-
About this article
Cite this article
Brian M. Fung, Brian E. Kadera, James H. Tabibian. 2021. Gastrointestinal Luminal Stenting: The Early US Experience with the Duodenal HANAROSTENT. Gastrointestinal Tumors. 8: doi: 10.1159/000510350
Gastrointestinal Luminal Stenting: The Early US Experience with the Duodenal HANAROSTENT
- Received: 21 April 2020
- Accepted: 17 July 2020
- Published online: 09 October 2020
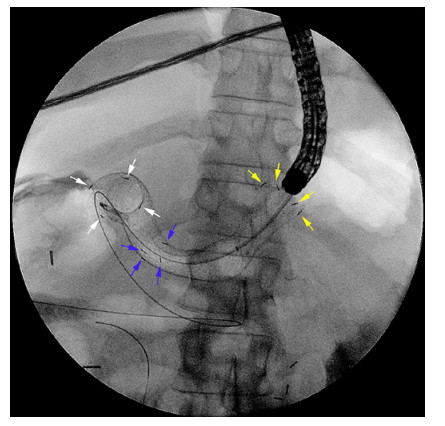
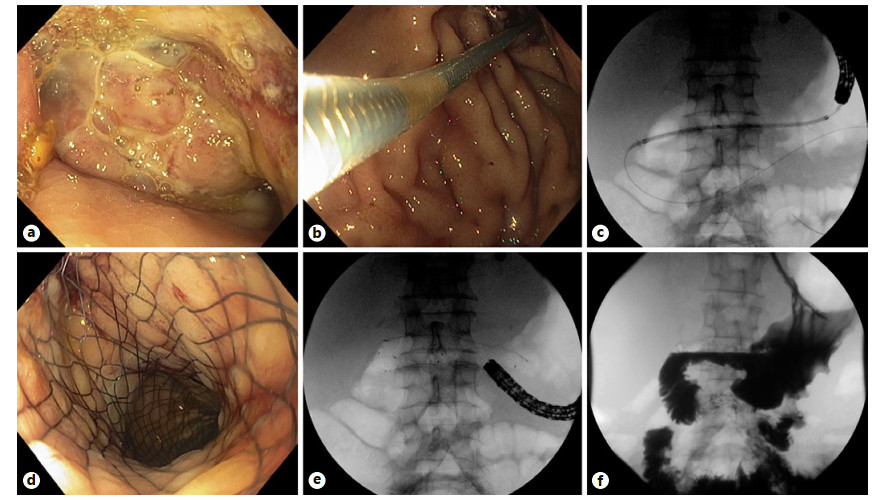
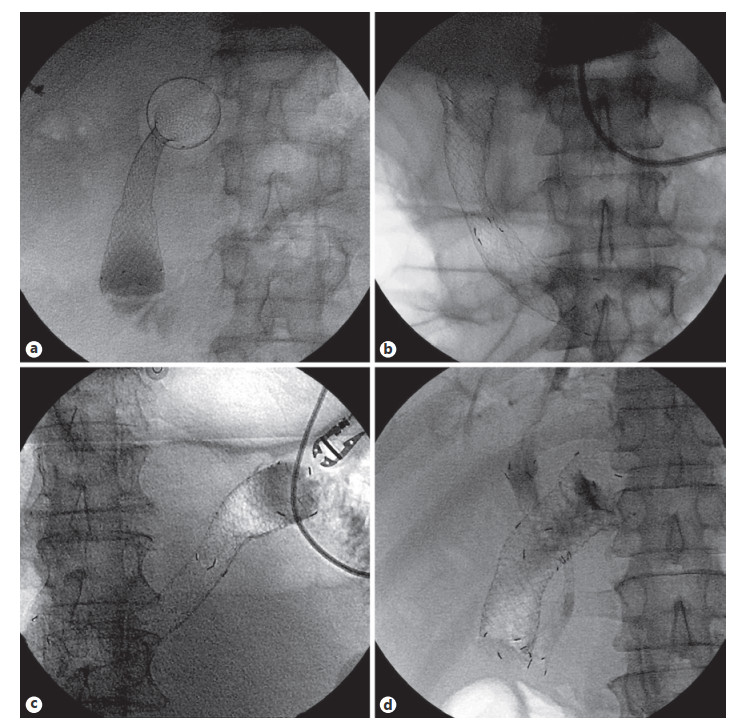
Abstract: Self-expandable metal stents (SEMSs) are frequently utilized for palliation of malignant gastric and/or duodenal outlet obstruction (GDOO). Re-establishing luminal patency with accurate SEMS positioning while limiting migration and adjacent tissue injury is an important technical consideration and aim. The duodenal HANAROSTENT® was introduced in the USA in 2019 and developed with these challenges in mind. As the first center in the USA to deploy the duodenal HANAROSTENT® in clinical practice, we herein examine our early experience with its use. Specifically, we describe 7 consecutive cases of malignant GDOO in which a duodenal HANAROSTENT® was placed for on-label use, defined as palliative treatment of malignant gastric and/or duodenal obstruction. All stents were 22 mm in diameter, with 5 being 90 mm and 2 being 120 mm in length. Technical and clinical success with duodenal HANAROSTENT® placement were achieved in all 7 cases (100%). In no case was stent adjustment required post-deployment. There were no stent-related adverse events, and no subsequent endoscopic procedures were necessary in any of the patients during a mean follow-up of 5 months (range 1–12 months). In summary, the duodenal HANAROSTENT® appears to perform well and be a promising alternative to other available duodenal SEMSs. As experience in the USA with this newly introduced duodenal SEMS grows, multicenter prospective data should be collected to better establish its relative safety and efficacy.