-
On October 16, 2025, Prof. Yafei Cai and Prof. Juan Li, as well as their research groups from Nanjing Agricultural University, were the first researchers in China to successfully vitrified a homemade clone of a Huaxi beef calf and to establish a precedent in the field of assisted reproduction. Huaxi cattle, the first indigenous beef breed in China, were chosen due to their adaptability, growth rate and high meat yield, making them ideal for cloning experiments. This is a breakthrough achievement that demonstrates the feasibility of zona pellucida-free (ZP-free) oocytes to achieve stable and efficient handmade cloning (HMC), followed by vitrification for embryo transfer (ET). It introduces new dimensions of cattle breeding within geographically and economically diverse agricultural areas[1].
HTML
-
One of the crucial steps of cloning by somatic cell nuclear transfer (SCNT) is the removal of all genetic material associated with chromosomes from the recipient oocytes. In the commonly applied traditional cloning (TC), chromosomes are usually removed by aspirating the cytoplasm under the first polar body (PB1) using micromanipulation. However, the cloning team used ZP-free bovine oocytes in HMC techniques[1]. These oocytes were prepared by enzymatically removing the ZP, and then oriented bisection was performed to remove the nucleus for enucleation without micromanipulation[2]. Subsequently, two cytoplasms with one donor cell, which was prepared from the fetal fibroblasts of a Huaxi beef calf, were fused into a reconstructed embryo, which was recorded as Day 0. These ZP-free cloned embryos were cultured further in vitro in the Well of the Well (WOW) system[3] (Fig. 1). On Day 6, the blastocysts were selected and vitrification was performed using Open Pulled Straw (OPS) for cryopreservation. When a synchronized surrogate of the recipient domestic cattle was ready, one OPS with two vitrified blastocysts was removed from liquid nitrogen for immediate ET[1,4]. This procedure resulted in the birth of a healthy cloned calf with a 100% rate in this pilot trial. Interestingly, the behavior and postnatal health of the calf were similar to those of naturally born calves[2,4].
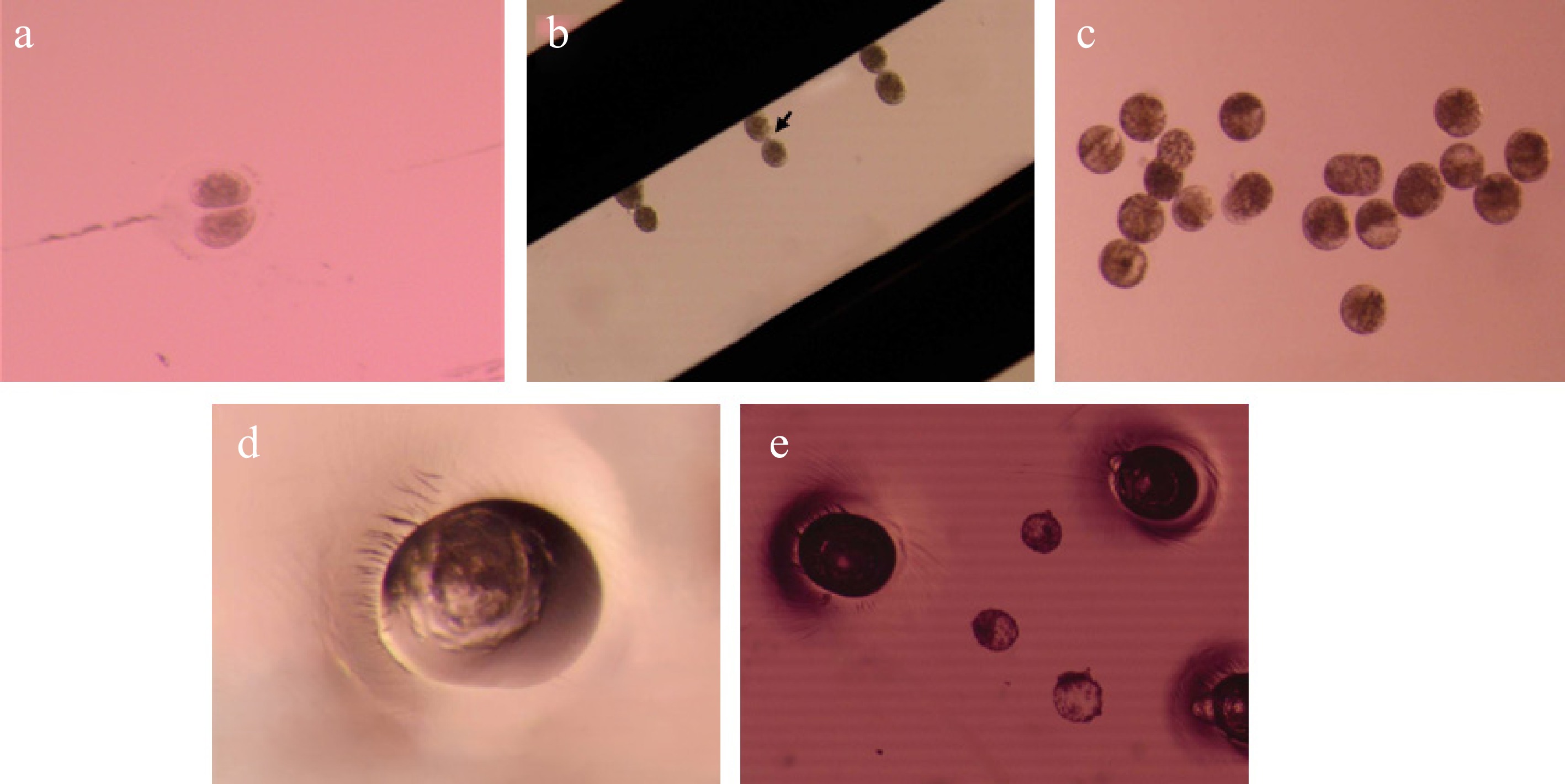
Figure 1.
