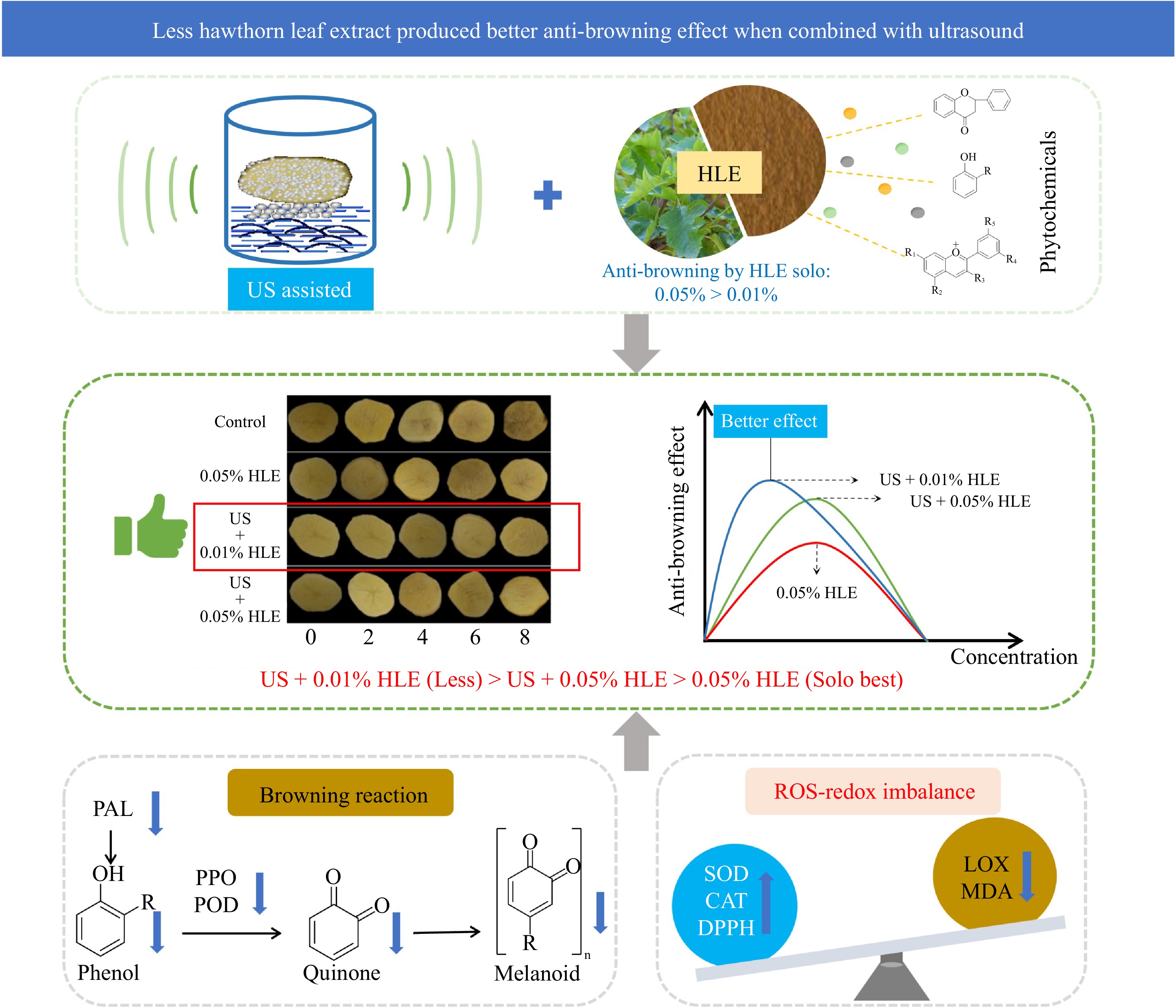
-
With the development of the fast-food and instant food industry, the demand for fresh-cut products (e.g. potato slices) with the characteristics of freshness, convenience, nutrition, and health is gradually increasing[1]. However, after mechanical operations such as cleaning and peeling, fresh-cut potatoes are prone to enzymatic browning after slicing, which restricts the advancement of the fresh-cut potato industry[2,3]. It is estimated that more than 50% of food products are wasted because of discoloration or browning[4]. Therefore, exploring novel anti-browning strategies is of great significance, and also makes sense.
Ultrasound (US), an excellent physical treatment, has been widely applied in the food industry for its mild price adjustment, convenient operation, low energy consumption, high efficiency, and environmental friendliness[5,6]. And there is no concern about the potential risks to human health from physically processed foods[7]. Numerous studies have indicated that it can effectively improve food quality, control enzyme activity, reduce agricultural residues, inactivate microorganisms, and activate the secondary metabolism and biosynthesis of plants[8−10]. Many studies have also demonstrated that US treatment delays the browning rate of fresh-cut potatoes, sweet potatoes, and mangoes while preserving their quality[8,9,11,12].
Additionally, to better satisfy the consumers' higher demand of food health, natural plant extracts have become a hot spot of current research[13]. Including purslane extract, cod peptides, Sonchus oleraceus L. extract, and seabuckthorn leaf extract, many natural extracts have proven to significantly postpone browning in fresh-cut products[14,15]. They improved antioxidant capacity, regulated the activity of enzymes associated with enzymatic browning, and maintained the integrity of protective membranes[16−19]. Hawthorn leaf extract (HLE) contains multifunctional phytochemicals, such as polyphenols, flavonoids, and other active substances. These natural phytochemicals usually serve as antioxidants, helping to play a role in resisting oxidation, and repairing cell damage[19]. Xu et al.[20] reported HLE functioned in antibacterial, hypotensive, and antioxidant biological activities. Notably, there are many investigations using US combined with chemical agents to regulate browning discoloration[9,21,22]. While only very few scholars have considered the combination of technologies using US and natural extracts to control browning.
This study was designed to explore whether the combination of US and HLE could influence enzymatic browning, and their best combination. Additionally, to explore its underlying mechanism, browning related enzymes (polyphenol oxidase: PPO, peroxidase: POD, phenylalanine ammonia-lyase: PAL), and reaction substance and products (the total phenol, soluble quinone, intermediate products, advanced products) have been determined. Furthermore, lipoxygenase (LOX), malondialdehyde (MDA), hydrogen peroxide (H2O2), and antioxidant system (catalase: CAT, superoxide dismutase: SOD, antioxidant capacity), were also evaluated.
-
For the study, fresh potatoes (cv. Netherlands) that were undamaged and of uniform size were obtained from a farmers' market in Tianjin. Hawthorn leaf extract (water extraction, extraction ratio 10:1, 1.5 h extraction, three times) was acquired from the Xi'an Zelang Biology Co., Ltd (Xi'an, China).
Wash, peel, and slice the purchased potatoes, then rinse the sliced potatoes with clean water. The potatoes were processed into 5 mm slices. Different concentrations of extracts were prepared and potato chips were then soaked in them. Primarily, the aim is to reveal whether the combination of US and HLE is a better anti-browning treatment than the single HLE treatment. At room temperature, the fresh-cut potatoes were treated as follows: control (distilled water, 5 min), HLE (0.05%, w/w, 5 min), US (CR-1018i, Shenzhen, China, intensity: 0.75 W/cm2, 5 min), US + HLE (US: 5 min; HLE: 0.05%, w/w). The optimal concentration of HLE and parameters of US are from the previous experiment and pre-experiment.
