-
Dietary protein and fat, as important macronutrients, have been consumed by humans in various forms. Dietary protein and fat are important dietary substances for maintaining human growth, and their nutritional value is not only determined by their composition (source and type), but also largely depends on bioavailability in the gastrointestinal tract (GIT)[1]. Water, proteins and lipids are the main components of mammalian muscle[2]. Dietary patterns in high-income countries have a higher proportion of animal foods, such as 62% of dietary protein for adults in the United States coming from animals[3]. The amino acid pattern of animal protein is close to that of human protein, which can be easily absorbed and utilized by the human body[1]. Due to the lack of lysine, methionine, and cysteine, plant proteins are generally considered less nutritious than animal proteins. Animal oil is rich in high unsaturated fatty acids EPA and DHA, and polyunsaturated fatty acids account for more than 30% of the total fat. Cholesterol in animal oil has important physiological functions for the human body and is an indispensable raw material for body metabolism[4]. Internationally, the pork industry accounts for 33% of total meat consumption and is consumed in Europe and Asia in the form of various processed products[5]. It is of great significance to study the nutritional characteristics of pork products.
The nutritional properties of food are not only affected by exogenous factors (such as interactions between different compounds), but also by endogenous influences (such as changes in the structural characteristics of nutrients during processing). The effect of the processing method of meat diet on the bioavailability of protein and fat is an important point to evaluate their nutritional value. Luo et al. found that cooking methods led to sarcoplasmic metalloprotein-mediated oxidation processes that destroyed meat protein digestibility[6]. The decrease of oxidative stability in salt-cured tuna meat was likely related to the loss of redox balance, and the oxidation and discoloration of lipids and myoglobin were intensified with the extension of salting time[7]. It was found that after emulsification, the dispersed phase of the system was a solid or liquid fat ball, and the continuous phase was an aqueous solution with dissolved (or suspended) salts and proteins. The process of fermented sausage accelerated the degradation of protein and delayed the oxidation of the product[8]. However, the effects of different processing methods on the structural properties of proteins and the digestion characteristics of fats and proteins are rarely explored.
To investigate the effects of different processing methods (boiling, emulsification, pickling, and fermentation) on the protein, fat properties, and bioavailability of pork. This project used methods such as protein and fat digestion and extraction, circular dichroism determination, thiobarbituric acid reactive substances (TBARS) analysis, potentiometry, and thin layer chromatography to study the samples. The results will provide new ideas for studying the structural states and digestive characteristics of proteins and fats in different pork meat products, and it provides new insights for other experimental studies on the impact of meat processing methods on nutritional health.
-
Porcine longissimus dorsi muscle was purchased from Sushi Meat Co., Ltd. (Jiangsu, China), and stored at 4 °C. The fermented sausage contains 27.9 g pork protein and 27.5 g pork fat per 100 g. Pepsin (3,000 U/mg) and pancrease (130 U/mg) were purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China) and stored at −20 °C. Pig bile salt (cholic acid ≥ 60%) and thiobarbituric acid was purchased from Yuanye Biotech Co., Ltd. (Shanghai, China) and stored at room temperature. Triton X-100 (Aladdin Biochemical Technology Co., Ltd., Shanghai, China), EDTA (Macklin Biochemical Technology Co., Ltd., Shanghai, China), Trichloroacetic acid (TCA) (Lin Feng Chemical Co., Ltd., Shanghai, China) and SDS (Solarbio Science & Technology Co. Ltd., Beijing, China) were stored at room temperature. If not indicated, the common reagents are analytically pure grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Preparation of meat products
Boiled meat
-
The longissimus dorsi muscle was cut into 5 cm × 5 cm × 5 cm and boiled at 100 °C for 30 min, ensuring that the central temperature reached 72 °C.
Emulsified sausage
-
Visible connective tissue was removed, and lean meat and backfat were minced separately through a meat grinder (8 mm diameter, TC12E, Sirman, Venezia, Italy). Meat (417 g), salt (10 g), triple phosphate (1.5 g), sodium D-isoascorbate monohydrate (0.25 g), nitrate (0.075 g) and ice water (50 g) were chopped at 3,000 r/min for 90 s. Backfat (83 g) and ice water (50 g) were added to the sample again to chop for 90 s at 3,000 r/min. Finally, 50 g ice water was added again and was chopped for 90 s at 3,000 r/min. The meat paste obtained through the above operation was loaded into collagen casings (Shuanghui Group, Henan, China) with a diameter of 21 mm. All processing temperatures were kept below 18 °C. The sausages were heated to a central temperature of 72 °C.
