-
The process of fruit abscission is crucial for the overall development of fruit crop species, both in terms of their vegetative and reproductive growth. This process has been a subject of immense interest and research in the field of plant biology, spanning from historical to contemporary times. Many woody fruit crop species produce a large number of flowers, which may be an adaptive strategy to attract pollinators and ensure offspring. However, once the fruits begin to set and develop, excess flowers abscise naturally[1]. Several woody fruit crop species undergo significant fruit drop during a process known as 'physiological fruit abscission'. For example, citrus and litchi experience fruit drop at different stages of development, with less than 1% and 5% of the fruits reaching full maturity, respectively[2−5]. Therefore, in the cultivation of litchi and citrus, physical practices such as girdling, as well as the application of synthetic auxin agents such as 2,4-D (2,4-Dichlorophenoxyacetic acid), are required to reduce fruit abscission. In other woody fruit crop species like apple, thinning practices, such as the application of chemical thinners including cytokinins (6-benzyladenine) and ethylene generating chemicals, are necessary to achieve commercially acceptable fruit and prevent the trees from entering an alternate year fruit bearing cycle[6]. In general, once developmental fruit abscission is complete, the remaining fruits can continue to develop until maturity, unless they are subjected to adverse environmental conditions or attacked by pathogens or pests, which may trigger new conditional abscission events. Fruits can also undergo preharvest abscission at the fully mature stage. Among fruit trees, apple, litchi, citrus, and peach are particularly susceptible to preharvest fruit drop. To address this issue, plant growth regulators are commonly used as a preventive measure[5,7−9]. It is worth noting that fruit drop can also occur during postharvest storage, posing a significant challenge to maintaining the quality of fruits after harvesting[10].
While plants have developed various efficient mechanisms to facilitate fruit abscission, these can present significant challenges when it comes to the commercial cultivation of fruit trees. Over the past few decades, significant progress has been made in understanding the molecular basis of organ abscission through genetic and mutant studies conducted on model plants like Arabidopsis and tomato. These studies have led to the identification of numerous molecular components that play a role in the regulation of abscission[11]. However, research on the mechanisms of fruit abscission in woody fruit trees has been relatively lagging. In this review, we will discuss the specificity of fruit abscission in woody fruit trees, combining the progress of organ abscission in non-woody fruit crops. We will focus on what signals are involved in fruit abscission, how these signals are generated and output, and how the abscission cues are perceived and transmitted by the abscission zone cells.
-