Then, we explored whether a lower dose of HLE combined with US could achieve a better or similar anti-browning effect. Also, at room temperature, potatoes slices were treated as follows: control (distilled water, 5 min), HLE (0.05%, w/w, 5 min), US + 0.05% HLE (US: 5 min; HLE: 0.05%, w/w), US + 0.01% (US: 5 min; HLE: 0.01%, w/w).
After the samples were processed, the fresh-cut potatoes were dried with gauze. Meanwhile, slices were transferred to a polyethylene freezer bag (28 cm × 18 cm, nine pieces in each bag) and refrigerated at 4 °C for 8 d, then the different indicators were assessed. Samples were taken every 2 d for index determination. Three parallel groups were set up for each group.
Color and browning index
-
The color of samples was evaluated via a chromameter (HP-200, Shanghai, China) with the CIE color system, which showed the L* (lightness), a* (reddish-greenish), as well as b* (yellowish-bluish) values. Additionally, the browning index (BI), and ΔE* were utilized to show the degree of browning and comprehensive color variation of slices[23,24]. Measurements were made three times at different points in each slice. The average of three measured results was taken.
$ {\text{ΔE}}^{\text{*}}=\sqrt{{\Delta }{\text{L*}}^{\text{2}}{+\Delta }{\text{a*}}^{\text{2}}{+\Delta }{\text{b*}}^{\text{2}}} $ (1) $ \text{}\text{BI}=\dfrac{\text{100×(}\text{x}{-0.31)}}{{0.172}} $ (2) where,
$\rm x=\dfrac{a^*+1.75L^*}{5.645L^*+a^*-3.102b^*} $ PPO, POD, PAL, and total phenols
-
The vitalities of PPO and POD were based on this procedure with little variation[16]. Two grams of samples were ground with (5 mL, 0.1 mol·L−1, pH 5.5) sodium acetate buffer (polyethylene glycol: 1 mmol·L−1, poly-vinylpyrrolidone: 4%, Triton X-100: 1%), in a centrifuge tube and the mixture was centrifuged at 4 °C (12,000 × g, 30 min). Then, the extracting solution was preserved to determine PPO and POD activities.
Acetic acid-sodium acetate buffer (4.0 mL, 50 mol·L−1, pH 5.5) was blended with catechol solution (1.0 mL, 50 mmol·L−1), and 0.1 mL of the extracting solution. Then, the value at 420 nm was gauged to count PPO. To measure the POD, the absorbance value at 470 nm was measured after mixing with guaiacol solution (3.0 mL, 0.025 mol·L−1), extraction solution (0.5 mL), and H2O2 solution (0.1 mL, 0.5 mol·L−1).
For PAL activity, 2.0 g freshly ground potatoes were weighed and borax buffer solution (5.0 mL, 0.1 mol·L−1, pH 8.8) was added. After blending, the solution was disposed at 12,000 × g for 30 min at 4 °C. Then, l-phenylalanine (0.25 mL, 20 mol·L−1), and boric acid buffer (1.5 mL, 50 mmol·L−1, pH 8.8) were added to a novel centrifuge tube and kept warm at 37 °C for 10 min. After heat preservation, 0.25 mL supernatant was blended into a centrifuge tube, which was rapidly blended and the initial value was recorded at 290 nm[5].
The slightly modified Folin-Ciocalteu was used to assay the total phenol content[13]. First, 2 g samples were blended with 5 mL 60% ethanol, and the mixture placed at 4 °C (12,000 × g, 10 min). Then, 0.5 mL supernatant and 4 mL distilled water were added into a new centrifuge tube. Next, 1.6 mL 20% Na2CO3 solution and folin-phenol (0.5 mL) were added to the tube and left in the dark for 25 min. Subsequently, the value was measured at 760 nm.
The units of PPO, POD, and PAL activities are all expressed in U·g−1, using g·kg−1 equivalent of gallic acid to represent the phenolic content of fresh weight.
Soluble quinone, intermediate, and advanced products
-
For soluble quinone variation, 2 g samples were evenly blended with 5 mL methanol. Then, the mixture was centrifuged at 4 °C (12,000 × g, 5 min) to gather the supernatant. Next, the absorbance value was recorded at 437 nm[25].
For intermediate and advanced products, 1 g of sample was blended well with 5 mL distilled water. After the solution was evenly mixed, it was placed at 12,000 × g for 5 min at 4 °C. The value of intermediate and advanced products was then determined at 294 and 420 nm respectively[26].
LOX activity, MDA, and H2O2 content
-
LOX activity was evaluated via a modified procedure[27]. Two gram slices were ground with 5 mL phosphate buffer (0.1 mol·L−1, pH 6.8) and placed at 4 °C (12,000 × g, 30 min). After that, sodium phosphate buffer (5.4 mL, 0.1 mol·L−1, pH 6.8), along with linoleic acid solution (0.2 mL, 0.5%) was placed at 30 °C for 10 min. Finally, 0.4 mL supernatant was added and the absorbance of the mixture determined. U·g−1 fresh weight reflected the LOX activity.
For MDA content determination, 1.0 g slices were ground with 5.0 mL trichloroacetic acid solution (TCA, 100 g·L−1), and placed at 4 °C (12,000 × g, 20 min). Next, 2.0 mL extracting solution and 5.0 mL thiobarbituric acid (TBA, 6.7 g·L−1) were mixed and boiled at 100 °C for 20 min. Then, cooled quickly and centrifuged at the same conditions for 5 min. Finally, the value of the supernatant was determined at 450, 532, and 600 nm.
For H2O2 content, 1.0 g of freshly cut potatoes were ground with precooled acetone (2.5 mL), and then placed at 4 °C (12,000 × g, 10 min). Subsequently, the supernatant (0.2 mL), was blended with 0.2 mL 10% (TiCl4-HCl: 1:9), and ammonium hydroxide (0.4 mL). Subsequently, the solution was placed under the same centrifugation conditions. Finally, the precipite was dissolved in sulfuric acid (6.0 mL, 2 mol·L−1) and the value of the mixture recorded at 412 nm.
The fresh weight of µmol kg−1 indicated the MDA and H2O2 content[25,28].
CAT and SOD activities, and antioxidant capacity
-
For CAT activity, 2.0 g fresh sample was ground with 5 mL of extraction buffer (pH 7.5) including dithiothreitol (DTT, 5 mmol·L−1) along with polyvinyl pyrrolidone (PVP, 5%). The extracting solution was collected after centrifugation at 4 °C (12,000 × g, 30 min). Next, the value at 240 nm was determined after blending H2O2 solution (5.8 mL, 20 mmol·L−1) and extracting solution (0.2 mL).
