[56]. This "on-off" or "ratiometric" sensing mechanism allows for the detection of a vast array of targets, from metal ions and small molecules to enzymes, pathogens, and disease biomarkers. For instance, CQDs functionalized with specific recognition elements can detect glucose levels for diabetes management, or identify pathogenic bacteria through selective binding events, offering rapid, sensitive, and low-cost diagnostic solutions.
-
Although the single-function carbon materials discussed previously demonstrate excellent performance in their respective fields, they often fall short when addressing complex real-world application scenarios due to their singular performance dimension. For instance, an efficient adsorbent may be incapable of degrading pollutants in situ; a high-performance electrode material may lack recoverability; a bioimaging probe may lack targeting or therapeutic functions. These limitations have catalyzed the necessary evolution towards multifunctional composites. Consequently, the current research frontier decisively moves beyond single-function materials towards the rational design of sophisticated, multifunctional composites, where torrefied biochar transcends its traditional role to act as a synergistic and enabling component. This paradigm shift is driven by the need for integrated solutions that can perform multiple tasks simultaneously or respond adaptively to complex environments. The unique properties of torrefied biochar make it an ideal building block for preparing these advanced materials.
The current research frontier decisively moves beyond single-function materials towards the rational design of sophisticated, multifunctional composites, where torrefied biochar transcends its traditional roles to act as a synergistic and enabling component[59]. This paradigm shift is driven by the need for integrated solutions that can perform multiple tasks simultaneously or respond adaptively to complex environments[16]. The unique properties of tunable surface chemistry, developing conductivity, and robust yet porous structures makes the torrefied biochar an ideal building block for creating these advanced materials. The research focus is on creating synergistic interfaces between torrefied biochar and other functional entities (metals, metal oxides, polymers, etc.), where the final composite exhibits properties that are more than the sum of its parts.
Magnetic carbon composites
-
The development of magnetic carbon composites represents a paradigm shift in the design of functional materials, moving from passive substances to intelligent, responsive systems that can be manipulated and recovered with precision[60]. At the heart of this innovation lies the synergistic coupling of a torrefied carbon matrix with magnetic nanoparticles, creating a composite that overcomes one of the most significant practical limitations of powdered carbons. The synthesis, functionality, and applications of these composites are multifaceted and have seen remarkable advances. The multifunctional composite systems based on torrefied biomass and their synergistic characteristics are listed in Table 4.
Table 4. Multifunctional composite systems based on torrefied biomass and their synergistic characteristics
Composite type Primary synthesis strategies Key synergistic properties Representative applications Magnetic carbon 1. Pre-/post-torrefaction impregnation with metal salts (e.g., Fe3+, Co2+) followed by thermal reduction.
2. Co-precipitation of magnetic nanoparticles (e.g., Fe3O4) onto carbon surface.1. High adsorption/catalytic capacity of carbon matrix.
2. Carbon matrix prevents nanoparticle agglomeration, enhances electron transfer, and stabilizes nanoparticles against leaching.1. Magnetically recoverable adsorbents/catalysts for wastewater treatment.
2. Magnetically guided drug delivery and magnetic hyperthermia for cancer therapy.
3. Resource recovery (e.g., precious metals from e-waste).Carbon/TiO2 hybrids 1. Sol-gel method using precursors.
2. In-situ growth/deposition of TiO2 from solution.
3. Chemical vapor infiltration (CVI).1. Pollutant pre-concentration by carbon adsorbent
2. Carbon acts as an electron shuttle, suppressing charge carrier recombination in TiO2.
3. Carbon can act as a photosensitizer, extending light absorption into the visible range.1. Highly efficient photocatalytic degradation of pollutants in air and water.
2. Photocatalytic water splitting for H2 production.
3. CO2 photoreduction to solar fuels.Carbon/SiO2 composites Sol-gel synthesis using silica precursors (e.g., TEOS), followed by ambient (xerogel) or supercritical (aerogel) drying. 1. Nanocomposite reinforcement: Carbon scaffold imparts mechanical robustness to brittle silica.
2. Thermal superinsulation: Synergy between silica's nanoporosity and carbon's role as an infrared opacifier results in ultra-low thermal conductivity.
3. Unique electrothermal properties: Combination of thermal insulation and electrical conductivity.1. Next-generation thermal superinsulators for buildings, pipelines, and aerospace.
2. Lightweight, insulating substrates for electronics.
3. Electrothermal insulation and self-sensing structures.3D-printable conductive
inks1. Dispersion of fine torrefied carbon powder into a polymeric binder matrix (e.g., PDMS for flexibility, PVDF for stability, PLA for biodegradability).