Similar to the abscission of other plant organs, fruit abscission typically takes place at distinct regions known as abscission zones (AZs). These AZs begin to form early on, alongside the development of lateral organs originating from the shoot apical meristem. Morphologically, they can be visually distinguished from the surrounding tissues. Different woody fruit crop species can have 1, 2, or 3 observed AZ locations[12−14]. For example, there is a single AZ for litchi and apple, and two and three AZs for citrus and olive to shed fruit, respectively (Fig. 1)[15].
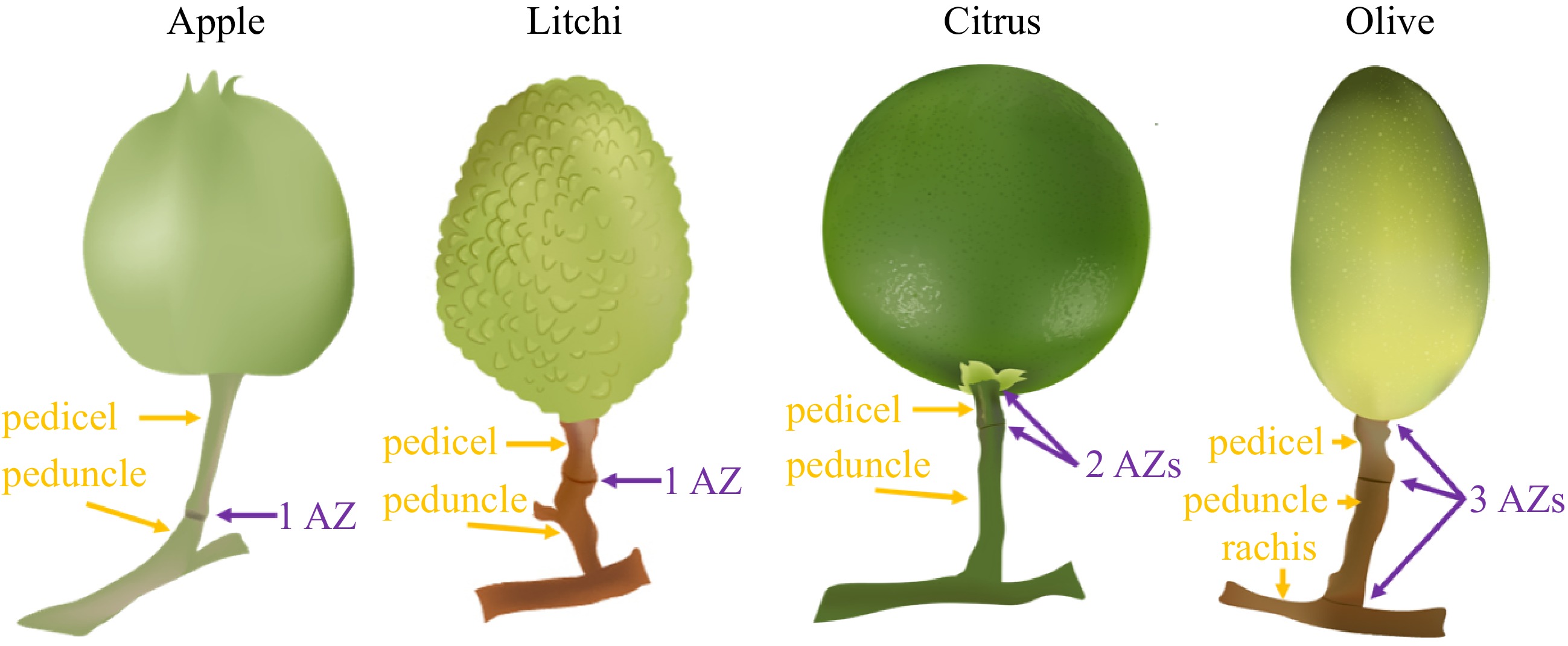
Figure 1.
The number of AZ varies among different woody fruit crop species. Apple and litchi fruits exhibit a single AZ localized at the junction region between the pedicel and peduncle. Citrus fruits possess two AZs localized at the junction between the fruit and pedicel (referred to as AZ-C) and the junction between the pedicel and peduncle (referred to as AZ-A), respectively. Olive fruits display three AZs localized at the junction between the fruit and pedicel, the junction between pedicel and peduncle, and the junction between the peduncle and rachis, respectively.
Once the AZ is formed, it acquires the ability to undergo cell separation. In plants, the process of organ abscission can be categorized into four distinct stages, including the differentiation and formation of the AZ, the perception of abscission signals by AZ cells, the initiation and completion of cell separation within the AZ, and the formation of a protective layer on the parent side[16]. The AZ, which consists of specialized tissue containing cells that are specifically targeted for abscission, has been a central focus in numerous studies on organ abscission. The development of AZ occurs early in the development of the organ that will eventually undergo abscission. Several important genes have been identified as necessary for AZ formation in various non-woody plant species. The transcription factors BOP1/2 have been identified as key players in the process of abscission zone (AZ) formation in Arabidopsis[17]. In tomato, three MADS domain transcription factors, JOINTLESS (J), MACROCAYLYX (MC), SLMBP21, and the ERF transcription factor ERF52 have been implicated in AZ formation[18−20]. AZ development in rice involves the crucial involvement of several transcription factors. These include the MYB transcription factor SHA1, the BEL1-like homeobox transcription factor qSH1 located on chromosome 1, and the APETALA2 transcription factors SHAT1 and SNB, both of which play significant roles in regulating seed shattering[21−24]. It is worth noting that while the ectopic expression of the apple MdJb gene in a J-deficient tomato mutant led to the restoration of functional pedicel AZs and the pear PsJOINTLESS was suggested to be involved in AZ development[25,26], the key genes regulating AZ development in woody fruit crop species have not yet been identified. In the past few decades, with the help of model plants such as Arabidopsis and tomato, many key genes involved in regulating organ abscission have been identified[11]. However, the mechanisms underlying the regulation of these key genes in AZ is still not clear, and little is known about the abscission cues that can be perceived by AZ cells. Moreover, there is a lack of consensus regarding the specific model of how abscission cues are generated and transmitted from the organ undergoing abscission to the AZ in woody fruit crops.
