-
Based on clinical research demonstrating that ceramide levels can predict the risk of cardiovascular events independently of cholesterol, Zhang et al. first identified the endogenous membrane receptors of ceramide C16:0 as CYSLTR2 and P2RY6. CYSLTR2 and P2RY6 are activated by ceramides, which trigger Gq recruitment, downstream activation of the inflammasome, and, ultimately, favor the formation of atherosclerotic plaques. Inhibition of CYSLTR2 and P2RY6 provides an innovative new way to reduce the atherosclerotic plaque burden without affecting blood lipid levels.
Atherosclerosis is one of the major causes of cardiovascular disease (CVD). While statins have achieved great success in reducing low-density lipoprotein cholesterol, the residual risk of inflammation remains a key factor leading to major adverse cardiovascular events[1]. In recent years, it has been proposed that ceramide may emerge as an independent CVD risk factor with lipid independence[2]. However, its precise pathogenic mechanism and its role as an extracellular signaling molecule have long been unclear. In a recent study in the journal Nature, Zhang et al. revealed that ceramides exacerbate atherosclerosis by activation of CYSLTR2 and P2RY6. Blocking the CYSLTR2 and P2RY6 receptor signaling might provide an appealing new therapeutic strategy for treating atherosclerotic diseases, especially in CKD patients (Fig. 1)[3].
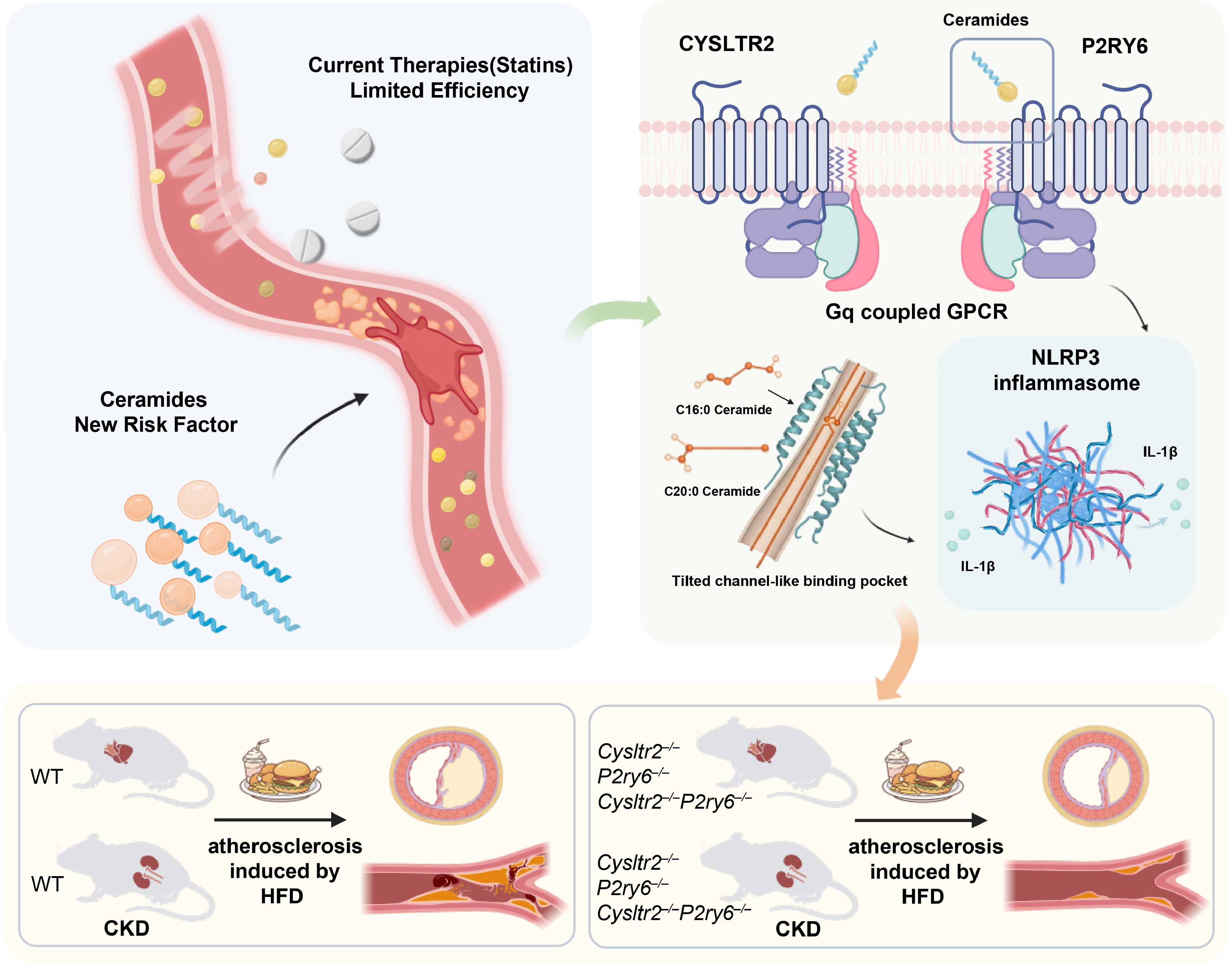
Figure 1.
