-
Hybrid vigor (also known as heterosis), referring to the phenomenon that F1 hybrid plants outperform their parents in most agronomic traits, is the foundation of modern agriculture. The seeds of hybrids however have to be renewed at each crop season due to the fact that F2 progenies segregate and lose uniformity[1] (Fig. 1a). Apomixis refers to a set of reproductive mechanisms that rely on avoiding meiotic reduction and fertilization of the egg cell to generate clonal seeds, and it has been discovered in at least 78 of 460 families of flowering plants[2]. The introduction of apomixis, asexual reproduction through seeds, into crop plants is considered as 'the holy grail of agriculture'[3]. As a proof-of-concept, hybrid vigor has been shown to be fixable by synthetic apomixis approaches[4−6], but several barriers, including low rate of the clonal seed production, reduced plant fertility and non-autonomous manipulation, have hindered the application of the technology in agriculture. Excitingly, a recent study by Vernet et al., employing an all-in-one system comprising of the apomeiosis system and the parthenogenesis inducer, has largely removed the barriers of application of synthetic apomixis in hybrid rice, and produced clonal seeds with efficiencies higher than 95% across several generations[7] (Fig. 1b).
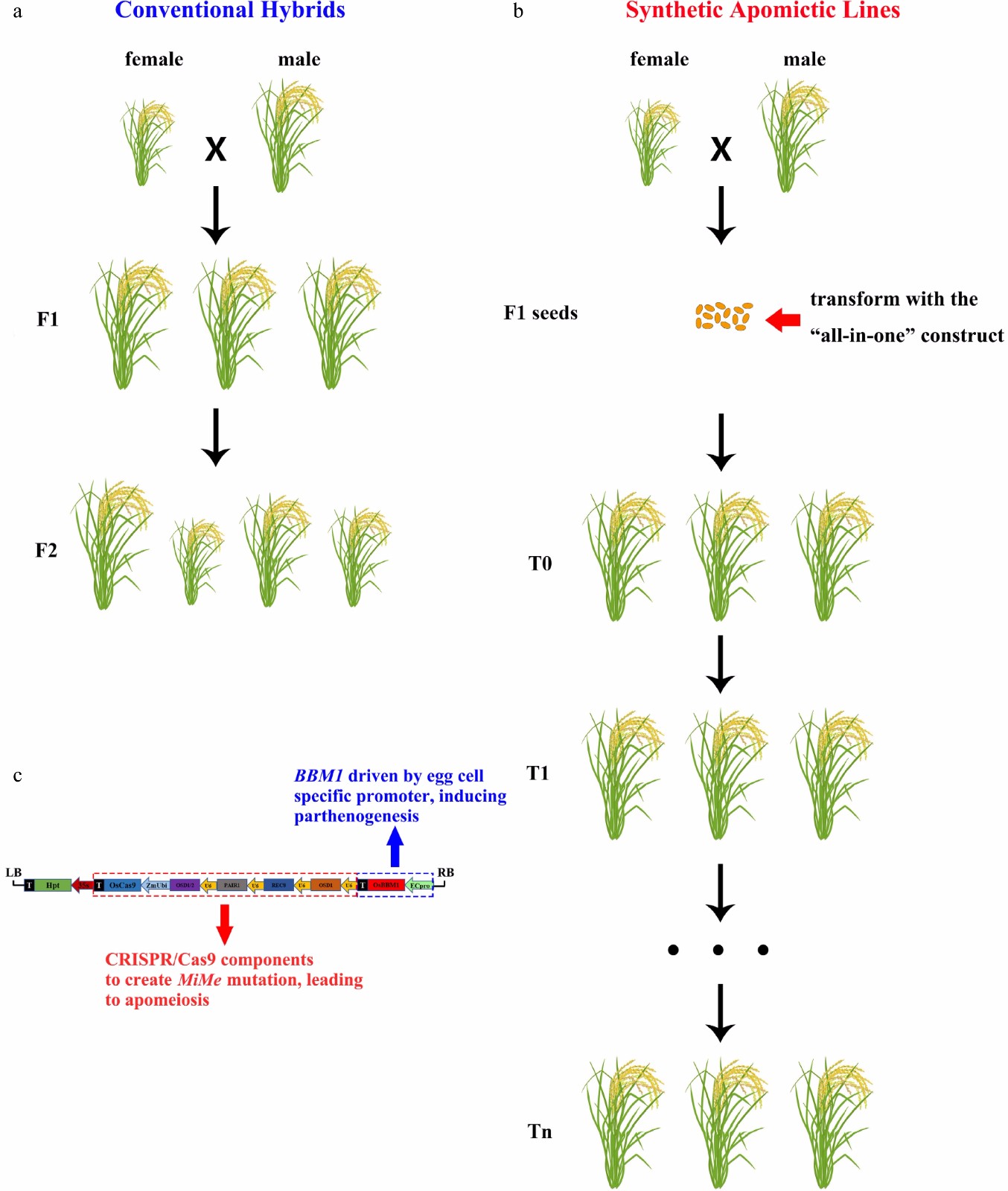
Figure 1.