Histologically, AZ cells are different from adjacent cells, as they are smaller, denser, and have little or no lignin deposition[5]. The AZ can function as an independent tissue that, upon receiving and perceiving abscission cues, initiates the cell separation process to complete organ abscission. Thus, the separation of AZ cells is remotely controlled by abscission cues from neighboring cells. But what are these signals and where do they come from? According to existing knowledge, it is understood that the main signals responsible for initiating the activation of the abscission zone originate from within the organ undergoing abscission[27−30]. In comparison to abscission of Arabidopsis floral organs, and rose petals, fruit abscission in woody plant species is generally more complex, as the process of fruit abscission involves changes in the activity of different tissues of the fruit itself and of other neighboring organs such as, for example, the sepals or the pedicel that may or may not contain the AZ[12,29,31−33].
Apple is considered as an interesting model species for studying early fruit abscission under endogenous and/or exogenous stimuli. The apple flowers and fruits grow in a cymose inflorescence, leading to a central 'King' fruit and less developed lateral fruits. During the early fruiting stage, there is a notable discrepancy in abscission potential between the lateral fruits and the 'King' fruit. This variation arises from the developmental differences observed in these fruits[9]. Several studies have revealed the major physiological and molecular events that lead to selective abscission of lateral fruits in the cymose, either occurring naturally or through thinning treatments[27−29,34]. Currently, there are two viewpoints regarding the nature of abscission cues and their generation sites in apples. One viewpoint suggests that abscission cues are triggered by an inhibition in polar auxin transport (PAT) in the fruit, primarily occurring at the junction between the pedicel and spur[35−37]. According to this viewpoint, also called primigenic dominance hypothesis which describes that the earlier developed sink organs inhibit the later developed ones. In detail, the early developed fruits (the 'King' fruit) always export more auxin than the subordinate fruits (the lateral fruits) and inhibit the auxin polar export of the lateral fruits, therefore, the lateral fruits are developmentally inhibited and go into senescence and fall off the tree[35−37]. The other viewpoint suggests that the reduction or even depletion of PAT is due to insufficient carbohydrate supply during fruit development. This leads to oxidative stress in the fruit, resulting in an increase in ethylene content in the flesh, which permeates into the embryo. As the ethylene receptors in the outer layer of the embryo cannot bind all the excess ethylene, the supersaturated ethylene gradually permeates into the embryo, causing damage and triggering embryo death. This suggests that ethylene produced in the fruit is an abscission cue preceding the reduction of PAT[27−29].
In the field of woody fruit crops, it is widely acknowledged that ethylene plays a crucial role in promoting the separation of cells in the abscission zone. On the other hand, the hormone auxin has been found to inhibit this process[5,11,38]. Currently, our understanding of the specific tissues involved in hormone synthesis and metabolism during fruit abscission is still limited. Different regulatory patterns may exist in different woody fruit crop species. For example, in avocado, it has been found that the ethylene production in fruits with defective seeds is 7−10 times higher than that in fruits with normal seeds, suggesting that defective seeds may be a source of increased ethylene production leading to fruit abscission[39]. Additionally, research has indicated a potential link between the accumulation of ABA in abscising avocado fruit and seed abortion[40]. In the case of mango, it has been suggested that the perception of ethylene by receptors in the pedicel, along with limited carbohydrate supply within the fruit pericarp, may contribute to the natural abscission of mango fruitlets[41]. In olive, there is an observed correlation between an increase in the content of 1-aminocyclopropane-1-carboxylic acid (an ethylene precursor) in the mature fruit abscission zone-C (AZ-C) and a decrease in the force required for fruit detachment from the tree[42]. Furthermore, in peach, when fruit abscission is induced, an increase in ethylene production has been detected in the abscission zone of the fruitlet, as well as in the proximal and distal tissues surrounding the abscission zone, indicating that both the abscission zone and its surrounding tissues can serve as sites of ethylene biosynthesis during fruit abscission[43]. In pear, it is found that the increase in abscission can be due to ABA-induced carbohydrate deficit[44]. In the case of persimmon, there is a proposed mechanism where ethylene, produced by the sepals, is believed to diffuse into other tissues of the fruit. This ethylene then acts as a secondary signal, triggering the production of more ethylene within these tissues through an autocatalytic process. Consequently, this surge in ethylene production primarily occurs within the abscission zone of the fruit, ultimately resulting in the detachment of fruitlets and other organs[45].