Schematic diagram illustrating the ceramide-CYSLTR2/P2RY6 pathway in atherosclerosis and its therapeutic implication. Ceramide-mediated activation of receptors CYSLTR2 and P2RY6 recruits Gq protein, leading to downstream inflammasome activation and promoting atherosclerotic plaque formation. Inhibiting CYSLTR2 and P2RY6 represents a novel strategy to reduce atherosclerotic plaque burden independently of blood lipid levels.
Ceramides belong to the sphingolipids and are formed by the connection of sphingosine chains and fatty acid chains of different lengths through amide bonds[4]. Clinical studies have shown that the levels of long-chain ceramides in circulating blood, such as C16:0, C18:0, and C24:1, can predict the risk of cardiovascular events independently of cholesterol[5]. However, it remains unclear how long-chain ceramides in circulation exacerbate the development of atherosclerotic cardiovascular disease and whether membrane receptors exist. In the present study, Zhang et al. confirmed that exogenous C16:0 ceramides exacerbated the formation of atherosclerosis, while caspase-1 knockout, IL-1β neutralizing antibodies, or IL-1 receptor antagonists could weaken this effect. Through a series of in vitro and in vivo experiments, including G protein signal quantification, bioinformatics analysis, and functional assays, CYSLTR2 and P2RY6 were identified as potential endogenous receptors for C16:0 ceramide-induced inflammasome activation. When these two receptors recognize ceramides, they trigger Gq recruitment, which in turn leads to the activation of inflammasomes, ultimately resulting in a significant intensification of the formation of atherosclerotic plaques. Inhibition or gene knockdown of CYSLTR2 or P2RY6 blocked C16:0 ceramide-induced IL-1β production and increased intracellular calcium levels. These findings indicate that genetic ablation of ceramide receptors or the use of receptor antagonists can effectively block the pathogenic effects of ceramides without affecting blood lipid levels.
With the findings that CYSLTR2 and P2RY6 mediated the ability of ceramides to exacerbate atherosclerosis, the authors found that deletion of Cysltr2 or P2ry6 significantly alleviated lipid accumulation and macrophage infiltration induced by C16:0 ceramide in the outflow track of aortas. The reduced plaque density was observed in Cysltr2−/− mice and P2ry6−/− mice, and Cysltr2−/− P2ry6−/− mice showed a more substantial reduction in a 12-week-long-term AAV-PCSK9 + HFD-induced atherosclerosis model. Moreover, the plasma ceramide levels in patients with chronic kidney disease (CKD) complicated with coronary artery disease (CAD) were elevated and positively correlated with the severity of CAD. Deletion of Cysltr2 or P2ry6 alone partially reduced plaque area and lipid accumulation, with no changes in plasma total cholesterol, triglyceride, or ceramide levels in the 5/6 Nx-induced CKD model. Of note, knockout of both Cysltr2 and P2ry6 further mitigated plaque formation. These findings also confirmed that knocking out CYSlTR2 and P2RY6 receptors or using CYSlTR2/P2RY6 receptor antagonists could alleviate the atherosclerosis process that aggravates kidney disease without interfering with lipid levels. This discovery results in a paradigm shift in that ceramides are not merely seen as pathogenic lipids but rather as extracellular signaling molecules that stimulate specific intracellular responses along a 'ceramide-GPCR signaling axis'. Among this pair of receptors, P2RY6 particularly demonstrates unique advantages and great potential as a promising therapeutic candidate.