Salt meat
-
The longissimus dorsi muscle (including backfat) was cut and dry-cured for two weeks (including five times salt), during which it is turned upside down so that the meat was fully immersed in the brine. The meat strips were air-dried under natural conditions for two weeks, vacuum-sealed to be measured and stored at 4 °C. It was boiled to a central temperature of 72 °C before use.
Extraction of sarcoplasmic and myofibrillar proteins
-
The soluble components of meat protein are mainly divided into water-soluble sarcoplasmic protein (SP) and salt-soluble myofibrillar protein (MP). Different meat products were ground and homogenized at 10,000 rpm for 1 min by adding nine times the volume of PBS solution (0.02 M, pH 6.5). The supernatant obtained after centrifugation at 12,000 g × 20 min was sarcoplasmic protein extract. The precipitate was washed three times by 25 mL PBS solution (0.03 M, pH 7.4, containing 0.1% Triton X-100), with the supernatant discarded each time. The precipitate was re-suspended in nine times the volume of PBS solution (pH 6.5, 0.1 M, containing 0.7 M of KCl) and homogenized again (10,000 rpm, 1 min) to obtain myofibrillar protein[9,10]. All operations were performed at 4 °C and the concentration of the protein solution was determined using a BCA protein assay kit (Biyuntian, Nantong, China).
Endogenous fluorescence assays
-
Fluorescence spectroscopy was used to determine the fluorescence intensity of tryptophan (Trp) and tyrosine (Tyr) residues in proteins from four meat products[9]. The protein solution of different meat products before and after heating was extracted by 0.1 M PBS buffer (pH 6.5, containing 0.7 M KCl). The endogenous fluorescence intensity of different protein solutions at 0.5 mg/mL, 25 °C was determined by fluorescence spectrometer (Varioskan Flash, Thermo, USA). The excitation wavelength was set to 280 nm with the slit width to 2 nm, and the fluorescence intensity of the samples at the emission wavelength of 300−450 nm was recorded.
Circular dichroism (CD) spectroscopy
-
Circular dichroism spectroscopy was used to detect the effects of different processing methods on the secondary structure of pork proteins[11]. The concentrations of all proteins were unified to 0.1 mg/mL and CD spectra at far-ultraviolet wavelengths of 190-240 nm were monitored by spectropolarimeter (J-1500, JASCO Corporation, Japan). The bandwidth was set to 1 nm and the temperature was 25 °C. The Yang secondary structure analysis software was used to determine the proportion of secondary structure in the measured data[12].
TBARS analysis
-
TBARS has been used to determine the extent of lipid oxidation in four meat products[13]. After mincing, 10 g of meat samples were taken and 50 mL of trichloroacetic acid (7.5%, containing 0.1% EDTA) was added and shaken for 30 min. The samples were filtered by double-layer filter paper, and an equal volume of 0.02 M thiobarbituric acid was added to 5 mL supernatant and heated in a boiling water bath for 40 min. The cooled mixture was centrifuged at 16,000 r/min for 5 min, and 5 mL of trichloromethane was added to the supernatant. The absorbance of the supernatant was measured at 532 and 600 nm respectively.
TBARS (mg/100 mg)
$ \text{=} $ In vitro digestion behaviour analyses of different meat products
In vitro digestion behaviour in the GIT model
-
The static digestion protocol of 2019 was referenced and the digestive fate of four meat products was simulated[14]. Simulated salivary fluid (SSF), simulated gastric fluid (SGF), and simulated intestinal fluid (SIF) are presented in Table 1. Before the experiment, all the simulated digestive fluids were incubated at 37 °C.
Table 1. Chemical composition of simulated digestive fluids in each stage of GIT model.
Chemicals Final salt concentration
in SSF (mM)Final salt concentration
in SGF (mM)Final salt concentration
in SIF (mM)KCl 15.1 6.9 6.8 KH2PO4 3.7 0.9 0.8 NaHCO3 13.6 25 85 NaCl − 47.2 38.4 MgCl2(H2O)6 0.15 0.12 0.33 (NH4)2CO3 0.06 0.5 − HCl 1.1 15.6 8.4 Oral phase: The minced meat product (3 g) containing equal amounts of protein and fat was added to 5 mL of SSF. The mixed sample of the oral phase was ground again for 1 min to produce a paste-like consistency. Since there were no carbohydrates in the samples, no additional salivary amylase was added.
Gastric phase: An equal volume of SGF (containing 0.15 mM CaCl2) was added to the mixed oral bolus and the pH of the system was adjusted to 3.0 using HCl (5 M). Pepsin was added and its enzyme activity in the final system reached 2,000 U/mL. The samples were mixed and cultured at 37 °C at 200 rpm for 2 h.