Carbohydrate shortage-induced increases in ethylene and ABA, accompanied by reduced IAA and perturbed polar auxin transportation (PAT), in fruits have been considered as important factors in fruit abscission in woody fruit crops[46−48]. In Citrus, studies have shown that application of IAA after removal of young fruits can inhibit pedicel abscission, which may be related to the inhibition of gene expression in ethylene synthesis and signal transduction by IAA through polar transport to the AZ, thereby inhibiting the transmission of abscission cues[12]. The technique of ringing branches is commonly employed in citrus cultivation to enhance fruit yield. Previous studies have provided evidence that ringing significantly decreases the rate of fruit abscission. This effect is mainly attributed to the increased availability of carbohydrates to developing fruitlets in branches that have been ringed[49]. Recent findings further suggest that the mechanism behind this process involves the induction of auxin export from fruitlets and its subsequent transport to the AZ-C where it inhibits activation, ensuring a continuous supply of carbohydrates to the fruitlet and ultimately preventing abscission[50]. In the development of litchi fruitlets, it is consistently observed that abscission waves occur during overcast and rainy weather conditions. To simulate these conditions, artificial shading or the application of photosynthetic inhibitors has been used, resulting in significant fruit abscission[51]. In a study conducted by Yuan & Huang, it was found that trunk-girdled trees during full bloom exhibited reduced intensity of abscission waves compared to ungirdled trees[52]. Furthermore, when litchi fruitlets were subjected to both girdling and defoliation, there was a significant decrease in soluble sugar content and IAA levels, while ethylene production was induced, leading to fruitlet abscission[33,53]. These findings suggest that carbohydrate deficiency may serve as the initial trigger for abscission in litchi fruitlets. Similarly, shade-induced or ABA-induced carbohydrate deficiency has been observed to induce fruit abscission in pear trees[44]. Recent findings in longan also suggest that carbohydrate stress reduces the levels of IAA but increases ethylene content and abscission rate in the fruitlets, which proposed that starved fruitlets release abscission cues primarily through weakened PAT rather than ethylene production[54].
