-
Wild tubers are an important alternative source of food which also have medicinal properties. Among them, Dioscorea (yam) is the fourth most staple edible root and tuber vegetable after potato, cassava, and sweet potato[1, 2]. It is a pantropical genus distributed in the tropical, subtropical, and temperate regions of the world comprised of eight genera and 880 species[3]. Among various genera, white yam (D. rotundata), water yam or greater yam (D. alata), yellow yam (D. cayenensis), trifoliate yam (D. dumetorum), aerial yam (D. Bulbifera) and Chinese yam or lesser yam (D. esculenta) are edible and suitable for human consumption. Yam species not only differ in their color (white, ivory, yellow, purple, etc.), flavor, but also in their shape with long and cylindrical sharps. Also the exterior texture of some species of yam tubers is rough and scaly[4, 5]. Yams are consumed both in the form of processed and cooked foods that includes flour, flakes, chips, fried yam, boiled yam, and dry-roasted slices or pounded yam[6, 7]. Yams are both nutritionally as well as medicinally important in providing food for local and tribal communities[8, 9]. The nutritional compositions and bioactive compounds of yams vary from species to species. Table 1 contains the nutritional information of yams. Tubers of yam contain starch, amino acids, proteins, lipids, fats, vitamins, and minerals[10−12]. Studies reported that minerals such as Potassium, Calcium, Magnesium, Iron, Zinc, and Phosphorus are present in the tubers of Dioscorea species. However, Sodium is not detected in most of the Dioscorea species[12, 13]. Dioscorea has been reported to provide more dietary protein than other roots and tuber crops, such as cassava[14]. Species also contain antinutritional substances such as total free phenolics, tannins, hydrogen cyanide, total oxalate, amylase, and trypsin inhibitor[15].
Table 1. Nutritional and antinutritional content in Dioscorea sp.
Nutrient Percentage (%) Reference Starch 75.6−84.3 [16,17] Protein 2.62−10 [16,18] Fat 0.22−1.71 [16,19] Crude fibre 0.17−1.8 [16,17] Amylose 15.1−27.0 [20] Aspartic acid 5.21−9.36 [21] Glutamic acid 3.20−8.12 [21] Lipid 0.03−10.2 [10] Ash 2.50−4.90 [22] Vitamin C 0.013−0.025 [17] Phytic acid 0.059−0.20 [17] Total oxalate 0.49 -0.78 [17] K 0.25−0.56 [23] Na 0.0041−0.018 [23] P 0.033−0.062 [23] Ca 0.14−0.047 [23] Mg 0.018−0.027 [23] Cu 0.0001−0.00021 [23] Fe 0.00039−0.0029 [23] Mn 0.00014−0.00035 [23] Zn 0.00022−0.00053 [23] Table 2 summarizes the medicinal properties of yams. Some levels of steroid saponins and sapogenins, such as diosgenin are present in Dioscorea tubers, which can be used as an anti-inflammatory, androgenic estrogenic, and contraceptive drugs[24]. Secondary metabolites discovered from root and tubers of Dioscorea sp. include dioscin, gracillin, saponins, phenanthrenes, anthocyanins, flavonoids, and phenolic acids etc.[24, 25]. Various secondary metabolites identified from Dioscorea sp. exhibited anticancer activity properties and were found effective in treating asthma, fever, diarrhea, swellings, scorpion stings, stomach pain, jaundice, and snake bites[24−26]. The rhizome of Dioscorea hemsleyi showed antidiabetic and antioxidant activity[27]. Dioscorea panthaica rhizome exhibited antitumor, anti-hypercholesterolemic effects, anti-platelet aggregation activity and antifungal activity[28,29]. Dioscorea membranacea rhizome used for anti-inflammatory activity[30], immunomodulatory activity, and anticancer activity[31,32]. The tuber and tendrils of Dioscorea hispida showed antioxidant activity[33] and revealed no toxicity on small intestine cells[34]. The tubers of Dioscorea antaly are used as the treatment of toxicity in embryo-larval development[35].
Table 2. Medicinal properties of Dioscorea sp.