2. Additives (surfactants, rheology modifiers) for optimal printability.1. Structural design freedom from 3D printing.
2. Shear-thinning rheology for extrusion-based printing and shape retention after deposition.
3. Multi-functionality: Can combine conductivity with flexibility, biodegradability, or high surface area.1. Custom-shaped, interdigitated micro-supercapacitors and battery electrodes.
2. Wearable and flexible strain sensors (electronic skin).
3. High-surface-area biosensors and conductive scaffolds for tissue engineering.Synthesis: strategic integration of magnetic phases
-
The synthesis of these composites is a critical step that dictates the dispersion, stability, and ultimately the performance of the magnetic component. The strategy involves incorporating Fe3+, Co2+, or Ni2+ salts into the process at different stages, each offering distinct advantages.
(1) Pre-torrefaction impregnation: In this method, the raw biomass is first soaked in a solution of the metal salt (e.g., FeCl3, Fe[NO3]3). The hydrophilic and porous nature of the biomass allows for capillary-driven uptake and distribution of the metal ions throughout its structure. Subsequent torrefaction serves a dual purpose: it carbonizes the biomass and, in the reducing environment created by the decomposing organic matter, reduces the metal ions to their magnetic forms[61]. For iron, this typically yields magnetite (Fe3O4) or maghemite (γ-Fe2O3). The key advantage of this method is the potential for a very uniform distribution of nanoparticles, as the metal ions are molecularly dispersed before carbonization.
(2) Post-torrefaction impregnation: Here, the already torrefied biomass is subjected to the metal salt solution. The torrefied material, with its developed porosity and surface functional groups, acts as an adsorbent for the metal ions. A second thermal treatment, often at a higher temperature than the initial torrefaction, is then applied to reduce the adsorbed ions to metallic (e.g., Fe0, Co0) or oxide nanoparticles[62]. This method provides greater control over the carbon matrix's structure before magnetic modification, and can prevent potential catalytic effects of the metal during the initial torrefaction, which might alter the carbonization pathway.
(3) Co-precipitation: This method is particularly effective for creating composites with a high loading of well-defined magnetic nanoparticles[63]. It involves the addition of a base (e.g., NaOH) to a suspension of torrefied carbon containing adsorbed Fe2+/Fe3+ ions, leading to the direct precipitation of Fe3O4 nanoparticles onto the carbon surface. This allows for precise control over the nanoparticle size and crystallinity.
The choice of magnetic metal is crucial. Iron oxides are most common due to their strong magnetism, low cost, and biocompatibility. Cobalt offers higher magnetic susceptibility but raises toxicity and cost concerns. Nickel is less common due to its weaker magnetism and potential toxicity.
Multifunctionality: a synergy of properties
-
The true power of these composites lies in the non-linear synergy between their components, resulting in functionalities that are more than the sum of their parts.
Function 1: Enhanced adsorption and catalysis. The torrefied carbon matrix is not a passive host. It provides the primary active sites for adsorption via its high surface area and porous structure, capable of capturing organic dyes, pharmaceuticals, and heavy metals through mechanisms like pore filling, π-π stacking, and electrostatic interactions[64]. Furthermore, the composite can be a powerful catalyst. The magnetic nanoparticles, particularly zero-valent iron or Fe3O4, can activate hydrogen peroxide (H2O2) or persulfates (S2O82−), to generate highly reactive radicals (e.g., •OH, SO4•−) in Fenton-like reactions. The carbon matrix enhances this catalysis by adsorbing pollutants close to the reactive sites, facilitating electron transfer between the oxidant and the pollutant, and even preventing the leaching of metal ions by stabilizing the nanoparticles.
Function 2: Magnetic separability and reusability. The embedded magnetic nanoparticles (typically > 10 nm in size to be superparamagnetic or ferromagnetic) impart a strong magnetic response to the entire composite. After the adsorption or catalytic reaction is complete, a simple external magnet can rapidly separate the spent powder from the aqueous solution within seconds[65]. This solves the long-standing challenge of filter clogging and material loss associated with conventional filtration or centrifugation. This facile separation is the cornerstone of the material's economic and operational viability, as it enables easy recovery and regeneration for multiple use cycles, dramatically reducing operational costs and secondary waste.
Advanced and emerging applications
-
The applications of magnetic carbon composites extend far beyond simple separation, venturing into sophisticated realms of environmental and biomedical engineering. Magnetically guided oil-spill cleanup is a method to sprinkle hydrophobic magnetic carbons over an oil spill. They rapidly absorb the oil, and the resulting oil-saturated composite can then be swiftly collected from the water surface using magnetic skimmers, offering a highly efficient and contained cleanup method[66]. These composites are ideal catalysts for wastewater treatment. They effectively degrade recalcitrant organic pollutants (e.g., antibiotics, pesticides) and can be magnetically retrieved after each batch, regenerated, and reused for numerous cycles without a significant loss of activity, overcoming the sludge formation issue of homogeneous Fenton processes. The composites can be functionalized with specific ligands to selectively adsorb precious metals like gold or palladium from electronic waste leachates. The magnetic separation allows for the efficient concentration and recovery of these valuable resources.