Intestinal phase: An equal volume of SIF (containing 0.6 mM CaCl2) was added to the mixed gastric chyme and the pH of the system was adjusted to 7.0 using NaOH (5 M). Bile salts and pancreatin were added to the system to achieve concentrations of 10 mM and 100 U/mL (trypsin activity), respectively. The samples were mixed and cultured at 37 °C and 200 rpm for 2 h.
ζ - Potential measurements
-
The electric charges of supernatant of meat products at different simulated digestion stages were studied by a Zeta Sizer Nano Zs90 Instrument (Malvern, UK). All samples were measured four times after 120 s equilibrium at room temperature.
Digestibility of protein
-
Samples from the end of the small intestine phase were mixed with triploid absolute ethyl alcohol and left for 12 h at 4 °C. The mixture was centrifuged at 10,000 g for 20 min to obtain the precipitate and 10 mL of protein extraction buffer (containing 10% SDS) was added. The mixture was ultrasonically treated at 50 °C for 2 h to completely dissolve and its protein concentration was determined by a BCA kit. Protein digestibility was calculated as follows:
Protein digestibility (%) = (W0 − W1) / W0 × 100
Where W0 represents the protein content of the sample before digestion, and W1 represents the protein content that has not been digested[15].
Thin-layer chromatography (TLC) analysis
-
The mixtures from the small intestine after simulated digestion were heated in a boiling water bath for 5 min to inactivate the enzyme activity. The samples were dried and then added with water, methanol and trichloromethane [1.5:2:4 (vol/vol/vol)]. The lowest layer of trichloromethane containing oil was taken, dried again and dissolved in 25 times the volume of trichloromethane. A 10 μL sample was taken for TLC analysis of lipid composition. A mixture of petroleum ether, ether, and acetic acid [70:30:1 (vol/vol/vol)] was used as a developing agent and fumigated with solid iodine for colour development[16].
Statistical analyses
-
All data were analyzed using Duncan's post hoc test by SPSS software (Ver. 26, SPSS Inc., Chicago, IL, USA) for correlation analysis. Graphics were generated through Origin 2021 (Origin Lab Inc., Massachusetts, USA). The significance level was set at p < 0.05.
-
Meat products are subject to mechanical forces, interactions of other food components (food formulation) and microbial fermentation during different processing processes, which may lead to changes in the structural properties of meat proteins and further affect their digestive properties[17]. Meat proteins may accumulate or fold during processing, affecting their digestive properties. We investigated the effects of emulsification (emulsified sausage), salt treatment (salted meat) and fermentation (fermented sausage) on the protein properties of pork, in which fresh meat (boiled meat) was used as the control group.
Analysis of endogenous fluorescence spectroscopy
-
