-
Medicinal plants are widely used to produce clinical therapeutic drugs or raw materials for drug preparations, because they are rich in various crucial bioactive substances, such as secondary metabolites (SMs), polysaccharides, starch, and other carbohydrates[1,2]. Medicinal plants have become important economic crops in several countries owing to their high demand. According to the China Statistical Yearbook (2022), China exported approximately 125,971 tons of medicinal plants valued at USD
${\$} $ ${\$} $ SM accumulation is affected by internal physiological factors, including plant variety, age, developmental stage, and different plant organs. For example, it has been reported that there are significant differences in the volatile components of the Citrus reticulata 'chachi' peel at different development stages[8]. The contents of C6-C9 alcohols and aldehydes are higher during the early stage (July−October), whereas the contents of terpenes, ketones, and esters are higher during the late stage (November−December)[8]. The biosynthesis of SMs in medicinal plants are also influenced by external environmental factors, such as light, water, temperature, salinity, pH, and microorganisms[9,10]. Environmental changes cause a series of physiological changes in plants, thereby affecting the accumulation of SMs. The total phenol content in Dracocephalum forrestii significantly increases upon treatment with blue and red light, especially in the case of caffeic acid, salvianolic acid I/H, salvianolic acid E, and salvianolic acid B[11]. Otherwise, some SMs can significantly enhance the adaptability of plants to the environment, including salt, drought, and disease resistance. For example, the active ingredients in Saposhnikova divaricate naringin dihydrochalcone, scopolin, deltoin, and overatorin can enhance plant resistance to drought stress[12].
Recently, increasing studies have shown that rhizospheric and endophytic microorganisms significantly affect SM synthesis in medicinal plants. With the rapid development of high-throughput sequencing technology, rhizospheric and endophytic microorganisms are reported to be closely related to the accumulation of SMs in medicinal plants[13, 14]. Owing to the advantages of easy cultivation and fermentation by microorganisms, they have great application prospects for improving the production of bioactive ingredients in medicinal plants. In this review, recent progress in understanding the role of microorganisms in the accumulation of SMs in medicinal plants are summarized.
-