Schematic illustration of the traditional hybrids and synthetic apomixis achieved by one-step installation. (a) Conventional hybrids show hybrid vigor in the F1 generation, but segregate in the following F2 generation; (b) Synthetic apomictic lines were created by introducing the all-in-one construct into the calli derived from F1 mature embryos. Once the apomixis was achieved, the major agronomic traits will never segregate across generations, which means these traits have been 'eternally' fixed. (c) Schematic map of the all-in-one vector (modified according to Vernet et al.[7]).
The successful installation of apomixis in sexual plants needs three requisites, i.e., omission of the meiosis (apomeiosis), parthenogenesis and normal endosperm development. The Mitosis instead of Meiosis (MiMe) system has been proven to be efficient in producing unrecombined and unreduced gametes with the identical genotype of the mother plants in both dicots and monocots[8,9]. Parthenogenesis induced by ectopic expression of Baby BooM (BBM) homologous genes in the egg cell has successfully been achieved in several cereal crops[4,10,11]. The previous attempt of synthetic apomixis in rice however was conducted in a two-step procedure: MiMe mutations by CRISPR/Cas9 system was introduced into a rice line ectopically expressing OsBBM1, and the fertile apomictic rice plants produced clonal seeds at a rate of 10%−30%[4].
Vernet et al. have now revealed that highly efficient synthetic apomixis could be installed into rice in a single step. A vector, carrying sgRNAs targeting three MiMe genes (PAIR1, REC8 and OSD1) and a cassette of OsBBM1 driven by egg cell-specific promoter (Fig. 1c), was transformed into F1 seed embryo-derived calli. In the T0 generation, MiMe mutants were first identified, and the ploidy of these MiMe mutants' progenies was examined. Under such circumstance, only when the apomeiosis and parthenogenesis were simultaneously achieved will plants produce diploid offspring, otherwise tetraploids will be produced. Surprisingly, most MiMe mutant plants produced diploid progenies at rates greater than 80% with several of them at a rate of more than 97%, indicating that the all-in-one system was highly efficient in producing clonal plants. Whole-genome sequencing of T0, T1 and T2 generations confirmed a faithful transmission of the heterozygous genome through the synthetic apomixis mode of reproduction[7]. Meanwhile, the all-in-one system also produced high-frequency apomictic events in the cultivar Kitaake, the same inbred material with up to 30% apomictic efficiency achieved by the two-step method. This indicates that the difference in the two systems arises from the architecture of the new construct or the simplified transformation procedure. The authors also demonstrated that the synthetic apomixis implements faithful reproduction of the F1 phenotype in apomictic lines, and no major differences in phenological, morphological and grain quality traits have been observed across generations (Fig. 1b).
The uncertainty in the system is the endosperm development, which might jeopardize high-efficiency clonal seed formation. In planta, upon arrival to the embryo sac, sperm cells need to be activated before double fertilization, and the process is governed by egg-cell secreted Egg Cell 1 (EC1) protein[12]. Parthenogenesis requires egg cells to initiate embryo development before fertilization, which might reduce EC1 protein abundance and in turn causes failure of the central cell fertilization and the subsequent endosperm development. However, Vernet et al. have shown that the hypothetical issue mentioned above didn't impact on the system at all[7]. Another concern of the system is the ploidy level of endosperm, which might affect the grain quality of the seeds. The apomictic endosperm is hexaploid instead of triploid as the endosperm was produced by the fusion of tetraploid central cell and diploid sperm cell. But Vernet et al. showed that even in wild type plants, 6C and 12C DNA peaks could be observed in endosperm due to the endoduplication, and this natural phenomenon does not have a major impact on grain quality[7].