SOD activity was assayed following a previously reported procedure[28]. Two gram slices were then blended with extraction buffer (5 mL, pH 7.8) containing DTT (5 mmol·L−1) along with 5% PVP. Then, the extracting solution was collected after centrifugation at 4 °C (12,000 × g, 30 min). Subsequently, sodium phosphate buffer (3.4 mL, 50 mmol·L−1, pH 7.8), metformin (0.6 mL, 0.13 mol·L−1), 0.6 mL nitro-blue tetrazolium chloride (NBT, 0.75 mmol·L−1), 0.6 mL ethylenediaminetetraacetic acid disodium salt (EDTA-Na2, 0.1 mmol·L−1), supernatant (0.2 mL), and riboflavin (0.6 mL, 0.02 mmol·L−1) were blended and illuminated under a fluorescent lamp (40 W) for 15 min. Then, the value was measured at 560 nm after stopping the reaction in the dark.
The index was assessed by the scavenging capacity of the 2,2'-diphenyl-1-picrylhydrazyl (DPPH) radical[10]. First, the ground potatoes were centrifuged at 4 °C (12,000 × g, 5 min) to collect the extracting solution. Next, 0.65 mmol·L−1 DPPH solution (4 mL) was mixed with 95% ethyl alcohol solution (1 mL) placed in the dark for 30 min, and recorded the absorbance at 517 nm (A0). Then, 1 mL of the extracting solution was blended with the ethyl alcohol solution (4 mL, 95%) to obtain absorbance values A1. Finally, the extracting mixture (1 mL) was blended with DPPH solution (4 mL, 0.65 mmol·L−1) to obtain the absorbance value A2. Formula: DPPH% = [1− (A2 − A1) / A0] × 100.
One unit of U·g−1 fresh weight represented the CAT and SOD activity[29].
Statistical analysis
-
The data obtained from the experiments were processed using one-way analysis of variance and Duncan's multiple range tests in SPSS 25.0. Significance was shown as p < 0.05. The data values were expressed by means ± standard errors (n = 3). The software version origin 2021b was used for drawing analysis.
-
Color is a prominent indicator which directly impacts peoples' desire to buy fresh-cut products or not. Surface browning is an especially important factor[10]. To validate the results of the US and HLE combinations, the appearance, color, and browning index of fresh-cut potatoes was estimated at 4 °C for 8 d based on a preliminary study. Figure 1a shows fresh-cut potatoes treated by US, 0.05% HLE were less brown compared with the control, especially by the US + 0.05% HLE treatment which exhibited the best appearance. In accordance with the overall appearance (Fig. 1b), the BI value of 0.05% HLE + US treatment kept a lower level, and this combination treatment displayed the lowest browning degree. Figure 1c and d demonstrated US + 0.05% HLE treatment maintained the highest L* value and lowest a* value, respectively. Meanwhile, the total color change ΔE* value of US + 0.05% HLE kept the lowest level during the whole storage (Fig. 1e). The results revealed that the US + 0.05% HLE treatment produced a better color quality and anti-browning effect than the individual application.
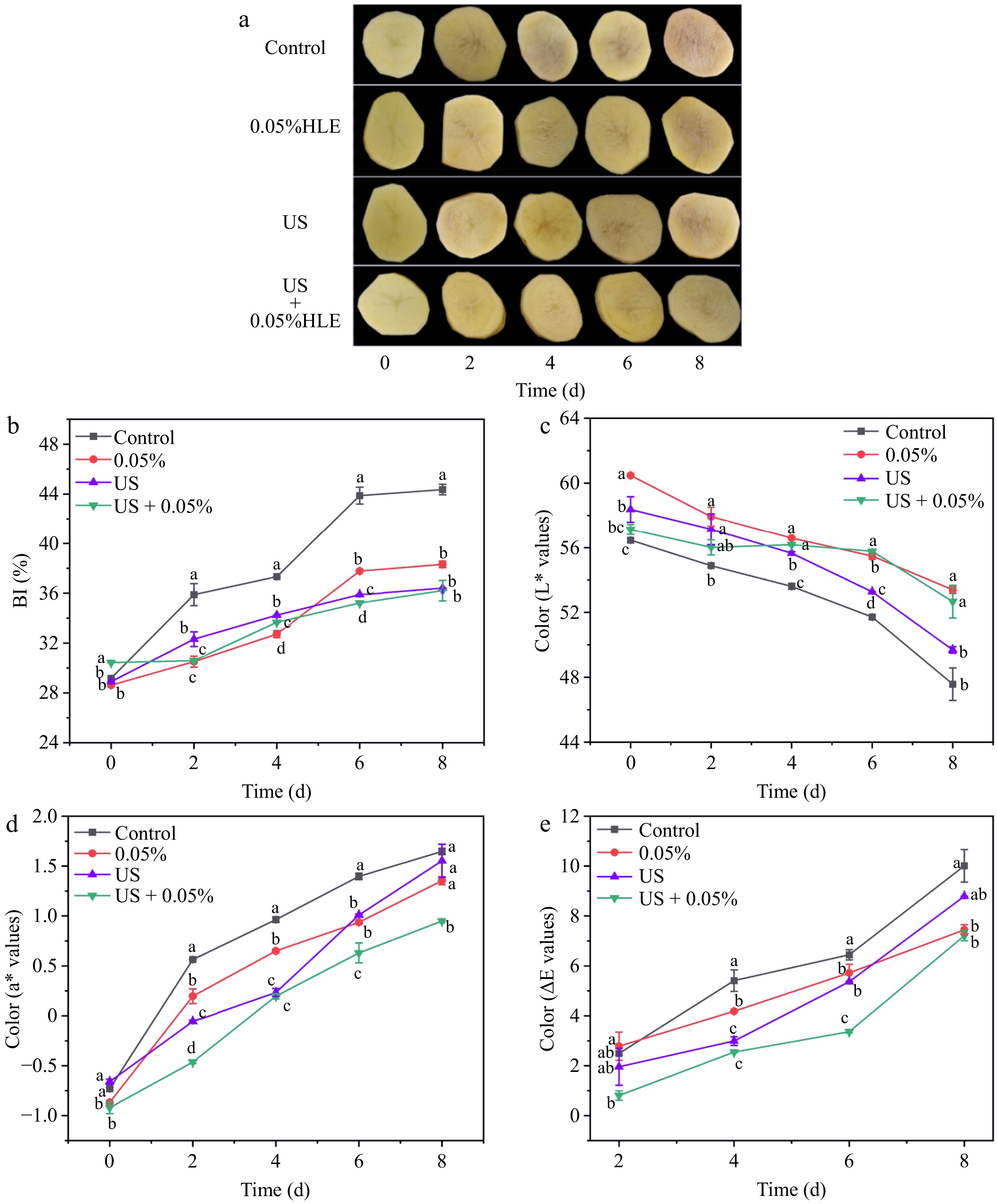
Figure 1.
(a) Effect of different treatments (Control: distilled water, HLE: hawthorn leaf extract, US: ultrasound, US + 0.05% HLE: the combination of ultrasound and hawthorn leaf) on appearance, (b) BI value, (c) L* value, (d) a* value, and (e) ΔE* of fresh-cut potato during storage at 4 °C for 8 d. Data are express as the mean ± standard error (n = 3). Different letters indicate significant differences between groups (p < 0.05).