Purine receptor P2RY6 is a G protein-coupled receptor that mainly responds to uridine diphosphate and drives inflammatory responses[6]. P2RY6 mediates the crucial role of ceramides in the inflammatory response of macrophages, which is a core link in atherosclerosis. The present studies have shown that ceramides efficiently trigger downstream Gq and Gi signaling pathways by directly binding to and activating the P2RY6 receptor on macrophages. The activation of this signaling pathway intensifies macrophage inflammation, leading to the explosive release of cytokines such as IL-1β, thereby creating a pro-inflammatory microenvironment within the vascular wall, which directly accelerates the formation and progression of atherosclerotic plaques. These findings add to the evidence that P2RY6 connects elevated levels of ceramides in circulation with local pathological inflammation of the vascular wall. Inhibiting the activity of P2RY6 can thus precisely suppress the inflammation within plaques without affecting systemic lipid levels, thus at least partly uncoupling systemic metabolism from adverse cardiovascular events.
The strategy to target P2RY6 represents a shift in the therapeutic paradigm, from reducing lipids to specifically interfering with pathogenic signaling. The traditional approach is to minimize the total amount of ceramides in the blood as much as possible, but this strategy is difficult due to the many important physiological processes in which ceramides are involved. A systemic and indiscriminate reduction in their levels may thus cause severe adverse effects. Targeting P2RY6 could provide a more precise functional selective intervention strategy. The research team confirmed through gene knockouts in mice that specific ablation of the P2ry6 gene in macrophages could significantly alleviate atherosclerotic lesions without affecting total ceramides levels. Other studies also confirmed that knockout of macrophage P2ry6 reduced plaque formation in atherosclerotic mice[7]. The therapeutic approach of targeted intervention for P2RY6 could potentially reduce the risk of off-target effects and enhance the safety window of treatment.
More importantly, P2RY6 has a relatively high druggability. The GPCR family is the largest and most successful drug target family in the human genome. Currently, more than 30% of marketed drugs act on GPCR[8]. This means that the development of small molecule inhibitors targeting P2RY6 can build on established platforms and workflows, as well as rich experience to draw upon. Compared with CYSLTR2, P2RY6 may have another layer of advantages. The natural ligand of CYSLTR2 also includes cysteinyl leukotriene, which is a key mediator in allergic reactions and asthma. Therefore, complete inhibition of CYSLTR2 may require a careful assessment of its potential impact on other functions of the immune system[9]. Together, these studies demonstrate that P2RY6 constitutes a promising therapeutic target in atherosclerosis.
However, several questions remain in order to establish P2RY6 as a new target in clinical practice. Firstly, the physiological consequences that may arise from long-term inhibition of P2RY6 in humans remain to be determined. Clinical trials conducted by Gliacure Inc. assessed the safety and tolerability of a single oral administration of GC021109 (NCT02254369) in healthy adults, providing a safety signal for P2Y6R-targeted therapy in clinical practice. Secondly, the role of P2RY6 in different cell types in atherosclerosis remains to be refined, such as vascular endothelial cells and smooth muscle cells. Furthermore, it remains unclear how to optimize lead compounds to achieve the best pharmacokinetic properties and safety[10,11]. In connection with these investigations, it is important to further elaborate on the spatiotemporal control of CYSLTR2 and P2RY6 signaling, particularly with regards to intracellular signaling neighborhoods, which have been shown to be important predictors of adverse drug reactions for other GPCR ligands[12]. Lastly, it is crucial to identify patient groups that can benefit the most from this therapy, such as those with high ceramide levels. However, given the highly conserved function of P2RY6 in both humans and mice, as well as the mature drug development experience of GPCR targets, these challenges are likely to be overcome.
Overall, this latest work of the team led by Prof. Wei Kong[3] not only reveals that CYSLTR2 and P2RY6 are ceramide receptors but also accelerates their therapeutic exploitation in clinical drug development. Particularly, P2RY6 is expected to be a potential new target for combating the risk of residual inflammation in atherosclerosis and inhibiting plaque due to its pleiotropic role in driving macrophage inflammation and foamification.
HTML
-
Not applicable.
-
The authors confirm their contributions to the paper as follows: conceptualization: Hu Q; writing of this manuscript: Li Y, Zhang C, Zhou Y; revision of this manuscript: Hu Q, Li Y, Zhang C, Zhou, Y, Lauschke VM. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
-
This work was supported by the National Key Research and Development Program of China (Grant No. 2023YFC2812500 to Qinghua Hu).
-
Volker M. Lauschke is co-founder, CEO and shareholder of HepaPredict AB, as well as co-founder and shareholder of Shanghai Hepo Biotechnology Ltd. The other authors declare no competing interests.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of China Pharmaceutical University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Li Y, Zhang C, Zhou Y, Lauschke VM, Hu Q. 2025. Novel strategies for lipid-independent atherosclerosis intervention. Targetome 1(1): e008 doi: 10.48130/targetome-0025-0008 |