The environment in which plants reside is filled with various microorganisms, including those in the soil and air, as well as microorganisms in plant roots, stems, and leaves. These microorganisms interact with and regulate many biological processes of plants. Among these microorganisms, rhizospheric and endophytic microorganisms most significantly affect the accumulation of SMs in plants[15]. The rhizosphere is a collective term for the narrow area surrounding plant roots, which is a complex ecosystem[16]. Endophytes are microorganisms that reside within the tissues and organs of plants and are generally present throughout the different stages of plant growth and development without causing obvious pathological damage to the plant[17]. Note that endophytes usually originate from rhizospheric microorganisms[18]. Plant roots secrete several organic compounds, including some primary metabolites such as carbohydrates, amino acids, organic acids, and SMs, which can provide energy for microbial survival. Therefore, compared to other plant parts, plant roots, and the rhizosphere are rich in various microorganisms. Microorganisms also affect many physiological processes in plants, including SM accumulation.
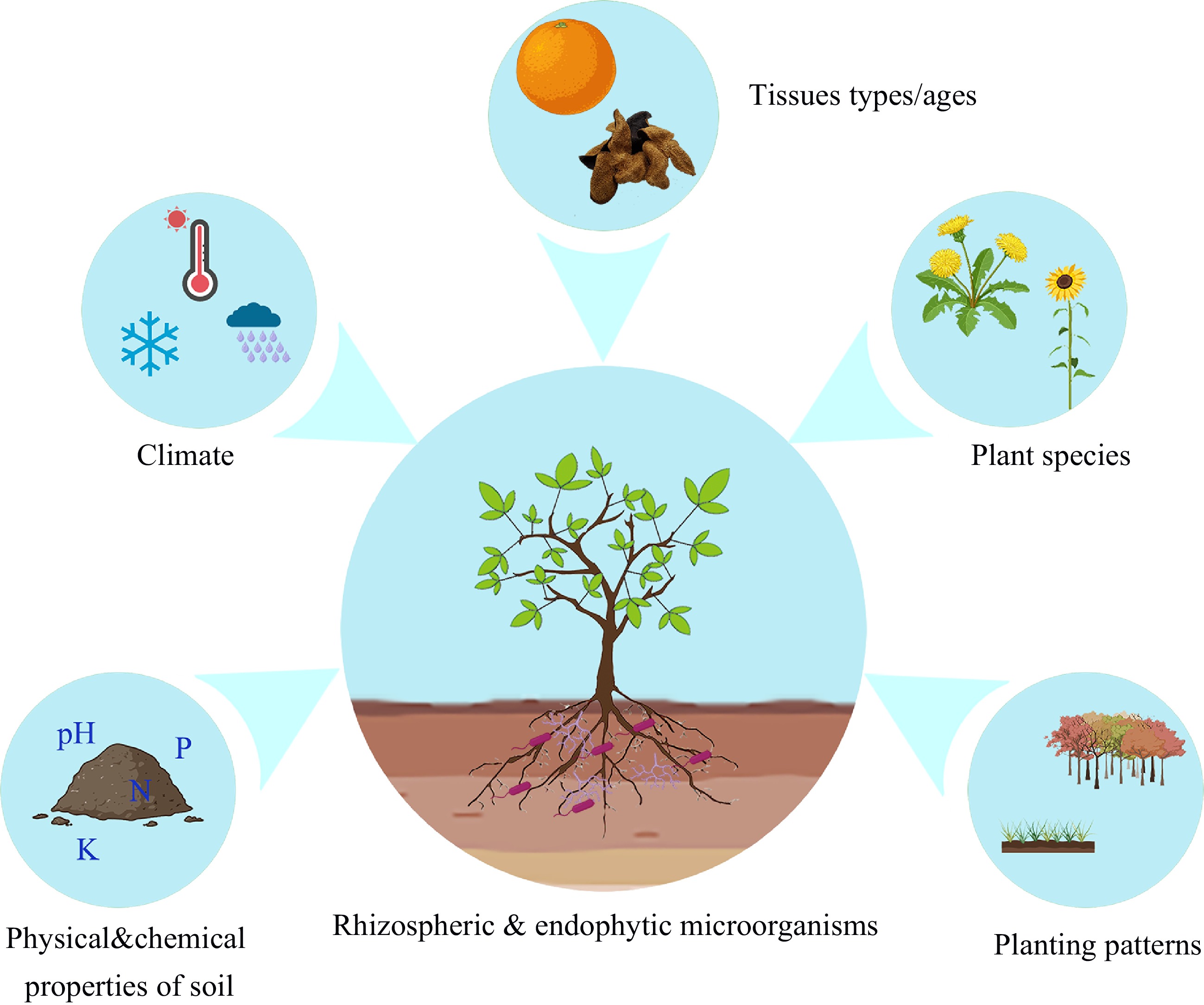
With the development of 16S rDNA, internal transcribed spacer amplicon sequencing, and macro-genome sequencing technologies, the diversity of rhizospheric and endophytic microorganisms in many medicinal plants has been revealed. Here, the studies on the rhizosphere and endophytic microorganisms of medicinal plants are summarized and the reported rhizospheric and endophytic microorganisms are listed in Supplemental Table S1. The composition of rhizospheric and endophytic microorganisms is influenced by multiple factors, including the physical and chemical properties of the soil, planting mode, developmental stage, and plant variety (Fig. 1). For example, the diversity of rhizospheric microorganisms in Fritillaria taipaiensis is affected by the pH, urease activity, available phosphorus, and organic matter content[19]. An analysis of the rhizospheric microbial composition of Panax notoginseng showed that disease-resistant bacteria and plant growth-promoting bacteria in farmland were significantly lower than those found in the forest[20]. Different developmental stages and plant species also affect the variety and abundance of rhizospheric microorganisms[21,22]. Reportedly, there is a high diversity of fungal communities in the rhizosphere soil of Epimedium koreanum Nakai at different growth stages from May to August, especially Ascomycota, Basidiomycota, and Mortierellomycota[23]. Differences in the composition and abundance of rhizospheric and endophytic microorganisms can affect many biological processes in plants, such as growth and development, disease resistance, hormone signaling, and the accumulation of SMs[24,25].