Tryptophan residues can be used as fluorescence tools to monitor and obtain information about protein molecular structure, interactions and microenvironment[18]. The endogenous fluorescence intensity of pork protein after different processing is shown in Fig. 1. In the unheated meat products, the intensity of endogenous fluorescence was: fresh meat > emulsified sausage > salt meat > fermented sausage (Fig. 1a). Interestingly, among the cooked samples, the meat protein in fermented sausages had the highest fluorescence intensity, boiled and salted meat were similar, while emulsified sausages had the lowest fluorescence intensity (Fig. 1b).
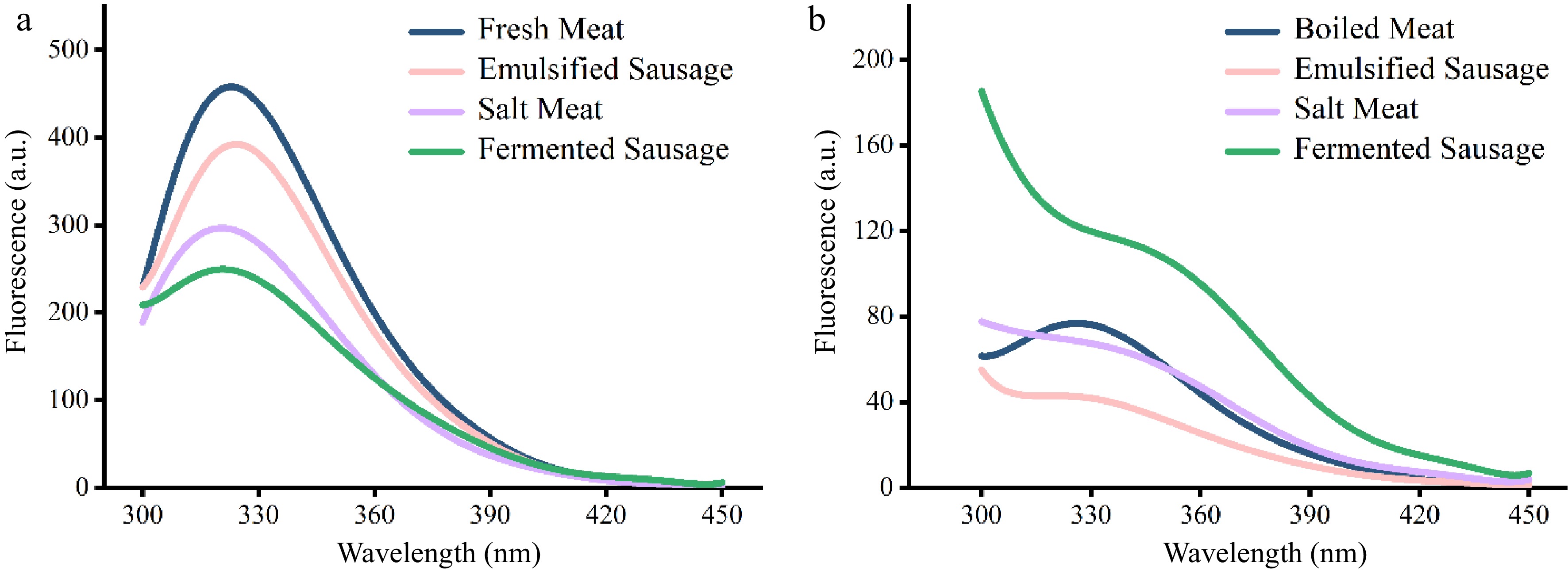
Figure 1.
Fluorescence spectroscopic analysis of proteins in different meat products. (a) Unheated. (b) After heating.
The endogenous fluorescence spectra reflected the changes of protein tertiary structure and the loss of tryptophan fluorescence could also be used as an indicator for protein oxidation degree[19,20]. The natural structure of proteins tends to have hydrophobic cores inside and hydrophilic groups on the surface. When tryptophan residues are attacked by reactive oxygen species and undergo oxidation reaction, the fluorescence intensity of proteins decreases[21]. The results showed that fermentation caused the most serious oxidation of pork protein, which was followed by salt treatment. The decrease of protein fluorescence intensity in emulsion sausage may be due to the interaction between fat and protein and the increased exposure of hydrophobic groups, which further leads to the aggregation of proteins[11]. A weak blue shift in the maximum fluorescence emission was observed in unheated salt meat and fermented sausage, implying a reduced polarity in the region near the tryptophan group.
Heat treatment is also considered to be an important factor in reducing the endogenous fluorescence intensity of proteins[19]. During the process of heating, the structure of myosin unfolded through denaturation and aromatic hydrophobic amino acid residues were exposed[21]. Proteins in fermented sausage retained high fluorescence intensity after heating, possibly due to the formation of a dense cross-linked network during fermentation that prevented protein aggregation and exposure of hydrophobic groups.
Changes in protein secondary structure
-
Circular dichroism is a special absorption spectrum used to quickly obtain the secondary structure of biological macromolecules. We investigated the effects of different processing methods on the secondary structure of SP and MP in pork (Fig. 2). The SP and MP in fresh meat showed negative peaks at 222 and 208 nm, positive peaks near 190 nm, and peaks in the far ultraviolet range in the "w" shape spectrum, indicating that the α-helix was the main secondary structure[22]. Fermentation treatments had the greatest effect on the α-helix in SP, meaning that more drain hydrophobic sites were exposed during fermentation[20]. The salt treated samples had the greatest influence on the α-helix content of MP (Fig. 2a). MP, as a salt-soluble protein, was more dissolved during the salting process. The loss of water during dry curing was probably the main cause for protein folding[1].

Figure 2.
CD spectral analysis of proteins in different meat products. (a) Sarcoplasmic protein. (b) Myofibrillar protein.