To determine whether a smaller concentration of HLE combined with US could realize a similar anti-browning result, a further combination experiment was conducted. Figure 2a showed the browning of fresh-cut potatoes was further alleviated by treatment of US + 0.01% HLE compared with the original treatment (US + 0.05% HLE). Figure 2b demonstrated this less combination treatment achieved a lower browning index than US + 0.05% HLE treatment. A similar browning pattern was observed in Fig. 2c and d which a higher L* value and a lower a* value was achieved by US + 0.01% HLE treatment. Especially on day 8, US + 0.01% HLE remained the lowest a* value, accounting for only 64.34% of the control group, and about 15.92% less than US + 0.05% HLE treatment. Figure 2e showed US + 0.01% HLE treatment maintained the lowest ΔE* value during the whole storage, which were 67.41%, and 79.87% of the control and US + 0.05% HLE group, respectively. Overall, it is suggested that the least browning development and maintenance of a higher L*, lower a*, ΔE* and BI values was attained by the US + 0.01% HLE treatment.
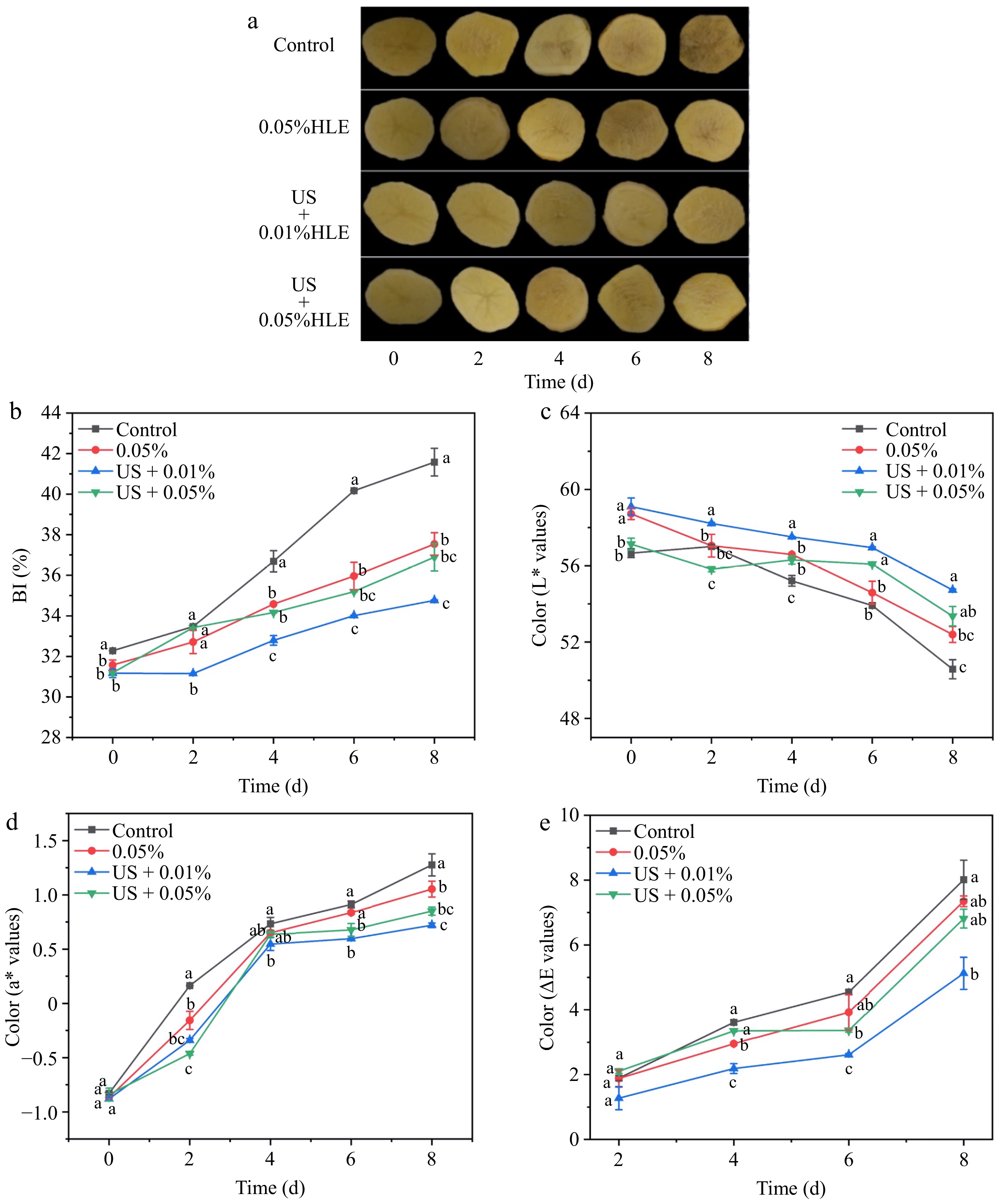
Figure 2.
(a) Effect of different treatments (Control: distilled water, 0.05% HLE: only hawthorn leaf extract (0.05%) and ultrasound coupling two concentrations of hawthorn leaf extract (0.01%, 0.05%)) on appearance, (b) BI value, (c) L* value, (d) a* value, and (e) ΔE* of fresh-cut potato during storage at 4 °C for 8 d. Data are expressed as the mean ± standard error (n = 3). Different letters indicate significant differences between groups (p < 0.05).
A suitable concentration of natural extract was needed to perform browning mediation in fresh-cut products, not the higher the better. Liu et al.[16] found that 0.1% cod peptides effectively retarded the browning of fresh-cut potato slices, and a higher concentration (1.0%) aggravated the browning, which activated higher POD and PAL activities. Similarly, our lab previously reported that 0.05% HLE better diminished browning development than 1.0% and 0.1%, and it was selected as the optimal concentration[19]. Additionally, ultrasound addition can reduce the minimum effective concentration of nature extract[13]. In other words, ultrasound application could help lower dose extract, and achieve a similar or better effect in some cases. Hallow et al.[30] reported that ultrasound can produce a cavitation effect and release strong sound energy, leading to various biological effects and the formation of sound holes. Temporary and reversible holes in the membrane then open up, allowing large extracellular molecules to enter the cell. Zhu et al.[13] found that US combined with lower purslane extract concentration (0.02%) produced lighter (less browning) slices than the optimal concentration alone (0.05%). Thus, a suitable concentration can perform a better browning mediation effect, no matter individual usage or combined with other technologies. Not the higher dose of nature extract, the better anti-browning preservation effect. When combined with ultrasound, less HLE produced a better anti-browning effect in fresh-cut potatoes. This may be the reason why US + 0.05% HLE treatment achieved more outstanding anti-browning result than the single 0.05% HLE, and US + 0.01% HLE treatment exhibited a similar or even better effect than their combination (US + 0.05% HLE) in this study.