Dioscorea sp. Disease Reference Dioscorea bulbifera Goiter, skin infections & oncological diseases [36] Dioscorea nipponica Arthritis, cough, asthma, & circulatory disorders, reduce pain & inflammation [37] Dioscorea villosa Intestinal disorders & hormone replacement therapy [24] Dioscorea birmanica Chronic diseases [38] Dioscorea septembola Treatment of gout [39] Dioscorea hispida Chemotherapy, blood glucose [40, 41] Dioscorea batatas, Dioscorea rotundata and Dioscorea communis Antifungal and antioxidant [42, 43] Dioscorea digitata Syphilis, gonorrhoea, hydrocele, goiter, piles, dysentery. [44] Dioscorea alata Piles, weakness, kill stomach worm [45, 46] Dioscorea belophylla (Prain) Fever, malaria, headache, and dysentery [47] Dioscorea dumetorum Jaundice [48] Dioscorea pentaphylla Digestive tract problems,
skin infections[24,49] Dioscorea pubera Weakness [49] Dioscorea hamiltonii Piles [50] Yams have been regarded as an underutilized crop for a long time and have rarely attracted the attention of researchers[51]. So far most of the research has been conducted on understanding the storage of yam tubers under different storage structures and growth conditions. Research also focused on the impact of various storage conditions on tuber physiology. Understanding the regulatory pathways underlying physiological and biochemical mechanisms during the storage of yam tubers will be helpful in manipulating tuber storage and processing quality. Recently, advances in transcriptomics, proteomics, and metabolomics, approaches have been applied in yams to understand the functioning of genes, proteins, and metabolites respectively involved in various physiological processes in yams[52, 53]. Transcriptome revealed genes involved in molecular mechanisms underlying various physiological and developmental processes in yams[54]. Likewise, the proteome produces a detailed analysis of key proteins and the information on regulation of yam storage proteins[53]. Identification and characterization of complete metabolomic profiling in yams are important to determine their diversity and suitability for food and medicine applications[55]. The present review summarizes insights into tuber dormancy, post-harvest storage losses, biochemical, and molecular approaches associated with yam tubers (Fig. 1).
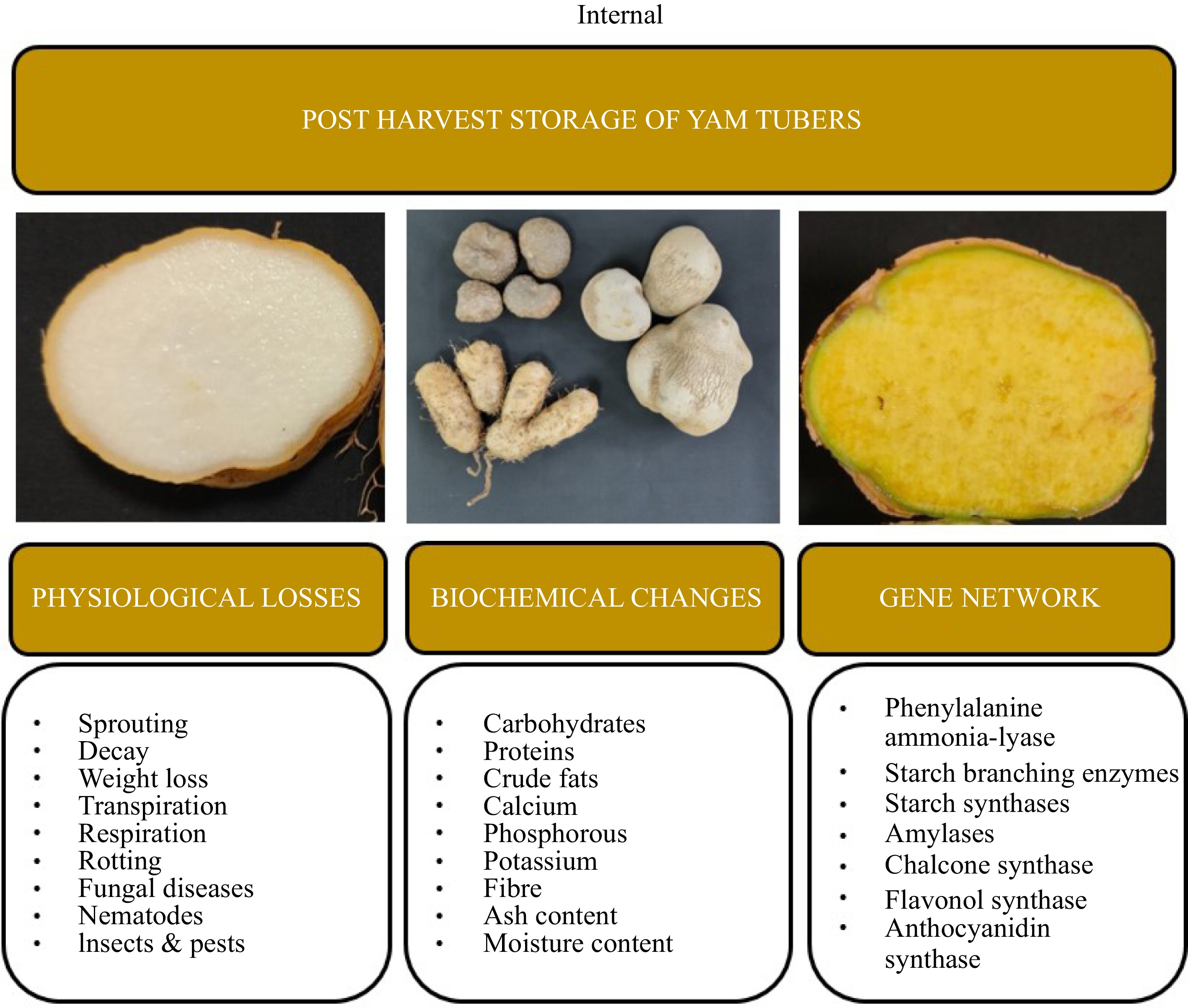
Figure 1.