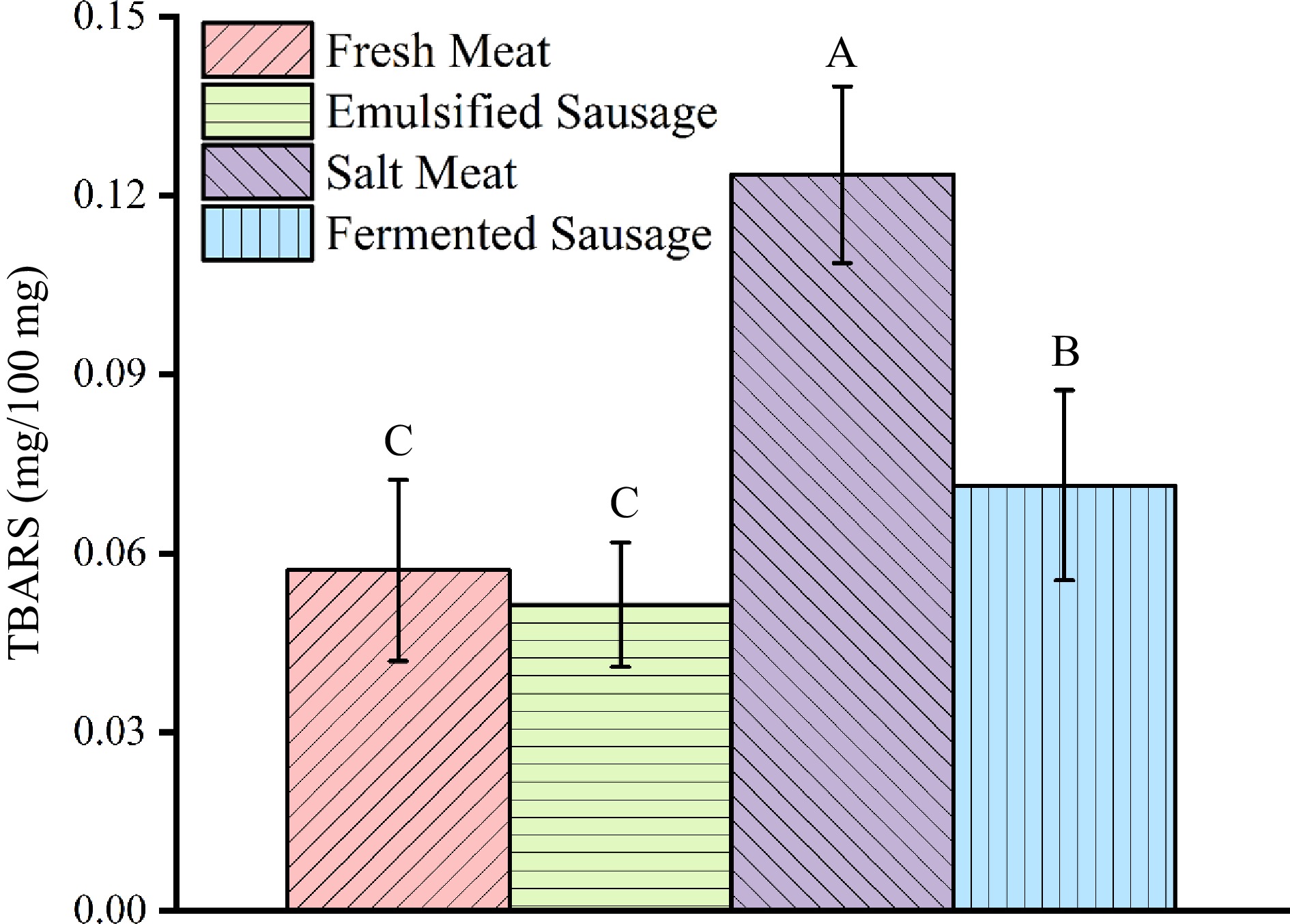
TBA content of pork fat in different meat products
-
In the second stage of autoxidation, peroxidation is oxidized to ketones and aldehydes, so TBARS is used to determine the extent of lipid oxidation[23]. 0.2 mg MDA/100 g is considered to be the limit value of TBA[24]. As shown in Fig. 3, the TBARS of fat increased during the processing of meat products, which were salted meat > fermented sausage > fresh meat ≈ emulsified sausage. The content of TBARS in fresh meat and emulsified sausage was measured on the day of purchase and showed low content. In the process of dry curing, more intense oxidation occurs in the salted meat, which leads to an increase in TBARS content. The TBARS content of fat in salted meat (0.124 ± 0.015 mg/100 mg) was significantly higher than that of other meat products.
Changes of ζ-potential in the GIT during dynamic digestion
-
Changes in the ζ-potential during digestion can provide information about interface properties[25]. In the stomach phase, except for the salt meat group, the digestive liquids of other meat products were had positive charges (Fig. 4a). Proteins were amphiphilic, and the ambient pH of the stomach was less than the isoelectric point of the protein (about pH 5) and dissociated the protein into positive ions[9]. The positive charge of boiled meat and emulsified sausage was significantly higher than that of fermented sausage. Salt meat carried a lower negative charge due to its higher NaCl content. The electric charge of each sample decreased after 2 h of gastric phase digestion compared with 1 h, and the boiled meat and emulsion sausage carried more positive charges than other samples.

Figure 4.
ζ-potential of four meat products during simulated digestion. (a) ζ-potential of the stomach phase. (b) ζ-potential of the small intestine phase.