Browning-related enzymes and the total phenol content
-
There is sufficient evidence that browning development is highly correlated with the activity of PPO, POD, PAL, and phenol content[16,25,29]. PPO is a representative enzyme that accelerates the oxidation of polyphenols to quinones and polymerizes them to produce melanin precipitation[16]. In Fig. 3a, US + 0.01% HLE, and US + 0.05% HLE treatment produced similar trends of PPO activity, which were the lowest level during storage. POD accelerates the oxidation of phenols by H2O2 to form quinones, which then polymerize to darker colored products[29]. Similar to PPO, POD in ultrasound combination groups maintained a lower level, regardless of the concentration (Fig. 3b). PAL, a major and rate-limiting enzyme in phenol metabolism, can induce the gathering of phenolic substrates and thus affect the enzymatic browning reactions[17]. The combination treatment consistently maintained lower PAL activity from day 2 to day 6 in Fig. 3c. And 0.05% HLE alone or combined with US significantly retarded PAL on the last day. Figure 3d revealed that the US + 0.01% HLE treatment significantly reduced the accumulation of phenols. On day 8, the total phenol content of US + 0.01% HLE were only 34.89% and 45.61% of the control group, and US + 0.05% HLE, respectively.
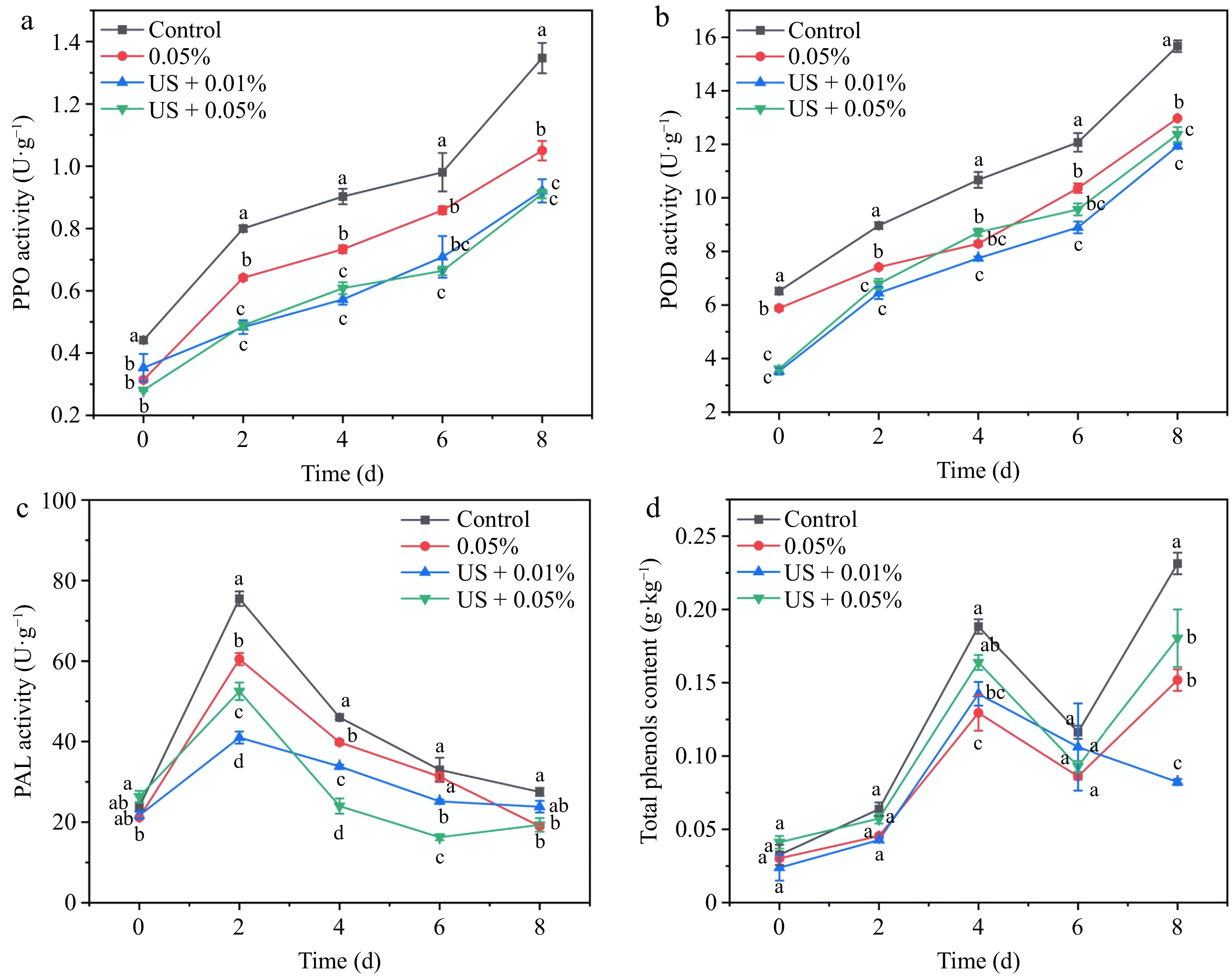
Figure 3.
Effect of different treatments (Control: distilled water, 0.05% HLE: only hawthorn leaf extract (0.05%) and ultrasound coupling two concentrations of hawthorn leaf extract (0.01%, 0.05%)) on activities of (a) PPO, (b) POD, (c) PAL, and (d) total phenol content of fresh-cut potato during storage at 4 °C for 8 d. Data are expressed as the mean ± standard error (n = 3). Different letters indicate significant differences between groups (p < 0.05).
In this study, US + 0.01% HLE greatly diminished the activity of PPO, POD, and phenol accumulation except for PAL when compared with US + 0.05% HLE. Usually, the higher the level of these enzymes and phenolics, the more browning, controlling their level may help to delay fresh cuts browning. Zhu et al.[13] found that the combined application inhibited activities of PPO and POD, and alleviated discoloration of potato slices. Dong et al.[31], explored short-term carbon dioxide treatment which lowered the level of PPO, PAL, phenolic content, and inhibited the browning of fresh-cut burdock. Thus, it is reasonable to believe that a lower level of these enzymes activities and induced substances may help US + 0.01% HLE combination produce a better anti-browning effect.
Browning products
-
Soluble quinone serves as an oxidation product in the enzymatic browning reaction, and its increase implies the accumulation of melanin in plants[23]. Soluble quinone accumulated with storage time, and the US combination group significantly reduced the content from day 4 to day 8 (Fig. 4a) regardless of the HLE concentration. Previous studies have found that the formation of intermediate and advanced products is usually accompanied by browning development of fresh cuts with the extension of storage time[26,32]. Figure 4b and c show that different treatment groups have certain effects on inhibiting intermediate and advanced products when compared with the control group. US + 0.01% HLE treatment produced lower contents of intermediate and advanced products, which were 77.40% and 76.23% of the control group on day 8, respectively.