Post-harvest storage losses, biochemical and molecular approaches associated with yam tubers.
Dormancy in yam tubers
-
After harvest, yam tubers undergo a natural dormancy period. Once the dormancy ends tubers sprout and new shoots are produced. Yam production is highly seasonal mainly due to tuber dormancy and the duration of tuber dormancy. Dormancy has economic and agricultural significance[52,56]. The duration of the dormancy period in yams varies from 30 to 210 d depending on species, genotype, cultural practices, growing, and storage conditions[57,58]. For example, D. cayenensis, exhibits a shorter dormancy period than D. rotundata. D. alata originating from Asia exhibits longer dormancy than D. rotundata originating from the savanna zone of West Africa. Dioscorea alata (greater yam) and Dioscorea rotundata (white yam) become ready for harvest by 8−10 months (240−300 d) after planting. The duration of tuber dormancy (between harvesting and sprouting) in Dioscorea alata is 98−126 d while it is 14−112 d in Dioscorea rotundata[57−60].
Duration of dormancy is very important which determines the time for which the tubers may be stored, and yam plantation programs can be planned. Therefore, depending on the final use and marketing strategies, tuber sprouting can either be delayed or broken by inducing early sprouting. Breaking tuber dormancy would be extremely useful to plant breeders and farmers so that more than one generation could be grown each year. On the other hand, prolonging the tuber dormancy period during storage has significance in terms of yam food quality[60]. Yam tubers are consumed either in the form of cooked or processed food products. For fresh preparations, long tuber dormancy is very important, and therefore, long dormancy is a desirable attribute in yam breeding[61]. However, the underlying metabolic changes and function of key genes that take place during dormancy and sprouting of yam tubers are not investigated in detail[61]. Ile et al.[58] proposed three phases of dormancy in D. rotundata yams: Phase I (tuber initiation to the appearance of tuber germinating meristem). Phase I of dormancy is the period during which no active meristematic activity (tuber germinating meristem) was observed and were therefore dormant. Phase I is the longest phase lasting for approx. 220 d, and an endo-dormant phase. Under this phase, plant growth regulators were unable to trigger tuber germinating meristem formation. The other two phases (II and III) are shorter (< 70 d in total) and proposed as endo-/eco-dormant phases. Phase II starts with the appearance of the tuber germinating meristem to initiation of foliar primordium. The duration of this phase was about 35 d. Plant growth regulators did not induce the formation of a tuber germinating meristem; however, they did influence tuber germinating meristem development (number of cell layers) and the rate/progress of shoot apical development. This phase ends with the appearance of the foliar primordium. Phase III of dormancy period initiates from the development of the complete shoot apical bud/foliar primordium to the appearance of shoot buds at the surface/sprouting loci. The duration of phase III is about 10 d. Both II and III are influenced by exogenous plant growth hormones as well as environmental conditions[58, 60].
Tuber dormancy is the major cause of ware and seed tubers' inability to sprout for a prolonged period, during which tubers remain dormant; incapable of developing internal or external shoot buds/sprouts for about 150 to 210 d depending on: species, genotype, and growing and storage environmental conditions[10−12]. Thus, this makes it impossible to have more than one crop cycle per year, thereby limiting yam crop productivity, and tuber availability for industrial use, and slowing down the rate of genetic improvement [13]. The ability to control yam tuber dormancy is necessary to provide uniform sprouting times that would enable farmers and industry to manage the crop production strategies so that two crop cycles can be managed. For instance, Dioscorea alata grows for 8−10 months and tubers remain dormant for 2−4 months. Hence two crops per year are practically not feasible. Ile et al.[58] concluded that phase I of dormancy lasts for 220 d. Two crop cycles of yams are feasible if the duration of dormancy Phase I (220 d long) is shortened, off-season plantation and more than one generation per year of yams is feasible[58]. This will be beneficial to yam industries as tubers will be available for round-the year processing and other purposes. Dormancy and sprouting in Dioscorea sp. can be controlled using gibberellic acids (GA3) (150 and 1,000 mg·L−1) and by inhibitors of GA biosynthesis such as 2-chloroethyltrimethyl-ammonium chloride (40 and 60 mL–1), Prohexadione-calcium (10 mg·kg−1) etc.[58, 61]. Hamandia & Carufurd[62] studied the effects of 2-chloroethanol (CLE, 4% and 6%), GA3 (150 and 1,000 mg·L−1), thiourea (2%), ethephon (1,000 mg·L−1), and CLE/thiourea combination on tubers of D. rotundata. Their results revealed that a combination of CLE/thiourea (6% and 2%) shortened the duration of sprouting by up to 32 d. GA3 (1,000 mg·L−1) prolonged the duration of sprouting in D. rotundata tubers. Gibberellins are used to break the dormancy in several crops however, it tends to prolong the duration of dormancy in some species of yam. A high concentration of GA3 (1,000 mg·L−1 prolonged dormancy while low concentrations (≤ 150 mg·L−1) of GA3 produced both shortening and prolonging effects in D. rotundata. Such an effect can be dependent on the age of the tubers[62]. However, these studies are inconclusive. Besides, the physiological, biochemical, and molecular control mechanisms involved in yam tuber dormancy are not investigated in detail. Studying the effects of hormones and their role during dormancy and sprouting will identify the hormonal network involved in governing these processes.