Biomedical and biotechnological applications
-
Concerning magnetically guided drug delivery, the composite can be loaded with a chemotherapeutic drug. Upon intravenous injection, an external magnetic field can be applied to a tumor site, guiding and concentrating the drug-loaded particles there. This "magnetic targeting" enhances drug efficacy at the target site while minimizing systemic side effects[67]. For hyperthermia therapy, when subjected to an alternating magnetic field, the magnetic nanoparticles within the composite generate heat. This can be used to locally ablate cancer cells by raising the tumor temperature to 41–47 °C. The carbon component can simultaneously deliver drugs, creating a powerful combined therapy. Regarding magnetic bioseparation, the composites can be surface-functionalized with antibodies or enzymes[68]. They can then be used to magnetically separate specific cells, proteins, or DNA from complex biological mixtures, serving as a powerful tool in diagnostics and biotechnology.
In conclusion, magnetic carbon composites, built upon the versatile platform of torrefied biomass, are a quintessential example of a multifunctional material. They seamlessly integrate the superior sorptive and catalytic properties of carbon with the remote controllability of magnetism, opening up transformative applications in environmental sustainability and advanced medicine. Future research is focused on improving nanoparticle dispersion, enhancing chemical stability against leaching, and developing smarter composites that respond to multiple stimuli (e.g., pH, temperature, and magnetic field) for even more precise control.
Carbon/inorganic hybrid composites
-
The frontier of multifunctional materials is powerfully exemplified by carbon/inorganic hybrid composites, where the tailored properties of torrefied carbon are synergistically combined with the unique characteristics of inorganic phases[69]. This is not a simple physical mixture, but a molecular-level integration that creates entirely new material systems with emergent properties, overcoming the limitations of each component[70]. The synthesis of these hybrids is a sophisticated process of material architecture, designed to leverage the complementary strengths of organic and inorganic matter for advanced technological applications. The design strategies and applications of multifunctional composites based on torrefied biomass are shown in Fig. 6.
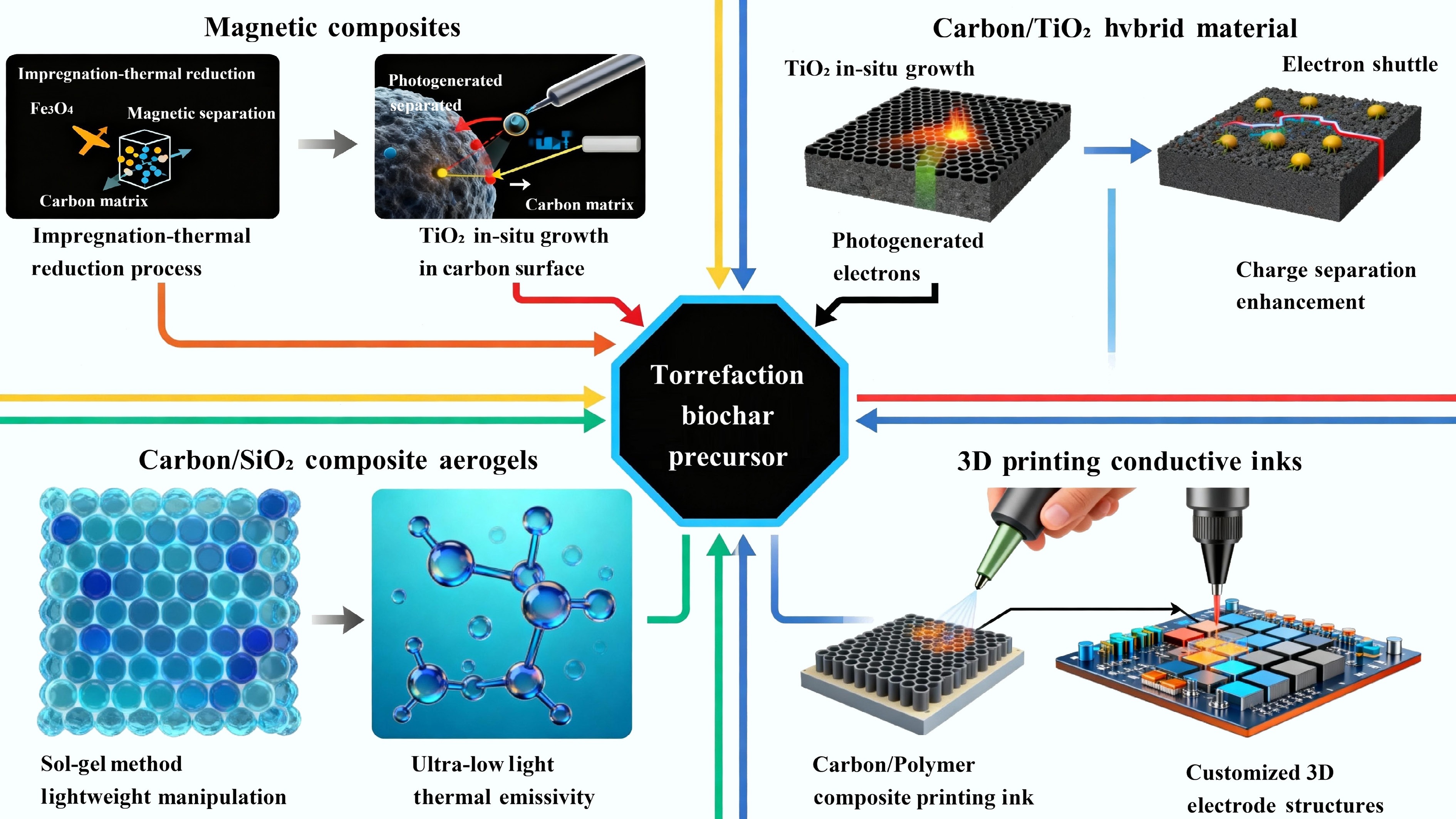
Figure 6.
The design strategies and applications of multifunctional composites based on torrefied biomass.
Synthesis: architecting the hybrid interface
-
The preparation of these composites relies on co-processing torrefied biomass with inorganic precursors through carefully controlled chemical pathways. The goal is to achieve a homogeneous dispersion and a strong interfacial bonding between the carbon and the inorganic phases.