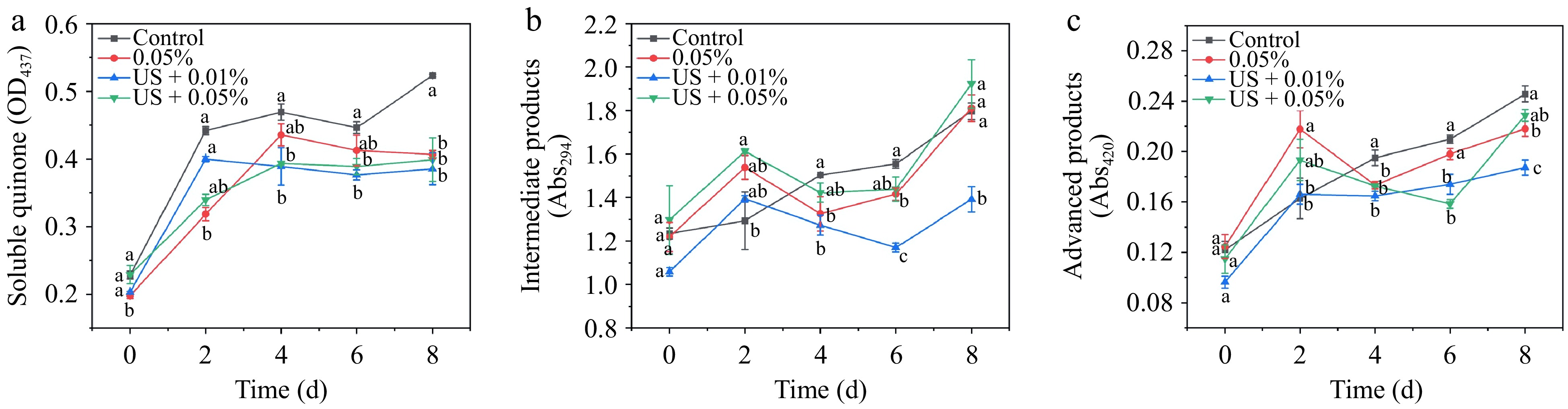
Figure 4.
Effect of different treatments (Control: distilled water, 0.05% HLE: only hawthorn leaf extract (0.05%) and ultrasound coupling two concentrations of hawthorn leaf extract (0.01%, 0.05%)) on (a) soluble quinone, (b) intermediate products, and (c) advanced products of fresh-cut potato during storage at 4 °C for 8 d. Data are expressed as the mean ± standard error (n = 3). Different letters indicate significant differences between groups (p < 0.05).
The production of soluble quinone, intermediate, and advanced products is not a straightforward reaction but an intricate reaction with browning development[32]. Quinones, as precursors of browning, combined with amino acids to generate black pigments and the accumulation of some intermediate and advanced products are also involved in the formation of melanoidins[23,25,26]. A previous study found that treatment of Sonchus oleraceus L. extract and US reduced the accumulation of soluble quinones, intermediate, and advanced products, and alleviated browning in fresh-cut potatoes[18]. Notably, the intermediate and advanced products increased gradually along with time in the control group. Three treatments enhanced their accumulation on day 2 and then alleviated products' increase during post storage. US + 0.01% HLE treatment effectively controlled the generation of intermediate products and advanced products, which may help control browning.
ROS-redox balance
-
Excessive reactive oxygen not only affects the antioxidant activity of food and biological systems, but also results in the formation of unsaturated fatty acids in phospholipids, lipid peroxidation, and the destruction of biofilm[29]. Among these treatments, the US + 0.01%HLE treatment maintained a lower H2O2 content during storage (Fig. 5a). Especially on day 4, the H2O2 content was only 65.91% of the control group and 88.59% of US + 0.05%HLE. LOX can be used as a specific and reliable indicator to reflect lipid peroxidation reaction, and its activity is related to plant senescence[33]. Figure 5b demonstrated LOX activity was observably suppressed by external treatments from day 4 to day 8, especially by US + 0.01% HLE treatment, which consistently maintained the lowest activity. MDA, as an indicator of lipid peroxidation, was usually used to denote membrane integrity[34]. In Fig. 5c, MDA accumulated with time, which was similar to that of H2O2 (Fig. 5a). Among four groups, US + 0.01% HLE treatment produced the lowest MDA content, while the control group produced the most.
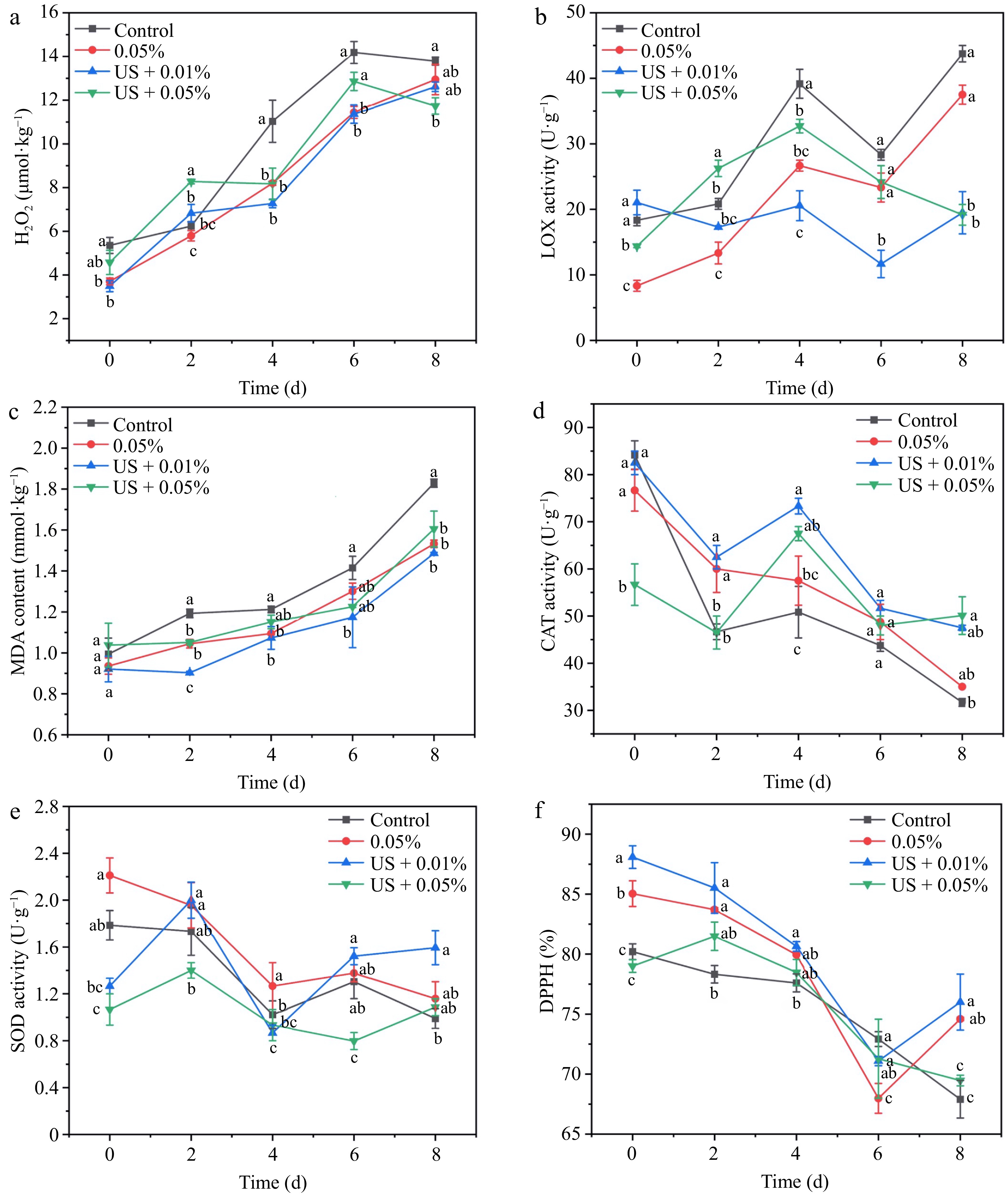
Figure 5.
