HTML
-
Neofomitella Y. C. Dai, Hai J. Li & Vlasák is a recently established genus under Polyporaceae (Polyporales), segregated from Fomitella Murrill by the presence of a distinct crust on the pileus, with cuticle extending from base to margin (Li et al. 2014). The genus is characterized by annual to perennial, pileate, sessile to effused-reflexed basidiomata with glabrous to velutinate, yellowish-brown, fuscous to almost black pileus surface, often with concentric zonations or sulcations, and buff to yellowish brown or pale grey, hard corky context, and poroid hymenium with white, cream to pale buff hymenial surface, and a trimitic hyphal system with clamped generative hyphae, absence of cystidia, and oblong ellipsoid to cylindrical, colourless, thin-walled, smooth basidiospores, that are acyanophilous, and inamyloid in Melzer's reagent in which species cause white rot on angiosperm wood (Li et al. 2014). Based on the molecular phylogenetic study (Li et al. 2014), Neofomitella is closely related with the genus Microporus P. Beauv. However, Microporus includes species with stipitate or infundibuliform basidiomata, and white to cream coloured context. Coriolopsis Murrill also shares some macroscopic and microscopic characters with Neofomitella, such as brown context, a trimitic hyphal system bearing generative hyphae with clamp connections, and hyaline basidiospores, and white rot type of wood decay (Gilbertson & Ryvarden 1986, Núñez & Ryvarden 2001, Hattori 2005). But, recent molecular phylogenetic studies (Li et al. 2014, Ji et al. 2019) considered Coriolopsis as polyphyletic, as its type species (Coriolopsis occidentalis (Klotzsch) Murrill) settled in Trametes clade, which is distinct from Neofomitella. Currently, five species (N. australiensis B. K. Cui & Xing Ji, N. fumosipora (Corner) Y.C. Dai, Hai J. Li & Vlasák, N. guangxiensis B.K. Cui & Xing, N. polyzonata Y. C. Dai, Hai J. Li & Vlasák, and N. rhodophaea (Lév.) Y. C. Dai, Hai J. Li & Vlasák) have been recognized in the genus (Index Fungorum 2021). Members of this genus have a tropical to subtropical distribution (Léveillé 1844, Ryvarden & Johansen 1980, Corner 1989, Núñez & Ryvarden 2001, Dai et al. 2011, Dai 2012, Li et al. 2014, Cui et al. 2019).
Sebipora Miettinen is a genus belonging to the family Gelatoporiaceae of Polyporales, established by Miettinan (Miettinen & Rajchenberg 2012). Major characteristics of the genus are annual, usually gelatinous, resupinate to pileate basidiomata, with a rubber like consistency, glabrous, azonate pileus that are white when fresh and cream, grey to brownish upon drying, and poroid hymenium with round to angular pores, white, homogenous context, and a monomitic hyphal system with clamped generative hyphae, either encrusted or not, and cylindrical, slightly bent, hyaline, thin-walled basidiospores that are usually guttulate, and inamyloid in Melzer's reagent. Species of Sebipora causes white rot on wood (Miettinen & Rajchenberg 2012). The genus is closely related to species in Tyromyces P. Karst. sensu stricto, in having infrequently encrusted hyphae that are metachromatic in cresyl blue, inamyloid in Melzer's reagent, and presence of cylindrical, guttulate basidiospores. However, the former produces a dimitic hyphal system in mature fruit bodies, and their contextual hyphae possesses thick-walled, finger like projections, characters that are absent in Sebipora. Phylogenetically, Sebipora belongs to the Cinereomyces clade, which is distinct from Tyromyces sensu stricto (Miettinen & Rajchenberg 2012). The genus consists of a single species, S. aquosa Miettinen (Index Fungorum 2021).