(1) Sol-gel synthesis: This is a highly versatile and common method, particularly for oxides like SiO2 and TiO2. In this process, the torrefied biochar (often in a powdered or dispersed form) is immersed in a solution containing the molecular precursor, such as tetraethyl orthosilicate (TEOS) for silica, or titanium isopropoxide (TTIP) for titania[71]. Through controlled hydrolysis and condensation reactions, the precursor molecules form a gel network that permeates and encapsulates the porous carbon structure. Subsequent drying and thermal curing result in a nanocomposite where the inorganic phase is intimately intertwined with the carbon matrix at the nanoscale[72]. The surface chemistry of the torrefied biochar, rich in functional groups, can actively participate in these reactions, leading to strong covalent C-O-Si or C-O-Ti bonds that are critical for mechanical stability and efficient electron transfer.
(2) In-situ growth/deposition: This method involves growing the inorganic phase directly from a solution onto the surface of the torrefied biochar. For example, to prepare carbon/TiO2 composites, the torrefied biochar can be dispersed in a solution of a titanium salt (e.g., TiCl4) or TTIP[73]. By controlling the pH and temperature, TiO2 nanoparticles or a continuous layer can be precipitated or crystallized directly onto the carbon surface. This method often yields a more direct and controlled interface, ensuring close contact between the photocatalyst and the conductive carbon support.
(3) Chemical vapor infiltration (CVI): For more advanced applications, techniques like CVI can be used to infiltrate the pores of a pre-formed torrefied biochar with gaseous inorganic precursors (e.g., silane for SiC formation)[74]. This allows for the precise coating of the internal pore walls with a uniform layer of the inorganic material, creating a highly complex and interpenetrating network.
The selection of the synthesis method is crucial as it dictates the morphology, interfacial area, and bonding strength, which ultimately govern the composite's performance.
Multifunctionality and synergistic mechanisms
-
The combination of torrefied biochar with titanium dioxide (TiO2) creates a powerful photocatalytic system that addresses the key shortcomings of pure TiO2: its rapid recombination of photogenerated electron-hole pairs, and its limited adsorption capacity for target pollutants.
With its high specific surface area and developed porosity, the torrefied biochar acts as a highly efficient adsorbent. It firstly concentrates pollutant molecules from a dilute aqueous or gaseous stream onto its surface, effectively increasing the local concentration of pollutants in the immediate vicinity of the TiO2 nanoparticles[75]. This pre-concentration step significantly enhances the probability of pollutant-photon interactions, drastically improving the overall degradation kinetics compared to a system where TiO2 is suspended in a bulk solution with dispersed pollutants. Electron shuttle and charge separation are the most critical synergistic effects for its functionalization. When TiO2 is irradiated with UV or visible light (if modified), it generates electron-hole (e−/h+) pairs. In pure TiO2, a large fraction of these pairs recombine within nanoseconds, releasing heat instead of driving chemical reactions. The torrefied biochar, with its graphitic domains and superior electrical conductivity, acts as an efficient electron acceptor and shuttle[76].