-
Compared to food crops, medicinal plants generally require longer growth times and have higher requirements for soil fertility, which restricts their high-quality and high-quantity production[26]. Farmers usually use large amounts of chemical fertilizers to increase the yield of medicinal plants, which can damage the soil microbial composition, reduce soil fertility, and lead to soil compaction[27−29]. It is known that rhizospheric and endophytic microorganisms can promote the growth and development of medicinal plants in multiple manners[14,30]. Phosphorus is a major nutrient for plant reproduction and growth. However, plants can only utilize a small amount of dissolved phosphorus in the soil (approximately < 1%)[31]. Scientists have isolated a phosphorus-solubilizing bacterium from the rhizosphere of Mentha viridi that promotes root growth and increases fresh and dry weights by releasing free phosphorus from rock phosphate[32]. The rhizosphere bacterium Bacillus amyloliquefaciens TB6 promotes root growth and significantly increases the fresh weight of Panax ginseng[33]. The rhizosphere microorganisms Bacillus subtilis and Bacillus velezensis isolated from Angelica sinensis (Oliv.) significantly increase plant height and fresh weight[34].
Endophytes reside in plants in symbiotic or parasitic manners, and most of them are derived from rhizospheric microorganisms. Endophytes promote the growth of medicinal plants by either providing some nutrients for plants or protecting plants from pathogen infection. Certain bacterial and fungal genomes, such as Rhizobium sp. WYJ-E13, Penicillium sp., Fusarium sp., Coniochaeta sp., Cladosporium sp. and Alternaria sp., contain genes related to nitrogen metabolism, hormone synthesis, phosphate metabolism, and root colonization[35,36]. Thus, they can provide nutrients for plants, which directly promote growth through nitrogen fixation, phosphorus acquisition, and siderophore production[37,38]. Certain endophytes (e.g. Fusarium genus TH15) synthesize sugars, amino acids, and vitamins to provide energy to plants[39]. Notably, endophytes can also protect plants from pathogenic infections. Han et al. found that Acrophialophora jodhpurensis MR-57 inhibits the spore germination rate of Fusarium equiseti and leads to hyphal deformation or degradation, thereby increasing the stem length, root length, root fresh weight, and dry weight of Saposhnikovia divaricate[40]. Reportedly, endophytes secrete hormones such as indole-3-acetic acid (IAA) to promote cell division or the expression of genes related to plant growth, thereby increasing root length, stem length, and root weight[39,41]. It was reported that Plectosphaerella cucumerina form Rumex gmelinii Turcz. can promote the expression of genes related to cytokinin and auxin synthesis to increase hormone accumulation and affect plant growth[42]. In addition, Pseudomonas sp., an endophyte from Zingiber officinale, can produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase, which hydrolyzes the precursor of ethylene synthesis, ACC, into α-Butyric acid and ammonia to reduce the accumulation of excess ethylene and that decomposed ammonia can increase nitrogen content in the soil[43,44].
Notably, some microorganisms can also simultaneously promote plant growth and the accumulation of bioactive SMs, and these microorganisms may have good application prospects. For example, the endophytic bacteria Mucilaginibacter sp. and Pseudomonas sp. isolated from Cannabis sativa L. not only promote plant growth but also significantly increase the accumulation of cannabinoids[45]. Azotobacter chloricum and Azospirillum brasilense, two rhizospheric bacteria from Mentha pulegium L., have been used in combination to increase the abscicic acid (ABA), protein, and soluble sugar contents in peppermint, enhance plant resistance to drought stress, and increase the phenolic, flavonoid, and oxidized monoterogene contents in Mentha pulegium L.[46]. Overall, rhizospheric and endophytic microorganisms can serve as crucial regulatory factors that modulate the growth and development of medicinal plants in multiple manners.
-
SMs are produced during the life activities of plant cells, and their production and distribution are generally specific to species, organs, tissues, growth, and developmental stages. SMs are the primary source of bioactive components in medicinal plants and are a class of compounds produced by medicinal plants that cannot be absorbed or converted into energy by the plant itself. The accumulation and synthesis of SMs are complex and regulated by internal factors in plants (e.g., gene expression, enzymes, hormones, different organs, and developmental stages) and external environmental factors (e.g., temperature, light, microorganisms, and insects)[10]. Some SMs are synthesized under stressful conditions and can promote plant resistance and adaptation to environmental changes, including biotic and abiotic stresses. Therefore, these SMs are synthesized to help plants adapt better to their environment[12]. During the past decade, rhizospheric and endophytic microorganisms have been shown to be highly related to the accumulation of SMs. While certain microorganisms can directly synthesize SMs[47], others can promote the biosynthesis of SMs using multiple strategies. In this review, the recent progress in understanding the role of microbes in regulating SM accumulation in medicinal plants is summarized.