Effect of different treatments (Control: distilled water, 0.05% HLE: only hawthorn leaf extract (0.05%) and ultrasound coupling two concentrations of hawthorn leaf extract (0.01%, 0.05%)) on activities of (a) H2O2 content, (b) LOX activity, (c) MDA content, (d) CAT activity, (e) SOD activity, and (f) DPPH of fresh-cut potato during storage at 4 °C for 8 d. Data are expressed as the mean ± standard error (n = 3). Different letters indicate significant differences between groups (p < 0.05).
CAT, as the guardian of the biological defense system, provides an antioxidant defense mechanism for organisms[29]. Figure 5d revealed that the activity of CAT decreased gradually, and US + 0.01% HLE treatment significantly alleviated its decline amplitude compared to the control group, especially from day 4 to day 8. SOD also synergistically resists the damage from reactive oxygen species and protects cells from toxicity. SOD activity showed an up-down fluctuation during storage (Fig. 5e). The SOD activity of the US + 0.01% HLE, US + 0.05% HLE, and 0.05% HLE treatment group was 1.76, 1.21, and 1.28 times that of the control group on day 8. DPPH is a relatively stable free radical, which is usually applied to evaluate the antioxidant capacity[13]. Figure 5f shows the antioxidant capacity gradually diminished during refrigeration, and treatments produced stronger capacity, particularly by US + 0.01% HLE treatment. Especially on day 8, the scavenging capacity of US + 0.01% HLE treatment was 76.00%, which was more than 1.13 times that of the control group.
Numerous research has demonstrated that the ROS-redox balance is a crucial factor in the regulation of enzymatic browning. You et al.[33], reported that short-period N2 treatment effectively delayed the browning degree in Chinese water chestnut slices, which may be related to lower contents of MDA, H2O2, and LOX activity, along with higher SOD and DPPH scavenging capacity. Wang et al.[35]found that salicylic acid spray delayed fruit senescence, reduced MDA content and PPO activity, and increased antioxidant enzyme activity. Magri et al.[36], reported that ascorbic acid combined with melatonin treatment delayed color changes of fresh-cut avocado fruits, improved ascorbate peroxidase, CAT and SOD activities, reduced PPO and guaiacol peroxidase activities, and decreased membrane disservice by influencing LOX. Thus, regulation of ROS-redox imbalance may help control enzymatic browning, maintain quality, and extend the storage period.
Schössler et al.[37] reported that potato is considered as a nonporous product, with only 2% of the intercellular space in the issue, and that the 'cavitation effect' produced by US would increase the mass transfer of dense nonporous tissue, change its original cell integrity, and better ensure product quality. Wloch et al.[38] reported HLE was beneficial to membranes. It is primarily positioned in the hydrophilic part of the membrane, changes the packing arrangement of the lipid polar heads, and successfully safeguards membrane lipids against oxidation. These modifications contributed to a stronger cell membrane and a higher stress resistance in erythrocytes. However, HLE may form a barrier that enhances and protects the membrane with none adverse effects. It has been previously identified that there are a total of 64 components in HLE including 34 flavonoids, 25 phenols, and five proanthocyanidins[18]. Alirezalu et al.[39] reported that HLE has large amounts of chlorogenic acid, vitexin, and vitexin 2-O-rhamnoside, which are usually used as natural antioxidants in the food industry. In our study, compared with the single treatment group, low or high concentration treatments, combined with US, effectively improved the quality of fresh-cut potato and cell wholeness, showing lower LOX activity, H2O2, and MDA contents, particularly the US + 0.01% HLE treatment.
Browning correlation analysis
-
In Fig. 6, correlation analysis demonstrated that the BI value was significantly positively correlated with the activities PPO (r = 0.91), POD (r = 0.92), and the total phenol contents (r = 0.81), soluble quinones (r = 0.75), as well as intermediate and advanced products (r = 0.60; r = 0.76), which indicated that the oxidative enzyme, browning substance, and products play a critical role in browning development (all p-values < 0.01). Additionally, LOX activity (r = −0.72), H2O2 (r = −0.89), and MDA content (r = −0.83) may also contribute to browning discoloration because their correlation coefficient with BI was close to −1 (p-values < 0.01)[40]. In terms of antioxidant level, a significant negative correlation (r = −0.74) was observed between the antioxidant capacity (DPPH radical clearance) and antioxidant enzyme activity (CAT: r = 0.44; SOD: r = 0.52) with BI (p values are all less than 0.01). It implies that the activation of CAT and SOD can enhance the antioxidant capacity, alleviate lipid peroxidation, and therefore control the browning of freshly cut potatoes[41]. Notably, all the detected parameters mentioned above influence browning except for phenylalanine ammonia-lyase. These outcomes illustrate that browning development can be delayed by improving antioxidant capacity, controlling the activities of oxidative enzymes, and reducing oxidative product accumulation.