The photo-generated electrons are rapidly transferred from the conduction band of TiO2 to the carbon matrix. This physical separation of electrons and holes drastically suppresses their recombination. The longevity of the holes (h+) left in the TiO2 valence band is thereby extended, allowing them to more effectively oxidize water or directly degrade pollutants[77]. The electrons in the carbon can be transferred to surface-adsorbed oxygen molecules, generating superoxide radical anions (•O2−), which are also potent oxidizing agents. Furthermore, the carbon component can act as a photosensitizer. It can absorb a broader spectrum of light, including visible light, and inject excited electrons into the conduction band of TiO2, thereby extending the photocatalytic activity into the visible range, which constitutes a much larger portion of solar energy[78]. These synergistic effects make carbon/TiO2 hybrids exceptionally effective for applications far beyond basic pollutant degradation, including photocatalytic water splitting for hydrogen production, and CO2 photoreduction into solar fuels.
Carbon/SiO2 composites for advanced thermal and structural applications
-
The integration of torrefied biochar with silica (SiO2) leverages the contrasting but complementary properties of both materials to create lightweight monolithic solids with exceptional multifunctional performance[4]. Using the sol-gel method with a TEOS precursor, a hybrid wet gel is first formed. If this gel is dried under supercritical conditions, the liquid is removed without collapsing the delicate pore structure, resulting in a hybrid aerogel. If dried under ambient conditions, a denser but still porous xerogel is formed. In both cases, the torrefied biochar becomes an integral part of the inorganic silica network.
To achieve the synergistic property enhancement, mechanical reinforcement is a promising method. Pure silica aerogels are notoriously brittle and fragile. By creating a reinforcing scaffold within the silica matrix to achieve torrefied biochar functionalization, this "nanocomposite reinforcement" significantly enhances the mechanical robustness, elasticity, and fracture toughness of the material, allowing it to withstand practical handling and structural loads[61]. Moreover, silica is an excellent thermal insulator due to its ability to minimize all three heat transfer pathways: solid conduction (through its nanoporous structure), gas conduction (by possessing pores smaller than the mean free path of air molecules), and radiation (via opacifiers). The carbon phase, while potentially increasing solid conduction slightly, can be optimized to act as an infrared opacifier, scattering radiative heat and further lowering the overall thermal conductivity. The result is a monolithic material with thermal conductivity often below that of static air (0.025 W/m·K), making it a thermal superinsulator. In addition, the conductive carbon network embedded within the insulating silica matrix creates a unique combination of properties. The composite remains a superb thermal insulator but gains electrical conductivity[79]. This enables applications such as electrothermal insulation (where the material can be heated by passing a current to prevent icing while still insulating) or self-sensing structures (where changes in electrical resistance can monitor structural health or stress).
The applications for carbon/SiO2 composites are vast, ranging from next-generation thermal insulation for buildings, pipelines, and aerospace vehicles, to serve as lightweight, insulating substrates for electronics or catalyst supports in high-temperature reactions. The exploration of carbon hybrids with other inorganics, such as alumina (Al2O3) for ultra-high temperature stability or calcium phosphate for biomimetic bone implants, further broadens the horizon of this exciting material class, showcasing the virtually limitless potential of combining torrefied carbon with inorganic chemistry.
3D printing inks and flexible electronics
-
The integration of torrefied carbon into the realm of 3D printing and flexible electronics represents a revolutionary leap from conventional material fabrication towards the digital, customizable, and architecturally complex design of functional devices. This approach transcends the limitations of traditional manufacturing, enabling the creation of structures with geometrically optimized, multi-scale porosity and form factors[80]. The core of this technology lies in the formulation of advanced functional inks, where carbon ceases to be just a powder and becomes the active ingredient in a versatile, formable material system.
Synthesis and ink formulation: a delicate balance
-
The synthesis of these functional inks is a sophisticated process of composite engineering, requiring a precise balance between material functionality and processability. The starting point is the production of carbon micro or nanoparticles with consistent morphology. Torrefaction and hydrothermal carbonization (HTC) are the two primary methods. Torrefaction produces a more aromatic, conductive carbon, ideal for applications demanding high electrical conductivity. HTC, on the other hand, often yields "hydrochar" spheres with a richer surface oxygen functionality, which can improve dispersibility in aqueous or polar binder systems[81]. These carbons are often subjected to further size reduction through ball milling or grinding to achieve a fine, uniform powder that prevents nozzle clogging during printing. The carbon particles are dispersed into a polymeric binder matrix at a high loading (the "filler" content). The choice of binder is critical and application-specific: Elastomeric Binders (e.g., Polydimethylsiloxane - PDMS, Styrene-Ethylene-Butylene-Styrene - SEBS): These are used for creating stretchable and flexible electronics. They allow the printed structure to withstand bending, twisting, and stretching while maintaining electrical connectivity.