Transcriptomics and metabolomic approaches identified the underlying genetic factors involved in the regulation of yam tuber dormancy. Using an untargeted GC-MS approach, profiling of two white yam genotypes (Obiaoturugo and TDr1100873) of D. rotundata identified differentially accumulated metabolites from dormancy induction to dormancy breaking phases[63]. Among all the metabolites, yam tuber dormancy induction and maintenance stages were found to be positively regulated by amines and biogenic polyamines, amino acids and derivatives, alcohols, flavonoids, alkaloids, phenols, esters, coumarins, and phytohormone. Dormancy breaking and sprouting stages were positively regulated by fatty acids, lipids, nucleotides, carboxylic acids, sugars, terpenoids, benzoquinones, and benzene derivatives in tubers of both yam genotypes[63]. Further linoleic acid metabolic pathway, phenylalanine metabolic pathway, galactose metabolic pathway, starch and sucrose metabolic pathway, alanine-aspartate-glutamine metabolic pathways, and purine metabolic pathway were significantly impacted yam tuber dormancy. Metabolomic approaches can be further explored to identify starch-sugar conversion process under storage. Such study can be utilized for understanding tuber dormancy mechanism in different yam species. In another study, profiling of non-structural sugar parameters, dry matter, and moisture content classified Obiaoturugo as a short dormant phenotype and whereas TDr1100873 exhibited a long dormant phenotype[64]. Their study concluded that sugar metabolites can be useful in manipulation of dormancy in different yam species. Detailed studies investigating the role of key candidate metabolites and associated genes will provide potential for manipulation of yam tuber dormancy using biotechnological tools to develop agronomically desired tuber dormancy phenotype[56].
Postharvest storage physiology of yam tubers
-
The perishability, pre-harvest factors, and postharvest storage losses of root and tuber crops are the major constraints in the utilization of these crops[65] for human consumption and industrial purposes. Although yam tubers are consumed as a fresh commodity, due to their nutritional and medicinal properties, the industrial demand for yams is increasing. Yams are best planted at the beginning of the rainy season. Planting is done between late January and April in West Africa coinciding with the start of the rains, although early plantings in November–December are also recommended. In India, it is planted between May and June. It grows in warm, sunny climates with temperatures between 25 °C and 30 °C. Short days of 10−11 h result in tuber formation, while days longer than 12 h favor vine growth. Yams require deep, loose, and textured loamy soil that is rich in organic matter. Dioscorea alata (Greater yam) and Dioscorea rotundata (white yam) become ready for harvest by 8−10 months (270−300 d) after planting. However, its production is limited by long-growing cycle (9−11 months), dormancy, and post-harvest storage losses[21, 58].
Post-harvest storage losses include weight loss, rotting loss, increased sugars, decreased nutrient levels, as well as processing quality. The losses can be high, which only affects not only economic value but also the seed planting material required by farmers. Aidoo[66] reported that due to the poor storability of yams, farmers often sell the tubers immediately after harvest at cheaper rates yielding low income or reduced profits. After harvesting depending on their final usage, short-term and long–term storage of tubers is mandatory. However, storage causes sprouting, transpiration, respiration, rotting, pathogen infestation, and other physiological losses[67, 68]. For long-term yam tuber storage and maintaining the chemical quality, a good storage structure with controlled conditions with proper ventilation system is necessary to maintain tubers in their most edible and marketable condition[12]. Good storage prevents moisture losses, spoilage by pathogens, attack by insects and animals, and controls sprout growth in yam tubers[12, 68].
Table 3 summarizes some examples of the effects of different post-harvest storage systems/structures, temperature, and storage duration on yam tuber quality. Previous studies revealed that post-harvest yam tuber quality is affected by cultivar type, storage structure, temperature, relative humidity (RH), chemical treatments, bio-pesticides, fungicides, storage structure, etc. Post-harvest rotting and weight losses of yams can be avoided using healthy seed material for plantation, crop rotations, fallowing, destruction of diseased, and unhealthy/infected seed/plant material, pre-treatment with nematicides (carbofuran granular), dipping of seeds in Nemacuron or treating seed material with wood ash[69]. Claudius et al.[69] reported that the use of pesticides, hot water treatment, and neem extract to seed tubers before plantation resulted in reduced post-harvest storage losses in Dioscorea rotundata.