At the stage of intestinal digestion, all samples showed a strong negative charge (Fig. 4b). This was mainly due to the addition of pancreatin and bile salts, as well as pH higher than the isoelectric point of protein[15]. Fermented sausages showed the most negative charge when digested in the small intestine for 1 h (−36.23 ± 1.96 mV). At the end of intestinal digestion (2 h), all samples showed similar negative charges, which means that different samples had similar interfacial stability.
Secondary structure of proteins in different meat products after digestion
-
The process of digestion disrupts the ordered structure of proteins and leads them to a more irregular development. The SP in all meat products no longer had α-helix structure after digestion in the small intestine (Fig. 5a & c). A high proportion of β-sheet (47.3% ± 7.5%) was still retained in fermented sausages, and the secondary structure of other samples was mainly random coil. This also meant that the proteins in fermented sausages may be more difficult to digest. Bai et al. found that higher levels of β-sheet would prevent the digestion of proteins, which can be attributed to the fact that β-sheet has a large number of hydrogen bonds and thus hinders the activity of proteases[26]. The MP of emulsified sausage, salt meat and fermented sausage still retained a certain proportion of α-helix, which was specifically: fermented sausage (47.9%) > salted meat (41.0%) > emulsion sausage (21.3%). The main structure of boiled meat was random coil and β-sheet. This meant that different processing methods affected the changes in the secondary structure of the protein during digestion, which in turn affected the digestibility of the protein.
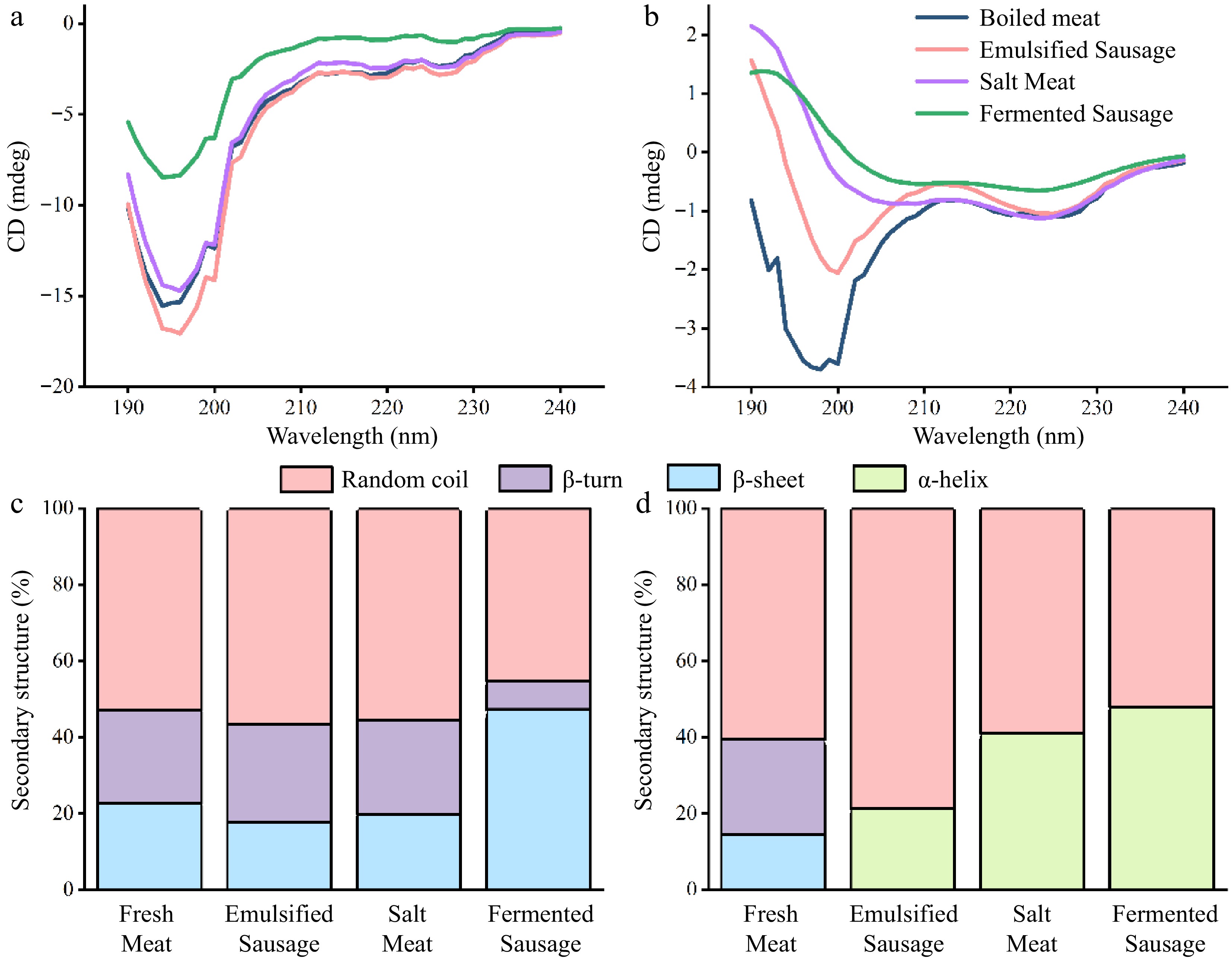
Figure 5.