Reduced fertility was sometimes noticeable in the MiMe mutants. In a previous attempt to generate MiMe mutants in rice, reduced fertility was observed when the triple mutant was generated in the Hwayoung and Nipponbare hybrid background (HW/NB)[9]. However, in another report, the fertility of MiMe mutants with a hybrid background is comparable to that of the inbred lines[6] . These results raised the possibility that, to some extent, the fertility depends on the genetic background. Reduced fertility was also observed by Vernet et al. in both control F1 plants and apomictic lines under experimental greenhouse conditions, retaining 25%−65% fertility and 27%−35% fertility, respectively. Vernet et al. have reasoned that the reduced fertility could be due to a detrimental interaction of the greenhouse environment with the Wild Abortive Cytoplasmic Male Sterility (CMS-WA) system used during F1 hybrid production. Another explanation could be incomplete penetration of MiMe in female meiosis, which means that a portion of meiocytes still enter meiosis II and produce non-viable female gametes. The third possibility is that reduced fertility is incomplete initiation of embryogenesis triggered by ectopic expression of BBM. Further investigation is needed to clarify the actual reason for the reduced fertility, which seems to be the only drawback of the system. Although the apomictic hybrids showed reduced panicle fertility, the identification of inbred Kitaake apomictic events with both high efficiency and high fertility is encouraging. Nevertheless, synthetic apomixis will have to be tested under more stringent situations before its actual application in agriculture. Meanwhile, another group has revealed that OsBBM4, one of the four BBM-like genes in rice, also induced parthenogenesis when ectopically expressed in egg cells. By combining egg-cell ectopic expression of BBM4 and the mutations of MiMe in a one-step procedure, synthetic apomixis was also achieved in Chunyou84 (CY84) hybrid rice. The apomictic events showed high seed-setting rate almost comparable to that of the normal hybrid CY84. However, the efficiencies of synthetic apomixis were relatively low in this approach, and further optimization is still needed[13].
Considering the high frequency and conservation of the MiMe and BBM modules, synthetic apomixis in other crop species seems possible. For cereal crops, since BBM-induced parthenogenesis was quite efficient[4,10,11], utilizing the 'all-in-one' system should be a reasonable attempt for synthetic apomixis. However, for dicotyledonous crops, verifying BBM-induced parthenogenesis will be a priority. There is likely a long way to go before parthenogenesis can be efficiently induced in egg cells, and by then synthetic apomixis could be considered in dicots[14].
In summary, Vernet et al. has presented an elegant system that allows the installation of high-efficiency synthetic apomixis in hybrid rice with a single-step transformation. The study has shown for the first time that apomixis can be synthetically introduced into rice, with almost full penetration, and the work has laid the fundamental ground for future efforts to fix hybrid vigor. With the current progress achieved in synthetic apomixis, all breeders should consider it a dream come true!
HTML
L. Yuan was supported by the National Youth Talent Program (A279021801) and the Key-Area R&D Program of Guangdong Province (2022B0202060001), M. Liu, S. Tian and J. Wang were supported by Natural Science Foundation of Shaanxi Province (2022JM-112 and 2021JM-089) and the Fundamental Research Fund from Northwest A&F University (Z1010422001, 2452022111 and Z1090219018).
-
The authors declare that they have no conflict of interest.
- Copyright: 2023 by the author(s). Published by Maximum Academic Press on behalf of Hainan Yazhou Bay Seed Laboratory. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Liu Q, Han D, Tian S, Wang J, Liu M, et al. 2023. Fixing hybrid vigor by synthetic apomixis: a dream come true. Seed Biology 2:2 doi: 10.48130/SeedBio-2023-0002 |