Table 3. Effects of post-harvest storage on yam tuber quality.
Species Treatment Parameter Quantitative data Reference Effects of storage structures, storage temperature and duration Dioscorea rotundata Underground pit structure
(25 °C, 24 weeks)Increased wight loss 13.2%−33.4% [70] Dioscorea rotundata cv. Oshei and Dioscorea dumetorum cv. Jakiri Prevailing tropical ambient conditions (18−31 °C, 16 weeks) Increased weight loss
Decreased in starch content31%
3.5%−4.5%[71] Dioscorea alata and Dioscorea esculenta (29 °C, 21 weeks) Increased weight loss
Decreased proteins, starch etc.−
−[74] Dioscorea
roundata cv. GiwaYam barn
(fluctuating temperature, 26 weeks)Increased rotting loss
Increased weight loss
Reduction in nutritional content12%
4.7%
−[75] Dioscorea cayenensis rotundata complex cv. Krenglè and D. alata cv. Florido Heap aired store and stored under prevailing tropical ambient conditions (26.5 °C, 26 weeks) Increased reducing sugars
Increased total sugars7%−9%
1%[80] Dioscorea cayenensis rotundata complex cv. Krenglè and cv. Kangba Heap aired store and stored under prevailing tropical ambient conditions (26.5 °C, 26 weeks) Increased amylase and cellulase
Decreased α-mannosidases and phospatases− [81] Effects of fungicides/pesticides/medicinal plants extracts etc. Dioscorea rotundata cv. Imola and cv. Akpaji Pre-planting treatment with neem extract, pesticides, and hot water
(17 weeks)Decreased rotting loss
Decreased weight lossApprox. 50% reduced
Approx. 50% reduced[69] Dioscorea rotundata cv.
PoirAmbient room temperature
(30 ± 5 °C, 22 weeks)
Trichoderma harzianumControl on fungal pathogens Paired treatments produced 2.22% (T. harzianum × F. oxysporum) and 6.67% (T. harzianum × A. niger) rots [77] Dioscorea rotundata cv. abii Medicinal plants and Apron plus 50DS (16 weeks) Weight loss
Pathogen infestation40% reduction
0 to 5% reduction[79] Dioscorea rotundata Medicinal plants extracts
(28−30 °C, 4 weeks)Rotting loss and
weight lossDecreased losses [82] Dioscorea sp. Medicinal plants extracts (variable temperature and storage structures) Control of diseases and pests Decrease in disease and pest incidence [83] Packaging yam tubers
-
Packaging is an important aspect of post-harvest storage and the transportation of produce from field to market. Appropriate packaging helps to minimize the losses during transport and storage. Reports on research on the packaging of yam tubers are not well documented. After harvest and curing, yam tubers can be packed in paper bags, wooden baskets/crates, gunny bags, perforates/non-perforated polyethylene or mesh bags. However, research on packaging with appropriate material along with recommended storage temperature and relative humidity of yam tubers is lacking. The effects of different packaging materials on short- and long-term storage and processing quality of tubers might be evaluated. This in turn will help decide appropriate packaging material and subsequently its effects on postharvest losses.
Storage structure, temperature, and relative humidity
-
Various storage structures have been suggested for the storage of yams. Yams can be stored in yam barns, platform storage, simple piles or clamps to storage in buildings etc.[72] when provided with proper ventilation. Dioscorea rotundata tubers stored in the open-sided structure showed reduced weight loss, sprouting, decay, pest damage and nutritional composition as compared to heaps on the floor and yam barns structures[12]. Oyewumi et al.[73] reported that yam tubers stored in the barn had the highest rotting loss, sprouting index, and weight loss while the tubers in the platform showed less weight loss compared to the barn after three months of storage.
The effects of storage structure on post-harvest storage affect the yam tuber quality. Storage in a well-ventilated room (24−28°C and 70%−90% RH) accelerated the sprouting after 60−90 d in D. alata and after 120 d in D. esculenta. In both species the moisture content of stored tubers significantly decreased throughout the storage period, resulting in losses in tuber fresh weight[74]. Ventilation due to airflow has been attributed to the reduction in growth rate and increased physiological damage of stored tubers[75]. Osunde & Orhevba[75] studied the effects of yam barn and barn with a fan to aid air circulation on nutritional and other qualities of Dioscorea roundata cv. 'Giwa'. A barn with a fan showed less physiological damage such as weight loss, rotten tubers etc., and nutritional content as compared to a simple barn after 6 months of storage period. Maalekuu et al.[12] demonstrated that barn, open-sided, and heaps on the floor the open-sided storage structure performed best with respect to weight loss, sprouting, decay, pest damage, and nutritional composition of Dioscorea rotundata (cv. Pona and Tela).