SMs directly synthesized by rhizospheric and endophytic microorganisms
-
With the development of high-throughput sequencing technology, scientists have discovered that the genomes of some endophytes contain genes or gene clusters related to SM synthesis, indicating that endophytes may possess the ability to synthesize SMs. These microorganisms can potentially be used for large-scale production of bioactive SMs[48]. Gibberella moniliformis, a fungal strain from the endophytes of Lawsonia inermis, can produce lawsone through in vitro fermentation[49]. In a study by Tanapichatsakul et al.[50], Neophyllotiopsis sp. and Diabothe sp. were isolated from the endophytes of Cinnamomum loureiroi. In vitro fermentation experiments have shown that eugenol, myristaldehyde, lauric acid, and caprylic acid are present in the supernatant of the culture medium[50], suggesting that these microbes can directly synthesize SMs. In addition, certain rhizospheric microorganisms of medicinal plants can synthesize SMs. For example, actinomycin X2, fungichromin, thalandin B, and antifungalmycin have been detected in the culture medium of Streptomyces SYP-7257 isolated from the rhizosphere of Panax notoginseng[51].
Genomic studies have identified many SM synthesis-related enzymes and pathways in microorganisms. Mining microbial genomes is important for studying SM synthesis and discovering new natural products[52,53]. Currently, numerous microbial genomes have been deciphered, including those of several rhizospheric and endophytic microorganisms from medicinal plants. Zhang et al. found that treptomyces netropsis WLXQSS-4, isolated from the Clematis manshurica Rupr rhizosphere, could synthesize alloaureothin. The genome contains genes involved in alloureothin biosynthesis, including aluA, aluB, aluC, and aluD[54]. The gene clusters aurA, aurB, aurC, and aurG are related to aurovertin biosynthesis in the genome of Calcarisporium arbuscula NRRL 3705 isolated from the fruit of Russulaceae. They also found that the genome contains gene clusters involved in the synthesis of aflatoxins, alternariol, destruxin, citrinin, and isoflavipucine[55]. The genome of Bacillus halotolerans Cal.l.30, an endophyte isolated from Calendula officinalis, encodes genes involved in the biosynthesis of kalimantacin A, bacillaene, fengycin/mojavensin A, bacillibactin, and subtilosin A[56]. A recent study by Su et al. showed that the physicochemical properties of soil regulate the accumulation of terpenes by affecting the composition of the rhizosphere and endophytic microorganisms in citrus. They further screened for an endophytic bacterium that could promote SM accumulation and performed whole genome sequencing of this strain. Bioinformatics analysis revealed that the genome of this bacterium encodes gene clusters capable of synthesizing terpenes[57]. These microorganisms have the potential for direct application in biological fermentation and provide a foundation for the discovery of new and efficient SM synthesis enzymes.
Plant hormone-induced SM accumulation affected by microorganisms
-
Plant hormones are small-molecule compounds that regulate plant growth, development, differentiation, and responses to environmental changes[58]. The accumulation or biosynthesis of SMs is induced by several plant hormones, such as auxin, salicylic acid (SA), and ABA[59]. Rhizospheric and endophytic microorganisms can regulate SM biosynthesis by producing plant hormones to manipulate host hormone levels. Here, we summarize the major plant hormones that are affected by microorganisms and modulate the accumulation of SMs.
IAA is an endogenous auxin commonly present in plants that regulates multiple biological processes, including cell division and elongation, lateral root development, adventitious root formation, fruit development, and senescence[60]. Some rhizospheric and endophytic microorganisms of medicinal plants can synthesize IAA to affect SM accumulation[61]. Bacillus muralis, Bacillus megaterium, Pseudomonas sp., Streptomyces sp., and Pantoea sp. isolated from Withania somnifera can affect plant endogenous auxin content by synthesizing IAA. Therefore, activating the expression of the methylerythritol phosphate pathway genes 1-deoxy-D-xylose 5-phosphate synthase (DXS) and 1-deoxy-D-xylose 5-phosphate reductoisomerase (DXR) to increase the synthesis of withanolide and withaferin-A[62]. The endophyte Pseudomonas fluorescens ALEB7B increases the accumulation of sesquiterpenoids in Atractylodes lancea by producing IAA. Consistent with this, exogenous IAA treatment also significantly increases the accumulation of β-caryophyllene, zingiberene, and β-sesquiphellandrene[63].