In conclusion, existing studies suggest that the weakening or depletion of PAT from the fruit to the AZ is a major form of fruit abscission cue in woody fruit crops. Under stress conditions such as carbohydrate shortage, fruits may release ethylene and ABA, thereby weakening or stopping PAT from the fruit to the AZ (Fig. 2). However, the specific tissues involved in hormone synthesis and metabolism in fruits, as well as the key regulatory genes, are far from understood.
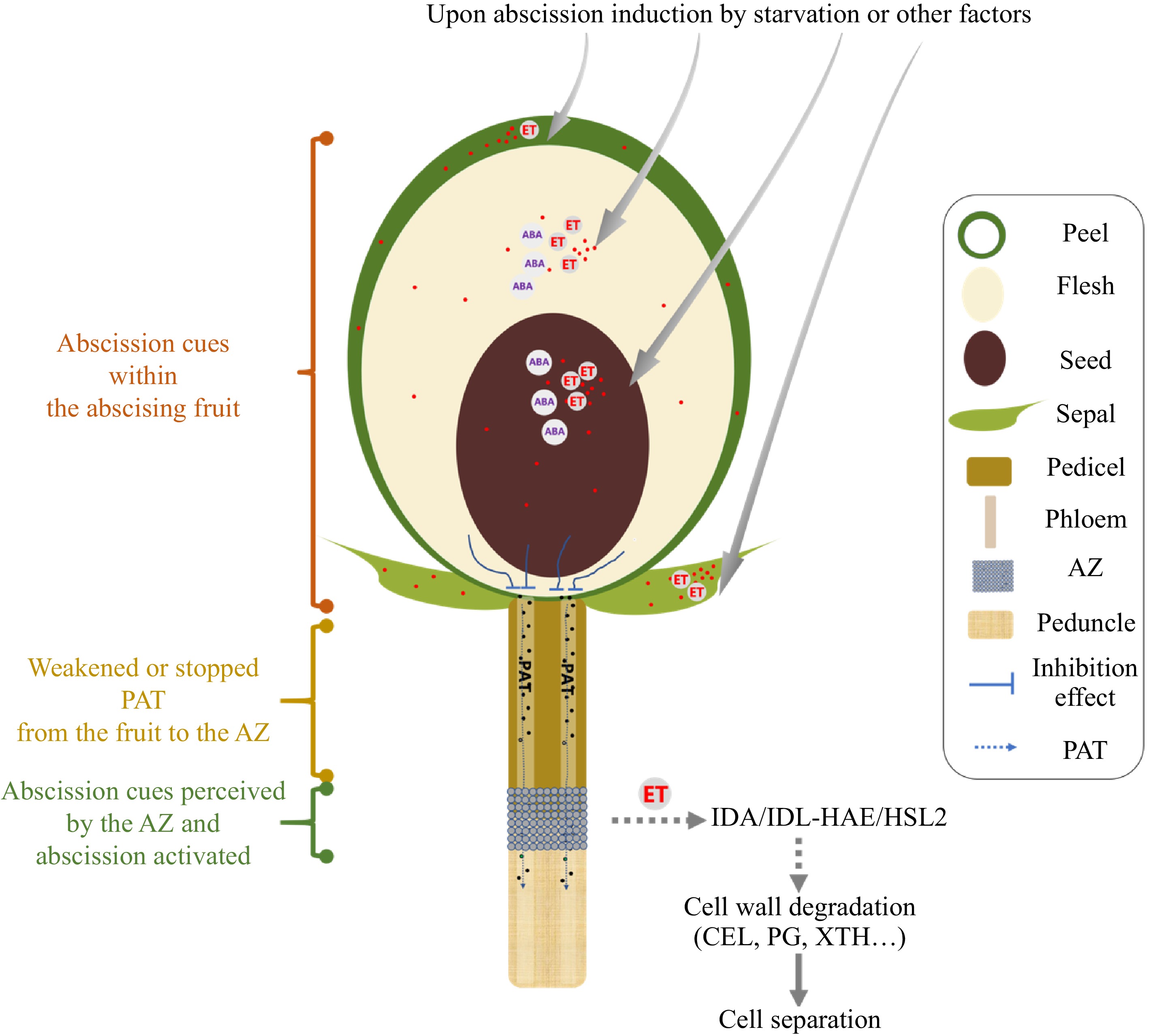
Figure 2.
A proposed model outlining the generation, transmission, and perception of abscission cues in woody fruit crop species. Upon abscission induction generally by carbohydrate shortage stress (starvation), the ethylene or abscisic acid (ABA) biosynthesis occurs within different parts of the fruit, depending on the species. For example, in apple fruitlets, the peel and flesh are responsible for the synthesis of ethylene and ABA. In avocado fruit, it is the seed, and in persimmon fruit, it is the sepal. The production of ethylene and ABA within the abscising fruit weakens or halts the polar auxin transport (PAT) from the fruit to the abscission zone (AZ). However, the precise mechanisms by which this occurs are currently unknown. When PAT is weakened or stopped across the AZ, it renders the AZ sensitive to ethylene or triggers the biosynthesis of ethylene within the AZ. In addition, both the ABA and ethylene (ET) generated in the fruit may also be transported to the AZ and participate in ethylene burst within the AZ. This ethylene production can be perceived by the IDA/IDL-HAE/HSL2 signaling pathway, likely through the binding of transcription factors involved in ethylene signaling. Subsequently, the cell separation process is initiated and is accomplished through the action of specific enzymes involved in the degradation of the cell wall, including cellulase (CEL), polygalacturonase (PG), and xyloglucan endotransglucosylase/hydrolase (XTH). Symbols used in the diagram are explained in the legend.