Ezeike[70] reported that weight loss of stored (under tropical conditions) D. rotundata tubers was affected by air temperature, relative humidity, length of storage, tuber weight, and geometry. Dioscorea rotundata cv. Os hei and Dioscorea dumetorum cv. Jakiri tubers stored under prevailing tropical ambient conditions (18−31 °C, 62%−100% RH) revealed that weight losses were maximum in Jakiri (35%) compared to Oshei (31%) tubers after 110 d of storage. The losses were attributed to the sprouting and dehydration of tubers in both cultivars[71].
Chemicals and medicinal plants extracts
-
Rotting of yam tubers and other postharvest diseases affects the quality and results in heavy losses. The application of synthetic chemical fungicides, bio-pesticides, plant growth hormones, and various storage methods was found beneficial in increasing the postharvest storage of yam tubers[76]. The use of synthetic chemicals such as thiobendazole, forcelet, mancozeb, borax etc. controlled the yam tuber rot in storage[77] Chemical fungicides and bio-pesticides had no harmful effects on the nutritional composition of yam tubers and were found to be effective in controlling physiological loss[72,78,79]. Bio-pesticides such as fruit powder of Azadirachta indica and Piper guineense significantly reduced the Dioscorea rotundata cv. abii tuber weight loss and controlled the yam rot as well as nematode infestation after 16 weeks of storage[79] Biocontrol agents such as saprophytic yeast and Trichoderma sp. helped in controlling yam rot[77]. Aidoo[72] reported that an improved pit storage system along with the coating of two chemical fungicides, Shavit F71.5 and Metalaxyl Mancozeb effectively controlled rotting losses in Dioscorea rotundata tubers.
Various storage conditions and methods have been applied to increase the shelf life of yam tubers. However, additional approaches are required to identify physiological, biochemical, and molecular mechanisms that are associated with post-harvest storage in different yam species and cultivars. Understanding physiological, biochemical, and molecular controls will help predict storage behavior and tuber quality of different yam species under storage.
Storage of yams triggers biochemical changes
-
Due to its perishable nature and high moisture content quality of yam tubers are affected due to physiological and biochemical changes during storage. Avery few studies reported biochemical changes that take place during storage of yam tubers. Ravindran & Wanasundera[74] reported that tubers of Dioscorea alata and D. esculenta cultivars showed a significant decrease in crude protein, starch, and vitamin C content after 60 d of storage (thin layers on the floor of a well-ventilated room with 24−28 °C and 70%−90% RH). Storage also reduced minerals and levels of oxalates in tubers of these yam cultivars[74]. Storage (18−31 °C) of Dioscorea rotundata (cv. Oshei) and Dioscorea dumetorum (cv. Jakiri) affected dry matter, sugar, and protein content in both species after four months of storage[71]. Afoakwa et al.[84] studied the effects of storage temperatures (ambient at 28 °C and cold room conditions at 4 °C for 24, 48, and 72 h) on chemical composition, as well as the biochemical and textural changes of two cultivars of Dioscorea dumetorum. The authors observed that the starch levels declined while sugars, fibre content, and textural properties increased under the storage temperatures after 72 h. Four months of storage in an open-air yam barn (27.4 ± 3.8 °C) altered the chemical composition and food quality of Dioscorea rotundata and Dioscorea alata. A total of 216 tubers of yam (Dioscorea Roundata cv. Giwa) stored in the barns for 6 months reduced nutritional parameters such as carbohydrate, calcium, phosphorus, crude fibre, crude fat, crude protein, ash, and moisture contents[75]. A study conducted by DJE et al.[80] demonstrated that total, reducing sugar, and dry matter content increased during 6 months of storage (26.56 ± 3 °C and 82% ± 5% RH) in two varieties of Dioscorea. Total sugar, reducing sugar, and dry matter content increased while minerals such as Zn, Ca, Mg and total phenolic compounds significantly decreased during storage[80]. Dabonne et al.[81] revealed that storage of Dioscorea cayenensis-rotundata (cv. Kangba and Krenglè altered the activities of amylases, cellulases and inulase, phosphatases and pNP-glycosidase enzymes. They observed that activities of amylase and cellulase were increased after 6 months of post-harvest storage (26.56 ± 3 °C and 82% ± 5% RH), whereas α-mannosidases, phospatases decreased in different tuber parts of yams. However, such studies were insufficient to investigate the role of such enzymes in storage of yam tubers. Three varieties of D. rotundata (alaako, Dodoro and Odo) stored under natural light- yam barn, or and darkness conditions significantly affected dry matter, free sugar, and starch content of the three tuber regions at 8 and 16 weeks in storage[85]. Comparative analysis conducted by Moses et al.[86] from D. rotundata, D. alata and D. cayenensis after 4 months of storage at ambient conditions revealed that total sugars increased in these species. Sugars, non-starchy carbohydrates and dry matter increased whereas starch, fat, and protein content decreased compared to freshly harvested yam tubers[20]. Authors also reported that the textural quality of pounded yam made from D. rotundata was more influenced than that from D. alata after storage.