-
Fruit bodies were collected from forest areas of the Kerala region of Western Ghats in India, during the monsoon seasons. Macroscopic characters of fresh specimens were recorded. Microscopic observations were made on materials stained using aqueous solutions of 3% phloxine and 1% congo red, and mounted in 5% aqueous KOH. Reaction of the basidiospores on treatment with Melzer's reagent and cotton blue was noted. Twenty basidiospores from each specimen were measured for obtaining the spore dimensions, range of spore quotient (Q, length/width ratio) and its mean value (Qm). All collections examined are deposited at the ZGC Herbarium, India. Current taxonomic names and systematic positions of all taxa used in this study are according to the Index Fungorum database (www.speciesfungorum.org.), accessed on 02 June 2021. Jayasiri et al. (2015) was followed to register faces of fungi numbers.
For DNA extraction from the specimens (ZGCVN689, ZGCVN775), NucleoSpinⓇ Plant Ⅱ kit (Macherey-Nagel 2014, Germany) was used. PCR amplification of ITS gene region was carried out using the primers ITS 1F and ITS 4R. Sequencing was done using BigDye Terminator v3.1 Cycle sequencing Kit (Applied Biosystems, USA). The sequencing PCR temperature profile consisted of a 1st cycle at 960C for 2 minutes, followed by 30 cycles at 960C for 30 sec, 500C for 40 sec, and 600C for 4 minutes. The newly generated ITS sequences were deposited in the GenBank database (www.ncbi.nlm.nih.gov) with accession numbers (MT365177, MT649883). Sequence similarity assessments were conducted using BLAST search in NCBI's GenBank nucleotide database (https://blast.ncbi.nlm.nih.gov/). The newly generated sequences and those taken from GenBank were aligned using MEGA X64 (Kumar et al. 2018). Sequences used in the dataset were selected from Miettinen & Rajchenberg (2012), Cui et al. (2019) and Ji et al. (2019). Laetiporus sulphureus (Bull.) Murrill was selected as an outgroup taxon for the dataset following Cui et al. 2019. Maximum Likelihood (ML) analysis was conducted with MEGA X64 using Tamura-Nei model (Tamura & Nei 1993). Phylogeny test was carried out using boot bootstrap method, based on 1000 bootstrap replicates. The aligned sequence data matrix was deposited in TreeBase (Submission ID: 26538, Reviewer access URL: http://purl.org/phylo/treebase/phylows/study/TB2:S26538?x-accesscode=500a205a67e40eb6a2031591ae0083a7&format=html).
-
Neofomitella guangxiensis B.K. Cui & Xing Ji, in Ji, Wu, Song, Liu, Si & Cui, Mycol. Progr. 18 (4): 599 (2019). Fig. 1

Figure 1. A basidiome of Neofomitella gunagxiensis. B Generative hyphae showing clamp connection. C Skeletal hyphae. D Binding hyphae. E Basidiospore. F Basidiomata of Sebipora aquosa. G Generative hyphae. H Generative hyphae showing clamp connection. I Basidiospores. Scale bars: A = 8 mm, B = 10 μm, C = 30 μm, D, E = 8 μm, F = 10 mm, G, H = 15 μm, I = 7 μm.
Index Fungorum number: IF828730; Faces of Fungi number: FoF09464
Basidiomata annual, small to medium sized, usually in clusters, soft and coriaceous when fresh, becoming harder on drying, light in weight, effused reflexed. Pileus 26-48 mm long, 20-35 mm wide, 3-5 mm thick, sessile, circular to irregular, covering the host, applanate, concentric zonations absent, irregularly wrinkled, glabrous, creamish white, light brown on bruising, tissue more brownish in KOH, margin even to slightly wavy. Hymenium poroid. Pores 4-6 per mm, round to slightly angular, oblique towards attachment region, cream, turning light brown on bruising, tubes up to 3 mm long, concolourous with pore surface. Context 1.5-2 mm thick, creamish white, homogenous. Odour not distinctive.