Thermoplastic binders (e.g., Polyvinylidene fluoride - PVDF, Polycaprolactone - PCL, Polylactic acid - PLA): These are suitable for fused deposition modeling (FDM), or direct ink writing (DIW) where thermal properties are the key. PVDF offers good chemical stability, while PCL and PLA are biodegradable, opening doors for transient implants[82]. Hydrogel binders (e.g., sodium alginate, gelatin): Used for bioprinting and creating soft, hydratable structures for biomedical interfaces. The formulation process involves thoroughly mixing the carbon filler into a solution or melt of the polymer. Additives such as surfactants (to prevent agglomeration), rheology modifiers (like fumed silica or clays to control flow), and plasticizers (to enhance flexibility) are often incorporated to fine-tune the ink's properties.
Multifunctionality: the convergence of form and function
-
The multifunctionality of these composite inks is what enables this technological paradigm shift, merging the process of manufacturing with the integration of performance. For extrusion-based 3D printing like DIW, the ink must be shear-thinning, due to its viscosity decreasing under the shear stress of being pushed through the nozzle, allowing it to flow, but it must instantly recover its high viscosity upon deposition to hold its shape and support subsequent layers (a property known as yield stress). The carbon filler and additives are crucial in achieving this non-Newtonian behavior[83]. The printed object is not merely a shape, but also a designed structure. It can be a lightweight lattice, a fractal-like electrode, or a porous scaffold with controlled pore size and interconnectivity, all dictated by the digital design file.
The torrefied biochar filler provides the essential electrical conductivity throughout the printed object. This occurs through the formation of a percolation network, which is a continuous pathway of touching, or proximally close carbon particles through which electrons can travel. The high carbon loading ensures that this network is robust[84]. Unlike metallic conductors, these carbon-polymer composites remain flexible and corrosion-resistant. The conductivity can be tuned by varying the carbon loading, type (with torrefied carbon generally offering higher conductivity than hydrochar), and degree of particle alignment during the extrusion process.
Advanced and emerging applications
-
The ability to 3D-print conductive carbon structures unlocks a new frontier of application-specific device design. Concerning the customized energy storage devices, this is a transformative application. It is now possible to 3D print entire micro-supercapacitors (MSCs) or battery electrodes with interdigitated architectures[85]. This design drastically reduces the ion travel distance, enabling ultra-fast charging and discharging (high power density). Furthermore, devices can be printed in custom shapes to fit the unused spaces within electronic housings, a concept known as "structural energy storage". Flexible, printed supercapacitors can be integrated directly into the fabric of wearable devices.
As for advanced sensing platforms, a 3D-printed grid of conductive carbon-PDMS composite can act as a highly sensitive strain sensor. When stretched or bent, the distances between carbon particles change, altering the electrical resistance (piezoresistive effect). This allows for the creation of "electronic skin" that can monitor human motion, joint movement, respiration, or pulse[86]. By functionalizing the carbon surface with specific enzymes or antibodies, 3D-printed electrodes can become highly sensitive and selective biosensors. The printed porous structure offers a massive surface area for biomolecule immobilization and analyte interaction, boosting sensitivity for detecting glucose, pathogens, or other biomarkers in point-of-care diagnostic devices. For electrically active tissues, such as bone, heart, and nerve, a 3D-printed scaffold made of a biodegradable polymer (like PCL) and conductive carbon is ideal[87]. The scaffold provides the mechanical support and the defined porosity for cell migration and growth, while the carbon conductivity can electrically stimulate cells to promote regeneration, e.g., accelerating bone healing or guiding neural axon growth. 3D printing allows for the fabrication of neural probes or electrode arrays that perfectly match a patient's unique anatomy, obtained from MRI or CT scans. This improves the interface quality and minimizes tissue damage, leading to more effective neural recording and stimulation for treating conditions like Parkinson's disease or paralysis[88].
The future of this field lies in multi-material printing, where conductors (torrefied biochar), insulators, and even other functional materials like semiconductors can be printed simultaneously to create fully integrated, complex devices straight from a digital file. The use of torrefied biochar as the conductive element ensures that this high-tech future is built upon a sustainable, low-cost, and high-performance foundation[89].