Biochemical changes in different yam species helped to understand the response of various yam species and cultivars associated with post-harvest storage. Detailed metabolomics approaches might be helpful in identification of key metabolites or metabolic biomarkers that can be utilized by yam breeders for predicating the storage behavior of different yam species/cultivars. Future research should be directed towards understanding the role of important enzymes involved in starch-sugar conversion during storage.
Molecular mechanisms associated with yam tuber storage and other physiological processes
-
Classical breeding approaches have been applied in developing improved yam cultivars. However, the main constraints in yam breeding are heterozygosity, dioecious, and polyploid nature, vegetative mode of propagation, poor seed set, non-synchronous flowering, and long breeding cycles[87,88]. Recent advances in genomics and transcriptomics helped in identifying the regulatory pathways and genes associated with various physiological processes involved in yams. Understating the molecular mechanisms is necessary to decipher the underlying gene network associated with tuber storage physiology and other related processes. Molecular mechanisms and important candidate gene function during yam tuber storage will be helpful in developing storage strategies that can be further utilized in breeding programs.
Transcriptome profiling identified candidate genes involved in starch and sucrose metabolism, and flavonoid biosynthesis
Starch and sucrose metabolism
-
Although starch is the major component in yam tubers, very limited studies on the molecular changes and the expression of genes involved in starch metabolism in yams are reported. Using the transcriptome approach, Siadjeu et al.[89] identified candidate genes involved in post-harvest handling of trifoliate yam (Dioscorea dumetorum). Transcriptome analyses of D. dumetorum tubers at 4 months after emergence, immediately after harvest, 3 d after harvest, and 14 d after harvest revealed genes encoding for CELLULOSE SYNTHASE A, XYLAN O-ACETYLTRANSFERASE, CHLOROPHYLL A/B BINDING PROTEINS, and an MYB transcription factor. Their studies confirmed that these genes were potentially involved in the post-harvest handling of yam tubers. In another study, Dioscorea polystachya subjected to transcriptome analysis during tuber development stages identified candidate genes involved in starch and sucrose metabolism, plant hormone signal transduction pathway, and flavonoid pathway[90]. Expression of granule-bound starch synthase, starch branching enzymes, α-amylase, β-amylase etc. revealed their significance in Dioscorea polystachya tubers starch metabolism. Likewise, the expression and correlation of sucrose synthase genes with sucrose metabolism revealed sucrose metabolism during the rapid growth of these tubers. Although this study identified important candidate genes involved in starch and sucrose as well as other metabolic processes associated with the molecular mechanism of tuber development[90] their functional significance remains to be elucidated.
Starch and sucrose metabolism are important processes in yam tubers. Hence, a detailed analysis of different yam species using various storage conditions may help in elucidating the role of starch and sucrose metabolism genes and respective enzymes in post-harvest storage and dormancy of yam tubers. Different yam species can be explored for allelic diversity and gene expression patterns of genes involved in starch and sucrose metabolism. Further research, perhaps using transgenic technologies is required to understand gene function at the species level.
Flavonoid biosynthesis
-
Color is an important criterion that determines consumer acceptance as well as its marketability. Dioscorea species not only vary in shape and other morphological characteristics but also differ in their flesh and skin color. Based on flesh color the species can be classified as white yam, yellow yam, and purple yam[91, 92]. Through transcriptome analysis, the elite purple-flesh tubers and the conventional white-flesh tubers of D. alata identified a total of 511 differentially expressed genes in two yam cultivars[93]. Authors reported that the expression of genes encoding chalcone isomerase, flavanone 3-hydroxylase, flavonoid 3′-monooxygenase, dihydroflavonol 4-reductase, leucoanthocyanidin dioxygenase, and flavonol 3-O-glucosyltransferase were found to be significantly up-regulated in the purple flesh tuber. Such studies provided valuable information of key genes that might be directly used to genetically manipulate the conventional, white-flesh tuber cultivars to enable them to produce purple flesh[93]. In another study, authors identified candidate genes related to flavonoid biosynthesis from mature tubers of Dioscorea cirrhosa which have a unique red color[94]. These red color tubers are rich in polyphenols, such as epicatechin, and catechins. Transcriptome profiling performed from four stages of color tubers identified 67 candidate genes related to the flavonoid biosynthesis pathway and three genes that played pivotal roles in proanthocyanin synthesis[94]. The expression of 13 enzymes (phenylalanine ammonia-lyase, cinnamate 4-hydroxylase, 4-coumarate-CoAligase, leucoanthocyanidin reductase etc.) directly involved in flavonoid biosynthesis was consistent with the changes in flavonoid metabolites such as luteolin-3-O-glucoside, luteolin-4-O-glucoside, aromadendrin-7-O-rutinoside, hesperetin-7-O-glucoside, and Tamarixetin[94].