Hyphal system trimitic, generative hyphae with clamp-connections, skeletal hyphae thick-walled, unbranched and binding hyphae thick-walled and frequently branched. Pileipellis interwoven at base and forming an irregular cutis made of hyphae that are 2-3 μm wide, hyaline, slightly (up to 1 μm) thick to thick-walled. Pileal trama interwoven, generative hyphae 2-4 μm wide, hyaline, smooth, thin to slightly (up to 1 μm) thick-walled, branched, with clamp-connections. Skeletal hyphae 2-5 μm wide, hyaline, weakly dextrinoid in Melzer's reagent, thick-walled (1 μm), unbranched, septations not observed. Binding hyphae 1.5-2 μm wide, hyaline, weakly dextrinoid in Melzer's reagent, thick-walled (1 μm), highly branched, septations not observed. Hymenial trama interwoven, generative hyphae 2-4 μm wide, hyaline, smooth, thin to slightly (up to 1 μm) thick-walled, branched, with clamp-connections. Skeletal hyphae 2-4.5 μm wide, hyaline, weakly dextrinoid in Melzer's reagent, thick-walled, unbranched, septations not observed. Binding hyphae 1.5-2 μm wide, hyaline, weakly dextrinoid in Melzer's reagent, thick-walled (1 μm), highly branched, branches short, septations not observed. Cystidia absent. Mature basidia not observed. Basidiospores 5.5-8 × 2-2.5 μm, L = 7 μm, W = 2.1 μm, Q = 2.6-4, Qm = 3.36, cylindrical to allantoid, hyaline, smooth, thin-walled, sometimes monoguttulate, inamyloid in Melzer's reagent, acyanophilous in cotton blue.
Material examined - India, Kerala state, Kannur district, Madayi Kavu, on dead branch of angiosperm tree, 07 July 2018, Vinjusha N, ZGCVN689.
Sebipora aquosa Miettinen, in Miettinen & Rajchenberg, Mycol. Progr. 11 (1): 144 (2012).
Index Fungorum number: IF519513; Faces of Fungi number: FoF09465
Basidiomata annual, small, in clusters, imbricate, soft, membraneous and coriaceous when fresh, becoming hard upon drying, light in weight, effused reflexed. Pileus 4-12 mm long, 6-50 mm wide. 2-3 mm thick, sessile, applanate to uneven, semicircular, concentric zonations absent, glabrous to hispid, white, pale brown on bruising, margin thin, undulate, lacerate, incurved on drying. Hymenium poroid. Pores 4-5 per mm, angular, absent along margin, white, pale brown on bruising, tube 1-2 mm long, concolourous with pore surface. Context 1 mm thick, white, homogenous. Odour not distinctive.
Hyphal system monomitic, generative hyphae with clamp-connections, thin to slightly (up to 1 μm) thick-walled, metachromatic in cresyl blue. Pileipellis a regular cutis made of hyphae that are 2-3 μm wide, hyaline, thin- to slightly (up to 1 μm) thick-walled. Pileal trama subparallel, generative hyphae 2-6 μm wide, hyaline, smooth, thin- to slightly (up to 1 μm) thick-walled, with frequent clamp-connections. Hymenial trama subparallel, hyphae 2-5 μm wide, hyaline, smooth, thin- to slightly (up to 1 μm) thick-walled, with frequent clamp-connections. Cystidia absent. Basidia 15-21 × 4-5 μm, clavate, 4-sterigmate, with basal clamp-connections. Basidiospores 5-7 × 2-3 μm, L = 5.8 μm, W = 2.5 μm, Q = 2-3, Qm = 2.37, cylindrical, usually curved at one end, hyaline, often monoguttulate, thin-walled, smooth, inamyloid in Melzer's reagent, acyanophilous in cotton blue.
Material examined - India, Kerala state: Palakkad district, Dhoni forest, on dead wood stump, 26 November 2018, Vinjusha N., ZGCVN775.
Taxonomy
-
BLAST search using the ITS sequence of the specimen, ZGCVN689 resulted in 100% identity with Neofomitella guangxiensis (GenBank numbers-MK192435, MK192434, MK192436). BLAST result also showed 99% similarity with Trametes cotonea (Pat. & Har.) Ryvarden (GenBank number- MH861271). BLAST search of the second specimen, ZGCVN775 showed 100% similarity with the sequences of Sebipora aquosa including that from the type material (GenBank numbers- KJ654615, HQ659243). BLAST search using the ITS sequence from the fruit body also resulted in 99% similarity with sequence of Ceriporiopsis subvermispora (JN116698).