-
If the decrease or depletion of auxin transport towards the abscission zone is considered an abscission cue, then how do the abscission cells sense this change? How do these hormonal changes transform into molecular events that trigger the abscission process in woody fruit crops? An important breakthrough in understanding the activation mechanism of organ abscission in plants involves the identification of the abscission mutant ida and the functionally redundant receptor kinases HAE and HAE-like2 in Arabidopsis[34,55−57]. In the ida mutant, the majority of floral organs, including petals, sepals, and stamens, remain attached during pod growth and maturation[55]. This phenomenon has also been observed in oilseed rape[58]. The IDA gene encodes a small protein with a conserved C-terminal domain that can bind to the HAE and HSL2 receptors[55,59]. Genetic studies have demonstrated that once IDA binds to HAE/HSL2, HAE/HSL2 can modulate KNOTTED1-LIKE HOMEOBOX (KNOX) transcription factors through a mitogen-activated protein (MAP) kinase cascade[34,60,61]. Although the knat2/knat6 mutant only exhibits a weak phenotype, it is suggested that KNAT2 and KNAT6, together with other transcription factors, induce the transcription of cell wall remodeling and degrading enzymes responsible for cell separation and subsequent organ abscission. Thus, IDA-HAE/HSL2 signaling is considered as a signal inducer for the final organ abscission[34,62−64].
To date, IDA-like and HAE-like genes have been identified in crop species and highly expressed in the AZ, including tomato[65−67], soybean[68], yellow lupine[69,70], rose[71], and woody fruit crops oil palm[72], citrus[73], and litchi[74,75]. Research has demonstrated that synthetic IDA peptides have the ability to induce premature abscission of floral organs in Arabidopsis[76], flower separation in yellow lupine[70,77], leaf abscission in poplar and mature fruit abscission in oil palm[78]. In addition, when IDA homologous genes from citrus (CitIDA3), litchi (LcIDL1), and rose (RbIDL1 and RbIDL4) are ectopically expressed in Arabidopsis, they can cause early flower abscission and rescue the abscission defects in ida[71,73,74]. Furthermore, the transformation of the litchi LcHSL2 into Arabidopsis hae hsl2 mutants can reactivate the abscission of floral organs[75]. Together, this evidence suggests that the function of the IDA-HAE/HSL2 signaling module is conserved in many species, including woody fruit crops. However, the detailed role of the IDA-HAE/HSL2 signaling module in these woody fruit crops still needs to be provided.
Considering that membrane receptor proteins are core signaling molecules that perceive changes in the extracellular environment (such as hormone level changes), it makes sense that the IDA-HAE/HSL2 signaling module is likely to be the core component for sensing the abscission cue. If this is the case, then how does the IDA-HAE/HSL2 signaling module receive the abscission cue? Continuous flow of auxin through the abscission zone can prevent ethylene synthesis and sensitivity, thereby inhibiting fruit abscission[79]. By promoting polar transport of IAA and downregulating the expression of the CitIDA3 gene, the occurrence of preharvest fruit abscission can be delayed or reduced[80]. Several investigations have reported that IDA homologous genes from various plant species, such as tomato, soybean, oil palm, citrus, tea, rose, and yellow lupine, exhibit activation during abscission induced by ethylene. Furthermore, the expression of these genes can be postponed by employing an ethylene inhibitor[65,69,71−73,81]. During fruit abscission in litchi, it has been observed that LcIDL1 and LcHSL2, which are located in the AZ, are also induced by ethylene[74,75]. Recent studies further demonstrated that low light intensity associated stress induces ethylene synthesis, which in turn increases the expression of the SlIDL6, ultimately promoting the abscission of tomato flowers[66]. The findings of these studies provide further support for the notion that the IDA-HAE/HSL2 signaling pathway is ethylene-dependent and functions downstream of ethylene to regulate the abscission process in various plant species, including woody fruit crops[30,82]. Consistently, the ethylene-responsive element (ERE) is found in the promoter of IDA-like genes, such as NtIDL6 in tobacco and SlIDL1, SlIDL7, and SlIDL4 in tomato. The expression of these genes is increased following AZ activation[67,83], implying that ethylene may directly regulate the IDA-HAE/HSL2 signaling module through transcription factors in its signaling pathway, thereby initiating the final abscission program (Fig. 2).
-
In the past few decades, research on model plants, particularly on Arabidopsis organ abscission[11], tomato flower abscission[18,20,66,84−88], and rose petal abscission[89−91], has successfully identified numerous molecular components that play crucial roles in the activation of organ abscission. However, due to the immature genetic transformation system in woody fruit crop species, the study of functional genes and regulatory mechanisms of fruit abscission in woody fruit crops lags behind. On the other hand, we are increasingly recognizing that certain elements seem to be common characteristics of fruit abscission processes in woody fruit crops, such as basipetal polar auxin transport, ethylene biosynthesis and perception. Moreover, the abscission zone, being a specialized tissue responsible for integrating abscission cues, has garnered significant attention in abscission studies. However, it is important to note that the abscission zone does not operate in isolation. In the study of fruit abscission in woody fruit crops, it is important to consider that adjacent tissues may play a significant role as sources of mobile signals that regulate the abscission process.