SA is a phenolic plant hormone that plays a crucial role in plant immunity and induces resistance to phytopathogens. It also regulates plant growth, development, photosynthesis, transpiration, ion absorption, and transportation[64,65]. Recent studies have shown that endophytes can perceive external stimuli in their hosts and activate a series of defense responses by secreting SA or regulating the content of endogenous SA, thereby protecting plants from invasion[66,67]. In addition to activating immunity, SA can activate several SM synthesis pathways. The exogenous application of SA to Dendrobium officinale can activate the expression of flavonol synthase (FLS) gene involved in flavonol biosynthesis metabolism pathways, thereby significantly increasing the total flavonol content in Dendrobium officinale[68]. SA and ABA have been detected in the fermentation supernatant of Sphingomonas paucimobilis ZJSH1, an endophyte isolated from Dendrobium officinale[41], suggesting that it may promote SM biosynthesis by increasing the SA or ABA levels in host cells. The endophytic fungus Fusarium sp. E5 from Euphorbia pekinensis increases isoeuphpekinensin content by enhancing host SA levels[69].
ABA is an important plant hormone of the isoprenoid (terpene) category. It plays a crucial role in plant responses to abiotic stresses[70,71]. Several rhizospheric and endophytic microorganisms can synthesize ABA or regulate host ABA levels, thereby affecting the accumulation of SMs via ABA-mediated pathways[72]. Exogenous treatment of Dendrobium officinale with ABA significantly increases the expression of genes involved in the methylethritol phosphate pathway, including DXS and DXR[73]. The endophyte Brachybacterium paraconglomeratum strain SMR20 from Leucojum aestivum L. reportedly regulates host ABA levels[74]. Several studies have shown that exogenous application of ABA on medicinal plants, such as Glycyrrhiza uralensis, and Salvia miltiorrhiza, significantly enhanced the accumulation of bioactive ingredients[74−76]. However, it still requires further studies to investigate whether these microorganisms regulate SM accumulation in medicinal plants by affecting ABA levels.
According to these reports, rhizospheric or endophytic microorganisms can synthesize plant hormones, thereby promoting SM accumulation in the host. However, current research on the mechanisms by which microbe-derived hormones directly or indirectly regulate SM accumulation in medicinal plants are largely unknown. The majority of studies have reported that microorganisms can synthesize hormones and that the exogenous application of hormones can increase the accumulation of plant SMs. We hypothesized that hormones synthesized by microorganisms enter host cells and activate multiple downstream signaling pathways mediated by hormones, including pathways related to the biosynthesis of SMs, ultimately affecting the accumulation of SMs (Fig. 2). Future research is needed to elucidate the molecular mechanisms by which microorganisms regulate SM synthesis by manipulating host hormone levels. To treat plants, hormones that can enhance the accumulation of SMs in plants can be directly applied, or microorganisms that can synthesize hormones can be used, thereby promoting the accumulation of SMs.