The phylogenetic tree (Fig. 2) shows the placement of our collections among other related taxa of order Polyporales. Two distinct monophyletic groups of Polyporales were recognized in the tree, such as core polyporoid clade, and Cinereomyces clade. In the ITS tree, one of our collections (ZGCVN689) settled near to Neofomitella guangxiensis with 95% bootstrap support, in the core polyporoid clade. Similarly, the other collection (ZGCVN775) clustered along with Sebipora aquosa with 98% bootstrap support, in the Cinereomyces clade.
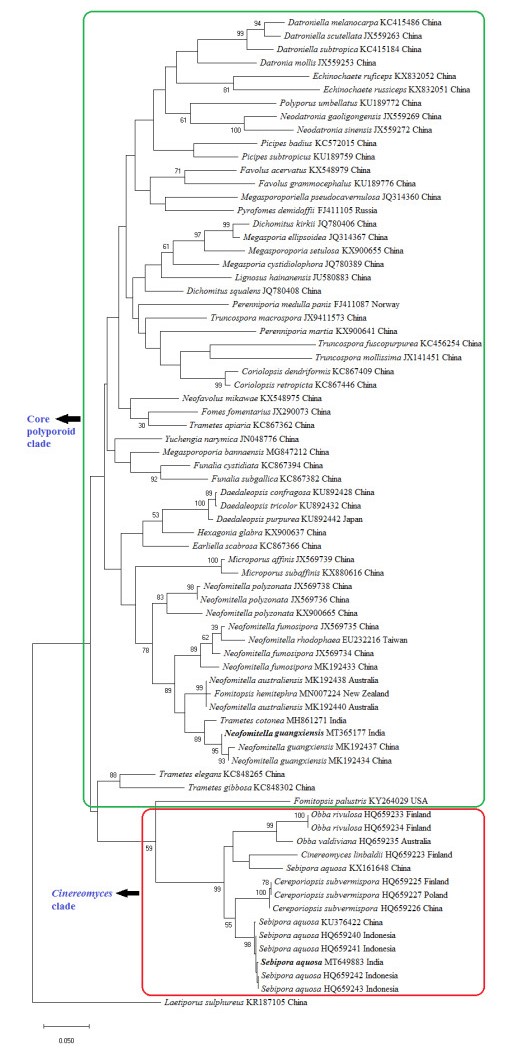
Figure 2. Maximum likelihood tree generated using nurITS sequence data. Values at the nodes indicate Maximum Likelihood bootstrap values. Bootstrap values above 50% are shown. Bold names represent the species collected from Kerala State, India. Clades are indicated in boxes. DNA sequence accession numbers and voucher localities are given along with the taxa.
-
According to Ji et al. (2019), Neofomitella guangxiensis is separated from other species in the genus by effused-reflexed to pileate basidiocarps, weakly dextrinoid skeletal and binding hyphae, and large basidiospores (5.5-7.5×1.8-2.2 μm). Morphological characters of the present specimen properly matched with the original description of the species by Ji et al. (2019), except for some variations. As per the description of Ji et al. (2019), the pileus of N. guangxiensis possesses concentric sulcations, and the hymenium produces fusoid cystidioles. However, the present collection lacks concentric zonations and sulcations on pileus, and fusoid cystidioles were not observed. Our collection resembles N. fumosipora (Corner) Y.C. Dai, Hai J. Li & Vlasák in morphology. However, the latter species produces smaller pores (7-9 per mm) and smaller basidiospores (3-4 × 1.7-2.2 μm, as per the descriptions of Hattori 2005, Li et al. 2014). Neofomitella rhodophaea also shows some similarities with the present specimen. However according to Ji et al. (2019), N. rhodophaea have smaller pores (7-9 per mm) and oblong ellipsoid basidiospores. Neofomitella australiensis and N. polyzonata are distinguished from our collection by the smaller basidiospores (3.8-4.9×1.8-2.3 μm in N. australiensis; 3.9-5 × 1.9-2.1 μm in N. polyzonata, according to descriptions of Ji et al. 2019, Li et al. 2014 respectively). In addition, pileus surface of N. polyzonata are velutinate, and buff-yellow to reddish brown.