In a recent review conducted by Botton & Ruperti[30], they provide a comprehensive overview of the role of ethylene in both the AZ and the abscising organ, shedding light on the potential connections between different stages of the abscission process. In this review, our focus is on exploring the signals involved in fruit abscission in woody fruit crops, their generation and output, and how the AZ cells perceive and transmit these abscission cues. We point out that one significant abscission cue in woody fruit crops is the weakening or depletion of the PAT from the fruit to the AZ. This process is usually triggered when the fruit experiences a shortage of carbohydrates, leading to the release of ethylene and ABA. Our analysis of the most recently published papers highlights that the ethylene signaling in the AZ activated by depletion of PAT could be directly sensed by the IDA-HAE/HSL2 pathway, thereby initiating the final abscission program.
Over the past few years, significant advancements have been made in the investigation of fruit abscission mechanisms specifically in woody fruit crops[14,50,92−97]. As an example, a recent study shed light on the role of a regulatory module comprising LcDOF5.6 and LcRbohD in the regulation of reactive oxygen species (ROS)-mediated fruitlet abscission in litchi. This finding provides valuable insights into the control of ROS homeostasis within the abscission zone, offering a deeper understanding of the underlying mechanisms[92]. However, major research perspectives including understanding how fruit produces abscission cues, how abscission zone cells perceive these cues, and the mechanisms of fruit abscission in woody fruit crops, are still to be addressed:
1. Determining where ethylene is synthesized in abscising fruit and how it is regulated.
2. Understanding how ethylene in abscising fruit inhibits polar auxin transport towards the abscission zone.
3. Using gene editing tools such as CRISPR/Cas9 to further validate the conserved role of the IDA-HAE/HSL2 signaling module in regulating fruit abscission.
4. Elucidating how the IDA-HAE/HSL2 pathway in the abscission zone senses the abscission cue and whether there is a direct connection between ethylene signaling and the activation of the IDA-HAE/HSL2 pathway.
5. Determining the downstream factors of the IDA-HAE/HSL2 signaling module. To date, the involvement of KNOX homologous genes in tomato and litchi has been reported in organ abscission. However, it remains to be determined whether these molecular factors are conserved across different fruit abscission systems, necessitating further investigation.
-
The authors confirm contribution to the paper as follows: study conception and design: Zhao M, Li J; analysis and interpretation of results: Zhao M, Shi CL, Li J; draft manuscript preparation: Zhao M. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This work was supported by grants from the Natural Science Foundation of Guangdong Province, China (Grant No. 2021B1515120082) and the Laboratory of Lingnan Modern Agriculture Project (Grant No. NZ NT2021004).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhao M, Shi CL, Li J. 2024. Abscission cues generated within the abscising organ and perceived by the abscission zone in woody fruit crops. Fruit Research 4: e014 doi: 10.48130/frures-0024-0007
Abscission cues generated within the abscising organ and perceived by the abscission zone in woody fruit crops
- Received: 09 November 2023
- Revised: 17 January 2024
- Accepted: 19 January 2024
- Published online: 02 April 2024
Abstract: From an evolutionary perspective, fruit abscission is an intelligent regulatory mechanism by which fruit trees adapt to their environment and ensure offspring. However, from an agricultural production standpoint, unwanted fruit abscission can cause significant loss in fruit yield and economic value. Therefore, investigating the mechanisms of fruit abscission has always been an important focus in the field of plant research. Acquiring a thorough comprehension of the underlying mechanisms responsible for fruit abscission is highly valuable for enhancing fruit crop breeding and optimizing harvesting practices. In this review, we focus on fruit abscission, particularly discussing the nature of abscission cues within the abscising fruit, how these signals are generated and transmitted, and how the abscission zone cells perceive and respond to these signals in woody fruit crops.
-
Key words:
- Abscission cues /
- Abscising organ /
- Woody fruit crops