The changes of protein secondary structure in different meat products were evaluated by CD spectroscopy. (a) CD profile of sarcoplasmic protein. (b) CD profile of myofibrillar protein. (c) Secondary structure content of sarcoplasmic protein. (d) Secondary structure content of myofibrillar protein.
Hydrolysis of proteins and fats
-
The proteins in animal muscles are mainly composed of sarcoplasmic proteins (water-soluble), myofibrillar proteins (salt-soluble), and collagen (insoluble), which have a unique and identical three-dimensional structure[1]. Lipids from different sources will further affect their nutritional properties due to their differences in the composition of fatty acids and triacylglycerol (TAG), among which pig fat (lard) contains rich saturated fatty acids (such as stearic acid)[27]. The determination of the degree of hydrolysis of protein and fat in different meat products is helpful to understand the influence of meat processing methods on the absorption and utilization of nutrients.
The results of pork protein digestibility under different processing methods are shown in Fig. 6a. The protein in emulsion sausage showed the highest digestibility (84.23% ± 0.32%) and the lowest in salt meat (77.04% ± 1.33%). Ding et al. reported that higher fat content in the food matrix promotes protein digestion, which can be attributed to the emulsification process which reduces the interfacial area of proteins and increases the accessibility of digestive enzymes[11]. The main reason for the decrease of protein digestibility in salt meat was that protein oxidation leads to the formation of a large number of carbonyl groups, which hinders proteolysis and reduces digestibility[28]. Dean et al. found that decreased protein digestibility during dry-cured duck processing can be associated with reduced initial oxidation levels and α-helix proportions, leading to impaired site recognition of aggregation and digestive enzymes[29]. This was consistent with the results in Fig. 2b, where the content and proportion of α-helix in MP of salt meat was significantly lower than in other meat products.
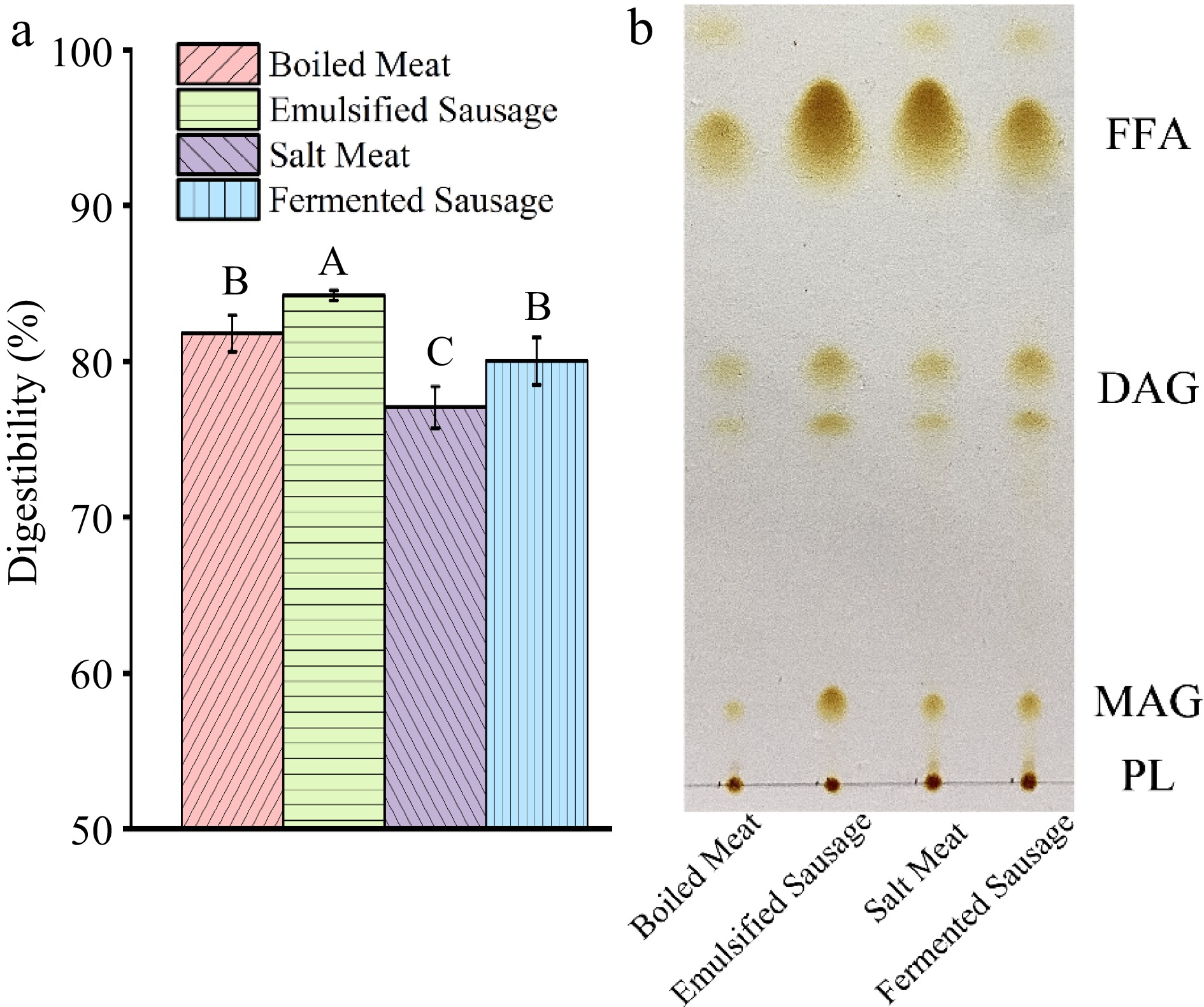
Figure 6.
Digestive characteristics of protein and fat in four meat products in vitro GIT digestive environment. (a) Intestinal digestibility of protein. (b) TLC analysis of lipids in the small intestinal phase.
The interesting result is that processed meat had a higher degree of fat breakdown during digestion than natural meat (Fig. 6b). The content of free fatty acid (FFA) was: emulsion sausage > salted meat > fermented sausage > boiled meat. In the natural structure of meat, fat molecules are embedded in muscle fibers, and the natural protein fiber matrix represents a strong structure[30]. When fat is trapped in the food matrix, the structure of the matrix is the main factor determining fat digestion. Emulsion sausages showed in vitro digestion behaviour similar to soft gel, and gel structure was degraded during digestion, allowing lipid droplets to be released from the matrix and hydrolyzed[31].