Flavonoids are an important class of secondary metabolites. Considering its health benefits, it is therefore necessary to understand the flavonoid diversity in different yam species. For this, the knowledge of genetic diversity of various flavonoids and their respective genes is necessary. Such information can be further utilized by breeders to develop yam cultivars with desired storage and processing characteristics.
Syombua et al.[95] reviewed the potential applications of clustered regularly interspaced short palindromic repeats/CRISPR-associated protein (CRISPR/Cas) system for yam improvement. Advances in genome editing tools such as CRISPR/Cas system will be helpful in developing yam cultivars with improved nutritional and medicinal properties. The authors mentioned that improvement of the yam germplasm through modern biotechnological tools such as genetic transformation and genome editing may allow direct manipulation of the genome. With the help of CRISPR/Cas system, genome manipulations can be conducted by designing strategies for yam disease and pest resistance, dormancy and storage-related traits, as well as the nutritional quality of yam tubers.
-
Due to their high nutritional and medicinal values, yams are considered as an alternative food source especially in developing countries. Both storage and dormancy are important physiological traits in yam tuber production. Storage and dormancy are influenced by yam species, agronomic conditions, storage type and duration, and biotic and abiotic factors. Therefore, understanding biochemical and molecular mechanisms underlying these processes is essential. Identifying important biochemical pathways, key candidate metabolites, and genes involved in regulating postharvest storage and dormancy will be helpful in determining and predicting storage as well as dormancy behavior of different yam species. Research can further be directed towards identifying starch to sugar conversion processes, related enzymes and key genes involved during post-harvest storage of different yam species and cultivars. Such candidates can be used as biomarkers which can be utilized in yam breeding programs. Manipulation of important genes using Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 (CRISPR/Cas9) will be helpful in elucidating the role of important genes involved in the regulation of yam tuber storage as well as dormancy. Incorporating the molecular techniques in yam breeding programs may enhance the post-harvest storage life without compromising their nutritional and processing quality.
-
The authors confirm contribution to the paper as follows: study conception and design: Datir S; data collection: Datir S, Kumbhar R; analysis and interpretation of results: Datir S, Kumatkar P; draft manuscript preparation: Datir S. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
The authors are grateful to Smita Godrej Crisha, Director, and Dr. Kranti Yardi, Head, Naoroji Godrej Centre for Plant Research, Shirwal for providing guidance, support, and infrastructure facilities. This research was supported by Naoroji Godrej Centre for Plant Research grant to SD under Wild Edible Plants Research Project.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Datir S, Kumbhar R, Kumatkar P. 2024. Understanding physiological and biochemical mechanisms associated with post-harvest storage of Yam tuber (Dioscorea sp.). Technology in Horticulture 4: e004 doi: 10.48130/tihort-0024-0001
Understanding physiological and biochemical mechanisms associated with post-harvest storage of Yam tuber (Dioscorea sp.)
- Received: 03 August 2023
- Revised: 10 January 2024
- Accepted: 14 January 2024
- Published online: 29 February 2024
Abstract: Yam (Dioscorea sp.) is an economically important staple root tuber crop of tropical and subtropical regions. Tubers are nutritious and contain carbohydrates, proteins, micronutrients, vitamins, and several other beneficial compounds. Yam species differ in their biochemical constituents, dormancy period, and storability. Tubers are dormant after harvest and the duration of the dormancy period in yams varies from 50 to 210 d depending on species, genotype, cultural practices, growing, and storage conditions. Depending on the final usage, tubers can either be stored for short- or long-term periods under proper ventilation with 15−17 °C/70%−80% relative humidity. Post-harvest storage losses are one of the major constraints in yam production that limits its usage for human consumption and industrial purposes. Long-term storage causes tuber sprouting, transpiration, respiration, rotting, pathogen infestation, and other physiological losses. Both dormancy and storage can be manipulated using the application of synthetic chemical fungicides, bio-pesticides, and plant growth hormones such as gibberellic acid along with the proper storage practices. As both dormancy and storage of yams result in physiological, biochemical, and molecular changes, understanding key metabolites, genes, and regulatory pathways associated with these processes is necessary. This can be achieved by measuring rotting losses, weight loss, starch, and sugar content along with the associated enzymes and expression of key genes involved in starch-to-sugar conversion. The present review summarizes insights into post-harvest storage losses, dormancy, metabolomic, and transcriptomic approaches associated with yam tubers.
-
Key words:
- Dioscorea /
- Dormancy /
- Metabolomic /
- Nutritional /
- Physiological /
- Storage