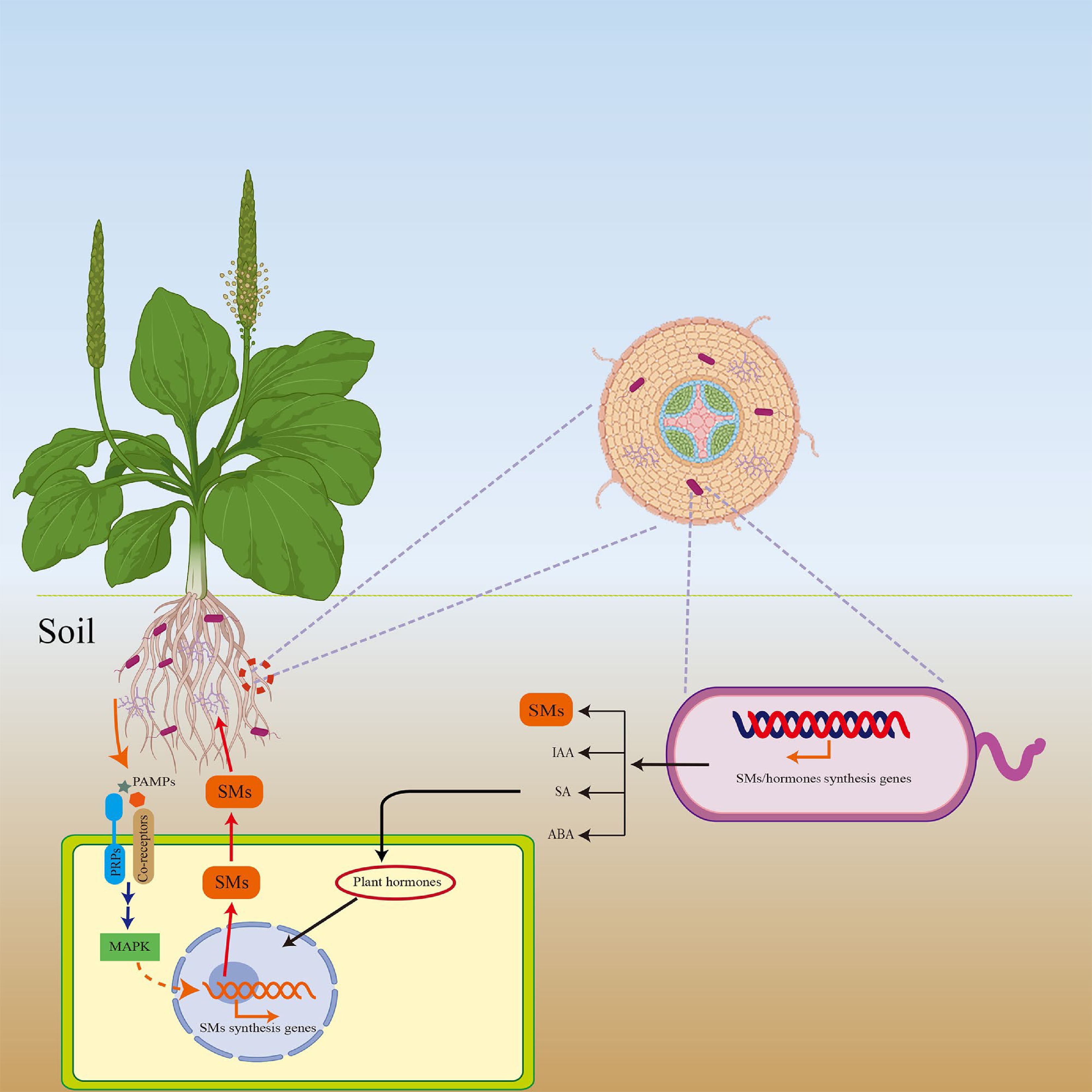
Figure 2.
Microorganisms regulate the accumulation of SMs in medicinal plants in multiple manners. Medicinal plants reside in a common environment with rhizosphere and endophytic microorganisms. While some rhizospheric and endophytic microorganisms directly synthesize SMs[103], others may promote the biosynthesis of SMs in medicinal plants by secrete plant hormones, such as IAA, SA and ABA into the host cells or activate host immunity-related pathways (e.g. MAPK) pathways[74,85,104,105]. In contrast, some SMs may promote host resistance or affected the microbial composition[106].
Promoting SM accumulation by activating plant immunity
-
Plants and microorganisms reside in a common environment, and plants can perceive the presence of microorganisms and respond accordingly. Plant cell surface-localized pattern recognition receptors (PRRs) recognize conserved molecular features, such as fungal cell wall components, chitin, and bacterial flagellin, and initiate innate immune responses against microbes. These conserved microbial features are called microbe/pathogen-associated molecular patterns (PAMPs/MAMPs)[77]. Upon recognition of PAMPs/MAMPs, plant cells activate a series of immune responses, including the production of reactive oxygen species (ROS), calcium influx, activation of mitogen-activated protein kinase (MAPK) and calcium-dependent protein kinase cascades, and transcriptional reprogramming[78]. Note that the PRR-mediated immunity can be triggered by MAMPs from most microbes, not only by pathogens. The activation of immune responses may activate the expression of genes related to SM synthesis, thereby affecting SM accumulation[79]. For example, the activation of plant immunity affects the metabolism of flavonoids, lipids, phenolic acids, and amino acids in Arabidopsis thaliana[80]. Fungi-derived MAMP chitin and bacterium-derived MAMP flg22 can induce ROS bursts and MAPK, which then regulate the accumulation of phenylpropanoids, flavonoids, and linoleic acid derivatives in rice (Oryza sativa)[81]. Similarly, the treatment of plants with chitosan, a fungus-derived MAMP, activate the production of immune signaling molecules ROS and NO and promotes the accumulation of lignin and the primary metabolites galactose and melibiose in chickpea (Cicer arietinum. L.)[79]. Some SMs, such as monoterpenes, can induce the activation of plant immunity and improve plant adaptability to environmental changes[82].