-
In this work, we explored the effects of different processing methods on the digestive properties of pork protein and fat. The processing method affected the secondary structure, tertiary structure and oxidation properties of the protein, and further changed the digestibility of the protein. The influence of processing methods on the degree of lipolysis was mainly attributed to the destruction of the initial food substrate and the oxidation characteristics of fats. The findings provide new insights into the impact of processing methods on the bioaccessibility of protein and fat in pork products. The interaction between proteins and fats will be further explored in future studies.
This study was supported by the Jiangsu Innovative Group of Meat Nutrition, Health and Biotechnology, the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No: KYCX22_0719) and the National College Student Innovation and Entrepreneurship Training Program (No: 202210307073Z).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang Y, Wang Y, Cai X, Huang Z, Li C, et al. 2023. Effects of processing methods on the properties and digestibility of protein and fat in meat products. Food Materials Research 3:27 doi: 10.48130/FMR-2023-0027
Effects of processing methods on the properties and digestibility of protein and fat in meat products
- Received: 21 June 2023
- Accepted: 29 August 2023
- Published online: 02 November 2023
Abstract: Protein and fat in foods of animal origin are important macronutrients for maintaining human growth and function. When measuring the nutritional properties of animal-derived diets, it is important to consider the effect of processing methods on their digestibility and nutritional properties. The purpose of this study is to investigate the effects of different processing methods on the properties and bioavailability of pork protein and fat. The molecular structure, oxidation degree and digestion characteristics of pork protein and fat in four processing methods (boiling, emulsifying, salting and fermentation) were studied. The results showed that the endogenous fluorescence and secondary structure of proteins were affected by the processing method. Fermentation and salting had greater influence on the properties of proteins. Salting caused a significant increase in the oxidation of pork fat. The potential and secondary structure characteristics of different meat products also showed differences during digestion, which ultimately affected their digestive characteristics. Salting and fermentation decreased the digestibility of pork protein, but increased the digestibility of fat. This finding may provide new insights into the structural states and digestive properties of proteins and fats in different meat products.
-
Key words:
- Digestibility /
- Protein /
- Meat products /
- Fat /
- Secondary structure /
- In vitro digestion