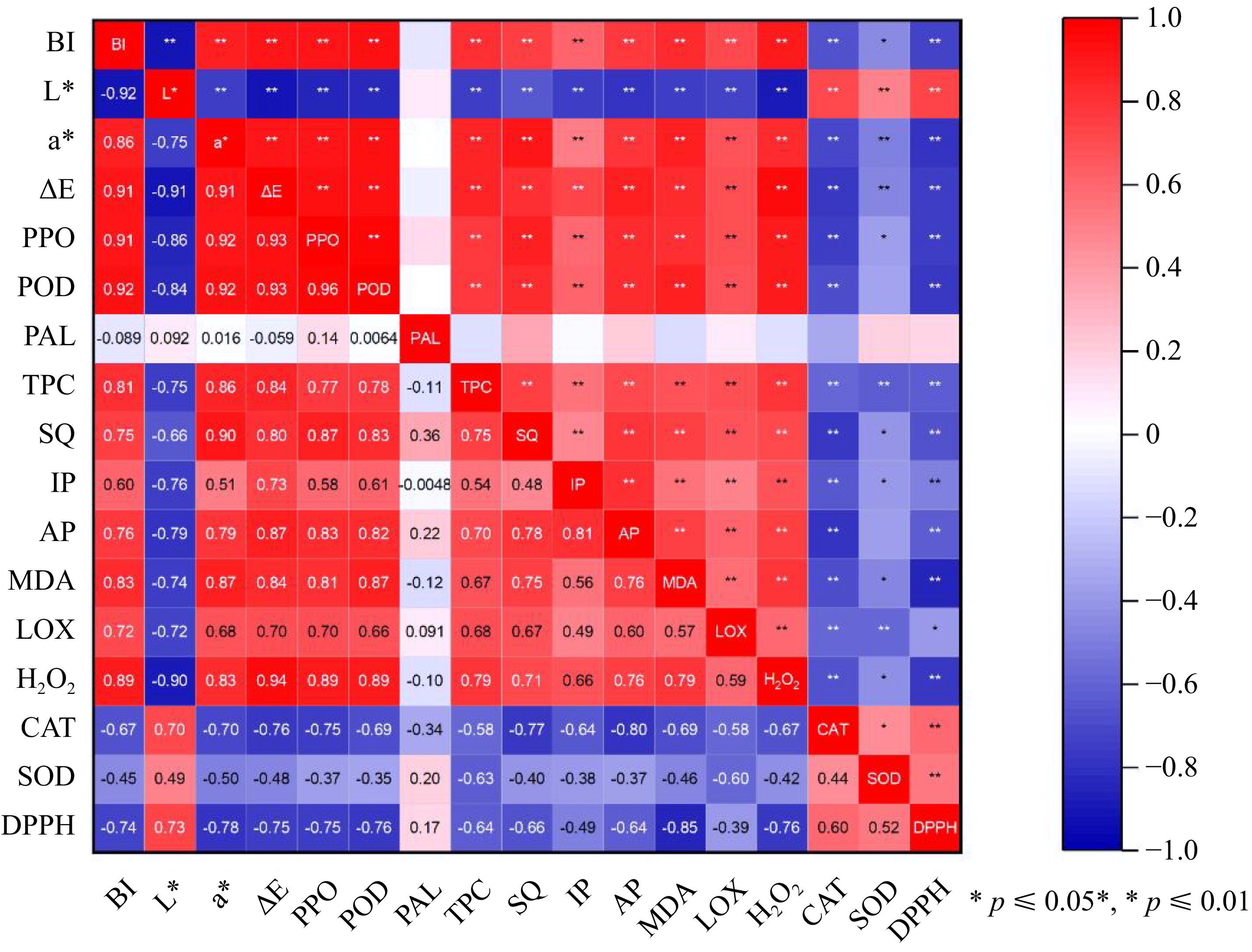
Figure 6.
Pearson correlation matrix of browning index (BI), color (L*, a*, ΔE*), enzymes (PAL, PPO, POD), total phenol content (TPC), browning products (soluble quinone (SQ), intermediate product (IP), advanced product (AP)), related membrane peroxides (LOX, MDA, H2O2), antioxidant enzymes (CAT, SOD), and antioxidant activity (DPPH). Different asterisks indicate different levels of significance (* p ≤ 0.05; ** p ≤ 0.01).
This study revealed that a suitable concentration can perform a better browning mediation effect, whether individual usage or in combination with other technologies. It is not the case that the higher the dose of nature extract, the better anti-browning preservation effect (Fig. 7). On the one hand, US + 0.01% HLE effectively reduced the activity of browning enzymes (PPO, POD), and significantly reduced the content of phenols, soluble quinones, intermediate, and advanced products. On the other hand, ROS-redox imbalance was impaired, evidenced by less MDA, H2O2, LOX activity, and higher CAT, SOD, and antioxidant capacity. Overall, US combined with low-dose extracts (0.01%) is a more promising browning regulation technique.
-
The results demonstrated that combined treatment better alleviated fresh-cut browning than US and HLE treatment alone at the optimized conditions, particularly with low concentration (0.01% HLE). It maintained a better color appearance and lower BI value. The US + 0.01% HLE treatment produced the least content of soluble quinones, intermediate, and advanced products. The greatest inhibition on the activity of PPO, POD, and PAL, along with the accumulation of total phenolics, was also achieved. Meanwhile, the lowest H2O2, MDA content, and LOX activity, and the highest activities of CAT and SOD were achieved by US + 0.01% HLE treatment. Correlation analysis demonstrated that all the detected parameters mentioned above influenced browning except for phenylalanine ammonia-lyase. It is suspected that controlling the key factors of the enzymatic browning reaction including enzymes, substance, and products may help US + 0.01% HLE treatment to produce the best browning alleviation effect. Additionally, modulation of the ROS-redox imbalance may also produce a synergistic and excellent anti-browning effect. Overall, ultrasound coupling extract (US + 0.01% HLE) is a promising technology for discoloration control in the fresh-cut industry. To our knowledge, this is the first report that has shown that less extract produces a better anti-browning effect when combined with US.
This work was supported by the Opening Research Grant, Biomedical Research Center, Northwest Minzu University (CM202101), and the Postdoctoral Research Foundation of China (2022M712375).
-
The authors confirm contribution to the paper as follows: study conception and design: Qiao L; writing-review and editing: Qiao L, Zhao Z, Wang H; data curation: Lu L, Zheng J; formal analysis: Ding G, Liu X; investigation: Qiao Z; data collection: Tian X; funding acquisition: Qiao L; project administration and supervision: Qiao Z; Tian X. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Qiao L, Zhao Z, Wang H, Zheng J, Lu L, et al. 2025. Less hawthorn leaf extract produced a better anti-browning effect when combined with ultrasound: a case study in fresh-cut potatoes. Food Innovation and Advances 4(2): 274−283 doi: 10.48130/fia-0025-0026
Less hawthorn leaf extract produced a better anti-browning effect when combined with ultrasound: a case study in fresh-cut potatoes
- Received: 20 August 2024
- Revised: 06 March 2025
- Accepted: 06 March 2025
- Published online: 30 June 2025
Abstract: In the fresh-cutting industry, enzymatic browning poses a significant challenge. Although many anti-browning approaches have been evaluated individually, there are few combined technologies reported with the characteristics of health and low cost. To explore whether ultrasound (US) coupled with hawthorn leaf extract (HLE) could function as an effective anti-browning technology, this study was conducted. Results indicated that the combined treatment (US + 0.05% HLE) delayed the browning rate better than the individual application in fresh-cut potatoes stored at 4 °C for 8 d. Interestingly, the lower concentration combinations (US + 0.01% HLE) produced better anti-browning than that of their optimal combination (US + 0.05% HLE). Not only the activities of polyphenol oxidase, peroxidase, and phenylalanine ammonia-lyase, but also the content of phenolic, soluble quinones, intermediate, and advanced products were significantly diminished. Additionally, a decrease of malondialdehyde and H2O2, as well as lipoxygenase activity, and an increase in catalase, superoxide dismutase, and antioxidant capacity were achieved by the lower combination treatment. A Pearson correlation analysis revealed that all of the above detected parameters, except for phenylalanine ammonia-lyase, influenced browning development. Compared with the optimal combination, there is less accumulation of phenolic, and the intermediate and advanced products produced by the lower concentration combination. Thus, it is inferred the combination (US + 0.01% HLE) mediated the browning reaction and alleviated the reactive oxygen species (ROS)‒redox imbalance, which synergistically produced a superior quality. Overall, ultrasound coupling with a less dose extract (US + 0.01% HLE) is a more promising technology for discoloration control in the fresh cut industry.
-
Key words:
- Enzymatic browning /
- Combination technology /
- Ultrasound /
- Hawthorn leaf extract /
- Fresh-cut potato