In the BLAST search, ITS sequence of the present collection showed 99% similarity with a strain of Trametes cotonea (Pat. & Har.) Ryvarden, reported from India. However, the latter can be morphologically separated from our collection by larger basidiospores (7-11 × 2.5-3.5 μm, Ryvarden & Johansen 1980). In the phylogenetic tree (Fig. 2), T. cotonea settled in a separate lineage from our collection. Phylogenetic analysis clearly reveals the identity of our first collection (ZGCVN689) as N. guangxiensis. All of the original collections of N. guangxiensis (Ji et al. 2019), were reported from fallen branches of angiosperm trees. Similarly, collection of the species from Kerala was also obtained from a fallen dead branch. So far, N. guangxiensis has been recorded only from its type locality, Guangxi Auto. Reg., Shangsi County, China. The present study forms the first report of the species outside its type locality.
Sebipora aquosa has been described from Indonesia, New Guinea and Yunnan Province, China (Miettinen & Rajchenberg 2012, Glen et al. 2014, Zhao et al. 2017). As per the descriptions of Miettinen & Rajchenberg (2012) and Zhao et al. (2017), basidiocarps of S. aquosa are usually gelatinous, however, the present collection lacks this character since the specimen was collected in slightly dried form. Morphologically, present specimen resembles Ceriporiopsis subvermispora (Pilát) Niemelä. According to the taxonomic descriptions (Lowe 1966, Niemelä 1985, Ryvarden & Gilbertson 1993), C. subvermispora always produces resupinate fruit bodies. However, the present collection produces effused reflexed basidiomata. Microscopically, C. subvermispora produces allantoid basidiospores with a width of 1-1.2 μm, whereas our collection possessed cylindrical basidiospores, which are 2-3 μm wide. In addition, C. subvermispora has eguttulate spores, while those of the present collection were mostly monoguttulate. C. subvermispora produces hyphae that swell in KOH (Miettinen & Rajchenberg 2012), but hyphal swellings were absent in the present collection. Moreover, C subvermispora is typically a North American species according to Miettinen & Rajchenberg (2012). Molecular phylogenetic analysis (Fig. 2) also clearly distinguishes C. subvermispora from our collection. Thus, morphological characterization and molecular phylogeny identifies our second collection (ZGCVN775) as S. aquosa.
According to the research of Miettinen & Rajchenberg (2012), substrates of S. aquosa were always a fallen angiosperm tree trunk. Zhao et al. (2017) also recorded the species from fallen angiosperm trunk. But Glen et al. (2014) reported the species on root of Acacia mangium. From India, an ITS sequence of S. aquosa has been deposited in the GenBank database (GenBank number: JQ746682), however the data remains unpublished and the reliability of the sequence identified as "S. aquosa" is also doubted. In India, S. aquosa has been isolated from corneal scrapings of keratitis patients (Nithya & Bhaskar 2013). However the paper (Nithya & Bhaskar 2013) does not provide a systematic account of the species, and neither the voucher material nor its molecular data has been deposited or made available. Hence, the present study forms the first systematic record of S. aquosa from India based on a voucher deposit and molecular sequence.
-
Vinjusha N. acknowledges support from the Kerala State Council for Science, Technology and Environment (KSCSTE) in the form of a research fellowship. The authors thank the Chief Conservator of Forests & Chief Wildlife Warden, Kerala, for permission for fieldwork in the forest areas of Kerala.
- Copyright: © 2021 by the author(s). This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| N Vinjusha, TK ArunKumar. 2021. Neofomitella guangxiensis and Sebipora aquosa newly recorded white rot polypores from India. Studies in Fungi 6(1):307−314 doi: 10.5943/sif/6/1/21 |