In medicinal plant studies, scientists have found that rhizospheric and endophytic microorganisms regulate the expression of genes related to the synthesis, metabolism, and accumulation of SMs by activating plant immunity. The endophytic fungal strain Penicillium oxalicum significantly enhances the expression of immune-related genes, such as the genes encoding the PRR protein FLS2 and calmodulin-like protein, in Gastrodia elata Bl. f. glauca S. chow tubers. Immune activation regulates the biosynthesis of flavonoids, diterpenoids, and phenylpropanoids in Gastrodia elata Bl. f. glauca S. chow[83]. Alternaria panax Whetzel is a pathogen isolated from Panax notoginseng that can activate ROS production and the MAPK cascade, enhance the expression of PAL, 4CL, CAD, and POX, and significantly increase the accumulation of p-hydroxyphenyl lignin, guaiacyl lignin, and total lignin[84]. It was reported that pathogenic microbes activate MAPKs, AP2/ERFs, and WRKYs in Syringa Pinnatifolia Hemsley, which enhances the expression of key enzyme genes in the biosynthesis pathways of sesquiterpenes and ligans, leading to the accumulation of SMs such as diterpenes, alistolane, aromadendrane, bisabalane, cadine, and ligans[85]. Collectively, plant immunity and metabolic pathways are closely related, and microorganisms or PAMPs/MAMPs can induce immune activation to promote SM accumulation (Fig. 2).
Several studies have reported that rhizospheric and endophytic microorganisms enhance SM accumulation, likely by activating plant immunity. However, the majority of studies have found, through multi-omics analyses, that SM accumulation is highly correlated with genes or pathways related to plant immunity, suggesting that microorganisms promote SM accumulation by activating host immunity. There are few reports on the molecular mechanisms by which microorganisms regulate SM accumulation through the host immune system. In the future, PAMPs/MAMPs derived from microorganisms could be directly used to enhance the quality of medicinal plants by activating the host immune system. This approach avoids the potential microbial invasion of the host. Considering production costs, weak or non-virulence microorganisms that express the PAMPs or MAMPs may have better application prospects.
-
During plant-microbe interactions, microorganisms may promote the accumulation of various SMs in plants, enhancing their adaptability to environmental stresses. Some plant SMs can protect plants from pathogenic microbe infections by activating the expression of resistance-related genes. Maize root exudates can recruit Bacillus amyloliquefaciens OR2-30, which secretes a lipopeptide called iturins to inhibit the formation and germination of Fusarium graminearum conidia, inducing the production of ROS and causing hyphal cell death[86]. Taxifolin is a flavonoid compound secreted by the root system of potatoes and onions. The tomato rhizosphere treated with taxifolin is enriched in Bacillus sp. B56, which inhibits the growth of Verticillium dahliae and reduces the incidence of verticillium wilt[87]. Many medicinal plants can synthesize some triterpenes compounds, such as Astragalus membranaceus Bge. var. mongolicus (Bge.) Hsiao and Nigella sativa[88,89]. Research has reported that triterpenoids, such as thalianyl palmitate, myristate and laurate, can selectively enrich Rhizobium sp., Hydrogenophage sp. and Herbaspirillum sp. in plants[90]. Tangeretin is a component rich in medicinal plants, such as citrus sinensis and citrus reticulata. It was reported that tangeretin treatment of rice can enhance its resistance to rice blast disease[91]. Phenolic acids are also one of the main active ingredients in many medicinal plants, such as Salvia miltiorrhiza and Lycium ruthenicum Murray[92,93]. Researchers have found that reducing the concentration of phenolic acids significantly inhibits the enrichment of pathogenic bacteria Ilyonectria in the rhizosphere of Panax notoginseng, while promoting the growth of beneficial bacteria Sphingomonas, Lysobacter, Massilia and Burkholderia, thereby reducing the occurrence of root rot disease[94]. However, whether these components can activate plant immunity in medicinal plants themselves remains to be further studied.
