Purpose: To report outcomes of transarterial radioembolization (TARE) using glass microspheres for the treatment of mixed hepatocellular-cholangiocarcinoma (HCC-CC) in a propensity-matched study.
Material and Methods: Between 2013 and 2019, 10 consecutive patients with histologically confirmed HCC-CC received TARE of a targeted territory using glass microspheres as a primary initial treatment. Baseline demographics in addition to tumor distribution, Child Pugh score, and BCLC were recorded. Tumor response was assessed according to modified RECIST criteria. The HCC-CC cohort was matched to the HCC cohort, and objective response and survival analysis was performed.
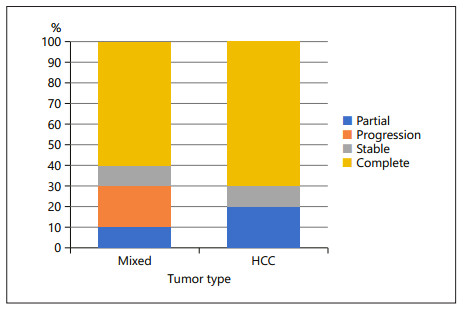
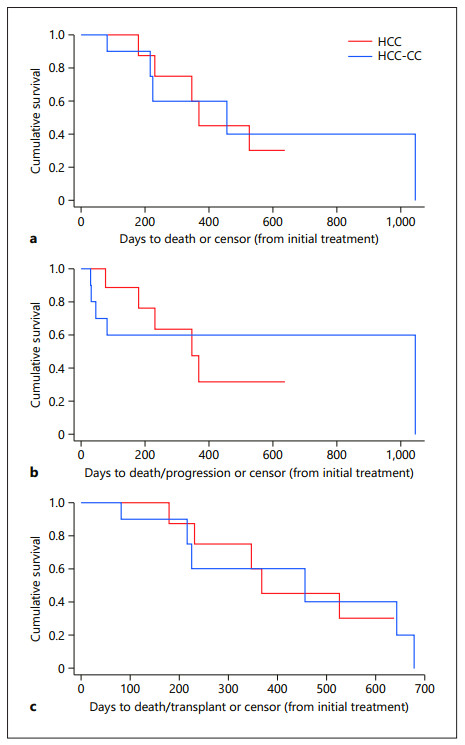
Results: In the HCC-CC cohort, patients had a 70% objective response rate (ORR), and in the HCC cohort, patients had a 90% ORR after matching (p = 0.54). The median overall survival (OS) for HCC patients was 12.3 months (95% CI: 6.0–17.4 months) in the matched population, and for HCC-CC patients, the median OS was 15.2 months (95% CI: 2.7–20.2 months) (p = 0.98). The median progression-free survival (PFS) for HCC patients was 11.6 months (95% CI: 2.53–19.3 months) in the matched population, and for HCC-CC patients, the median PFS was 15.2 months (95% CI: 2.7–20.2 months) (p = 0.94). The median transplant-free survival (TFS) for HCC patients was 12.3 months (95% CI: 6.0–17.4 months) in the matched population, and for HCC-CC patients, the median TFS was 15.2 months (95% CI: 2.7–20.2 months) (p = 0.98).
Conclusions: While outcomes of combined HCC-CC are poor and optimal treatment remains undefined, TARE appears to represent an effective locoregional treatment with survival outcomes similar to that of HCC treated by TARE.