-
Biomass torrefaction has firmly established itself as a pivotal and versatile technology in the sustainable production of functional carbon materials. It masterfully addresses the inherent weaknesses of raw biomass by producing a stabilized, energy-dense, and hydrophobic solid with a deoxygenated and aromatized structure[61]. This torrefied material is not the final product but an engineered precursor whose properties can be tailored for downstream functionalization. As detailed in this review, this approach enables the synthesis of high-performance materials for a multitude of applications:
(1) Energy storage. To prepare hierarchical porous biochar material for supercapacitors with high capacitance and outstanding cyclability.
(2) Environmental remediation. The production of highly efficient adsorbents and, notably, powerful metal-free or metal-supported catalysts for advanced oxidation processes.
(3) Biomedicine. The synthesis of biocompatible carbon quantum dots and nanocarbons for advanced imaging, drug delivery, and sensing.
(4) Advanced composites. Serving as a key component in multifunctional materials, such as magnetically separable sorbents/catalysts and conductive 3D-printable composites.
Despite significant progress, several challenges and exciting opportunities lie ahead:
(1) From empirical to predictive synthesis. Future work should focus on developing a deeper, fundamental understanding of the relationship between biomass composition, torrefaction parameters, precursor structure, and final material performance. The integration of in-situ characterization techniques and machine learning could enable the predictive design of carbon materials with pre-defined properties.
(2) Process intensification and scale-up. Currently, most research remains at the lab scale. The development of continuous, efficient, and economically viable torrefaction and integrated activation reactors is crucial for commercial translation. Life-cycle assessment (LCA) and techno-economic analysis (TEA) studies are needed to validate the environmental and economic benefits of the entire value chain.
(3) Exploration of novel functionalization pathways. Beyond KOH activation and N-doping, there is room to explore novel functionalization methods, such as plasma treatment, microwave-assisted activation, and templating methods to create even more precise pore architectures.
(4) Expansion into new application frontiers. The potential of torrefaction-derived biochar in emerging fields like CO2 capture and conversion, electrocatalysis for fuel cells and water splitting, and as components in next-generation batteries (Li-S, Na-ion) warrants extensive investigation.
(5) Standardization and safety. As these materials move towards commercialization, issues of standardization (quality control) and the environmental health and safety (EHS) of torrefied carbon nanoparticles need to be proactively addressed.
Totally, biomass torrefaction is far more than a mere pretreatment; it is a powerful platform technology that unlocks the latent potential of lignocellulosic waste, transforming it into the advanced carbon materials that will underpin a more sustainable and technologically advanced future. Most current research remains at the laboratory scale. A central challenge in advancing this technology towards practical application lies in developing continuous, efficient, and economically viable processes and reactors for scaled-up torrefaction and integrated activation. Crucially, future work must move beyond mere performance optimization to systematically integrate TEA and LCA. TEA is needed to quantify the full-process costs from feedstock collection to final product production, identify key levers for reducing energy consumption and capital expenditure, and assess economic competitiveness at different production scales. LCA is required to comprehensively evaluate the environmental footprint (e.g., greenhouse gas emissions, water consumption) from "cradle to grave", ensuring that the entire value chain, starting from biomass waste, delivers genuine environmental benefits and avoids potential burden shifting. The combination of TEA and LCA will provide a solid foundation for decision-making regarding process route selection, scale-up design, and policy formulation, serving as an indispensable bridge connecting laboratory innovation to market application.
As torrefaction-derived carbon materials, particularly carbon quantum dots (CQDs) and porous carbon nanoparticles, demonstrate significant potential in biomedical fields such as bioimaging, drug delivery, and biosensing, establishing standardized characterization protocols and a comprehensive Environmental, Health, and Safety (EHS) assessment framework tailored to them becomes unprecedentedly urgent, and a prerequisite for their clinical and commercial translation. Firstly, material standardization norms must be established. The diversity of biomass feedstocks and fluctuations in torrefaction/post-processing parameters can lead to batch-to-batch variations in the final nanomaterials regarding size, shape, surface chemistry, zeta potential, and impurity content (e.g., metal residues). A unified set of stringent characterization standards (drawing from ISO, ASTM, etc.) must be developed to ensure batch-to-batch consistency, reproducibility, and quality control, forming the foundation for reliable biological studies and subsequent regulatory approval. Secondly, systematic toxicology and biocompatibility assessments are imperative. Although many studies report the "good biocompatibility" of biomass-derived carbon materials, this conclusion is often based on limited in vitro cell tests or short-term in vivo models.