SM is also known to affect the composition or abundance of the rhizospheric or endophytic microbial communities in crops[95−97]. For example, researchers have found that SMs in tobacco can regulate the composition of microbial communities. Nicotine is the primary alkaloid in tobacco leaves that defends against insects[98]. Benzoxazinoids synthesized in corn are indole-derived compounds, which can significantly inhibit pathogenic fungi such as Blumeria, Ramularia, Puccinia, and Filobasidium in the buds through their accumulation in the body[99]. In tobacco, isoquinoline alkaloids can significantly reduce the number of pathogenic bacteria Ralstonia solanacearum in soil, thereby reducing the occurrence of tobacco wilt disease[100]. However, there are still few studies on the impact of medicinal plant SMs on the composition of microbial community, and the mechanisms remain to be further investigated.
-
Microorganisms play essential roles in SM accumulation in medicinal plants. These microorganisms promote plant growth and development by establishing symbiotic relationships with plants or suppressing pathogen infection. Many rhizospheric and endophytic microorganisms can promote SM biosynthesis in various manners, including the direct synthesis of SMs, secretion of plant hormones, and activation of host immunity (Fig. 2). In contrast, SMs may regulate the composition and abundance of microorganisms. Interactions between microbes and medicinal plants provide an important perspective for understanding the accumulation of bioactive SMs in medicinal plants. However, the precise mechanisms through which plant SMs and microbes regulate each other remain unclear. Future research will require in-depth analyses of the molecular mechanisms underlying these interactions. In recent years, Salvia miltiorrhiza and Echinacea purpurea have been known to have good medicinal effects, rich medicinal ingredients, and relatively mature experimental research systems. They have been proposed to be model plants for studying medicinal plant-microbe interactions[101,102]. Future studies on the microbiomes of these two medicinal plants may facilitate breakthroughs regarding the interactions between medicinal plants and microorganisms. Future studies should also focus on the development of efficient and high-quality microbial fertilizers to improve the yield and quality of medicinal plants. Multiple microbial strains be combined to develop microbial fertilizers that can simultaneously promote the growth, accumulation of active ingredients, and disease resistance of medicinal plants. The accumulation of certain SMs is induced by microbially derived PAMPs/MAMPs, which can be produced through large-scale fermentation.
-
The authors confirm contribution to the paper as follows: study conception and design: Liang X, Wu H, Yu J; literatures collection and analysis: Bai M, Wang C, Yu J; draft manuscript preparation: Yu J, Liang X. All authors approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work was supported by Key realm R&D Program of Guangdong Province (2020B020221001), the Laboratory of Lingnan Modern Agriculture Project (NZ 2021024), the Open Competition Program of Top Ten Critical Priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2022SDZG07), the Chinese Natural Science Foundation (32270381), and 2021 Traditional Chinese Medicine (Medicine of South China) Industry Talents Project-Innovation Team of South China Medicine Resources, Yunfu (YRCB(2021)2).
-
The authors declare that they have no conflict of interest. Hong Wu is the Editorial Board members of Journal Medicinal Plant Biology. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of the Editorial Board members and their research groups.
- Supplemental Table S1 Diversity of rhizosphere and endophytic microorganisms in medicinal plants.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yu JB, Bai M, Wang C, Wu H, Liang X. 2024. Regulation of secondary metabolites accumulation in medicinal plants by rhizospheric and endophytic microorganisms. Medicinal Plant Biology 3: e011 doi: 10.48130/mpb-0024-0011
Regulation of secondary metabolites accumulation in medicinal plants by rhizospheric and endophytic microorganisms
- Received: 23 July 2023
- Revised: 21 March 2024
- Accepted: 25 April 2024
- Published online: 26 June 2024
Abstract: Medicinal plants contain numerous bioactive secondary metabolites (SMs) that can be used for the treatment and prevention of diseases. SM concentration is a crucial standard for evaluating the quality of medicinal plants. SM accumulation is affected by multiple factors, including genetic background, climate, soil physical and chemical properties, and environmental changes. In recent years, increasing studies have indicated that rhizospheric and endophytic microorganisms, play an essential role in regulating the accumulation of SMs in medicinal plants. While some microorganisms establish symbiotic relationships with medicinal plants to promote plant growth. Other microorganisms can directly synthesize SMs or promote plant SM biosynthesis through multiple strategies, such as activating plant immune signaling pathways and secreting plant hormones into host cells to manipulate hormone-mediated pathways. In contrast, SMs may improve plant resistance to environmental stresses, thereby affecting the composition of rhizospheric and endophytic microorganisms. In this review, we summarized the recent progress in understanding the role of microorganisms in regulating SM accumulation in medicinal plants. Further studies should focus on the application of utilizing microorganisms to enhance the accumulation of bioactive SMs in medicinal plants.