For clinical translation, more rigorous guidelines must be followed, involving comprehensive preclinical safety evaluations, including long-term in vivo toxicology, biodistribution, metabolic pathways, excretion mechanisms, and potential immunogenicity studies. Special attention must be paid to nano-size effects, surface-chemistry-dependent toxicity, and potential long-term impacts from degradation products. Finally, guidelines for assessing and managing life-cycle environmental and occupational exposure risks need to be established. This encompasses evaluating potential exposure risks to workers, consumers, and the environment during the production, transport, use, and disposal of these nanomaterials, and developing corresponding safe handling and containment measures. Proactively addressing the standardization and EHS challenges does not hinder innovation, but rather constructs a credible, reliable, and responsible translation pathway for these promising sustainable nanomaterials. It is a crucial step in moving them from the laboratory to the marketplace and, ultimately, benefiting society.
-
In summary, this review has comprehensively articulated the critical role of biomass torrefaction as a foundational technology for the functionalization and application of sustainable carbon materials. By undergoing a controlled thermal treatment in the 200–300 °C range, raw biomass is transformed from a heterogeneous, oxygen-rich, and unstable resource into a superior carbonaceous precursor. This torrefied material exhibits a stabilized, aromatized structure with a reduced atomic O/C ratio, which is ideally suited for subsequent activation and functionalization processes. The resultant advanced carbons demonstrate exceptional performance across a diverse spectrum of high-value applications. These include high-energy-density supercapacitors, efficient adsorbents, and catalysts for environmental remediation, and biocompatible agents for biomedical imaging and therapy. The frontier of this field lies in the design of multifunctional composites, integrating magnetic, photocatalytic, or structural properties for next-generation technologies. As research progresses from empirical studies towards predictive design and scalable production, torrefaction is poised to be a cornerstone of the circular bio-economy, effectively bridging the gap between abundant biomass waste, and the pressing demand for high-performance, sustainable materials.
-
The authors confirm their contributions to the paper as follows: all authors contributed to the study conception and design; material preparation, data collection and analysis were performed by Wei Han; the first draft of the manuscript was written by Congyu Zhang; and all authors commented on previous versions of the manuscript. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
-
This work was supported by China Postdoctoral Science Foundation (2023MD744171), and high-level talent introduction project of Northeast Agricultural University. The authors also acknowledge the financial support of the Open Research Fund of State Key Laboratory of Geomechanics and Geotechnical Engineering Safety (SKLGME022030), and the Technological Project of Heilongjiang Province "the open competition mechanism" to select the best candidates (2022ZXJ05C02).
-
The authors declare that they have no conflict of interest.
-
Torrefaction enables tailored precursor engineering for advanced carbon materials.
Torrefied biochars achieve high capacitance and stability in supercapacitors.
Torrefaction-derived materials excel in adsorption and catalytic pollutant degradation.
Biomedical applications include bioimaging CQDs and drug delivery systems.
Multifunctional composites enable magnetic recovery and 3D-printable electronics.
-
Full list of author information is available at the end of the article.
- Copyright: © 2026 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Han W, Wang Y, Wang L, Xie P, Liu T, et al. 2026. A comprehensive review of biomass torrefaction as a versatile platform for the synthesis of functional carbon materials. Sustainable Carbon Materials 2: e007 doi: 10.48130/scm-0026-0002
A comprehensive review of biomass torrefaction as a versatile platform for the synthesis of functional carbon materials
- Received: 06 November 2025
- Revised: 04 January 2026
- Accepted: 10 January 2026
- Published online: 10 February 2026
Abstract: Biomass torrefaction, a mild pyrolysis process at 200–300 °C under an inert atmosphere, has emerged as a pivotal pretreatment technology for solid biowaste conversion and functional carbon materials production. This process fundamentally transforms raw biomass through deoxygenation and aromatization, with improved material properties. The chemical composition and structural heterogeneity of the biomass feedstock significantly influences the deoxygenation and aromatization pathways during torrefaction, thereby tuning the pore structure, surface chemistry, and electrochemical performance of the resulting carbon materials. This engineered intermediate is highly amenable to subsequent activation and functionalization, enabling the synthesis of high-value carbon materials. Torrefaction-derived porous biochar exhibits exceptional performance as electrodes in supercapacitors, delivering high specific capacitance and cycling stability due to its hierarchical pore structure and tunable surface chemistry. Furthermore, these materials serve as highly efficient adsorbents for pollutants and, when doped with heteroatoms or metals, as potent catalysts for degrading organic contaminants via advanced oxidation processes. In the biomedical field, controlled carbonization yields biocompatible carbon quantum dots for bio-imaging and drug delivery. The current research frontier is shifting toward multifunctional composites, such as magnetically separable catalysts and 3D-printable conductive inks. By providing a versatile and sustainable platform for precursor engineering, biomass torrefaction effectively bridges the gap between abundant renewable resources and the pressing demand for high-performance, sustainable materials across energy, environmental, and biomedical sectors. This review comprehensively summarizes the current research progress and forecasts the future trend to contribute a potential solution with great practical significance.