Key steps in the handmade cloning (HMC) process using ZP-free bovine oocytes. (a) Enucleation of a ZP-free oocyte by oriented bisection. (b) Cytoplasts positioned for fusion with a somatic donor cell (the arrow indicates the somatic cell placed between two cytoplasts). (c) Post-fusion reconstructed embryos undergoing parthenogenetic activation and initial development in vitro. (d) Blastocyst within a single well of the WOW culture system. (e) Expanded Day 6 blastocysts.
-
The approach presents various operational benefits over previous SCNT protocols. It avoids the use of micromanipulation devices and makes ET a single-step operation. HMC has long held the promise of democratizing SCNT without any use of high-priced micromanipulators and highly technical procedures[1]. Day 6 blastocysts continued to exhibit optimal growth accordingly, which was associated with high levels of developmental competence[5]. Conventionally, ET often requires thawing and morphological evaluation and, in some cases, introduces stress and increases labor[3]. Even though blastocysts prepared using ZP-free oocytes[1] would be more susceptible to cryoinjury because of the lack of the ZP as a structural barrier, our optimized vitrification protocol successfully preserved the blastocysts' integrity and allowed successful re-expansion after thawing, allowing direct ET without in vitro testing. In this model of cloning of ET, a major bottleneck in SCNT or IVF procedures is overcome: Operational complexity[5]. Conventional protocols require evaluating the embryos' viability, vitrifying the embryos in vitro and further thawing and re-evaluating the embryos prior to final transfer, which introduces additional cost and risk[1]. Our vitrified embryos are immediately ready to transfer from storage straws to the recipient cow in field conditions, even in remote or high-altitude areas. The procedure is applicable to beef and dairy cattle reproduction systems, and it has been proven by a successful post-transfer development to produce a healthy calf.
-
This breakthrough is a milestone in practical cloning. The birth of a healthy and behaviorally normal cloned calf has shown that HMC can be both scientifically and operationally viable (Fig. 2). This can be used as a replicable approach for the development of livestock biotechnology worldwide. We developed a technique that uses ZP-free oocytes, vitrification-ready embryo handling and simplified ET procedures to provide a cost-effective, field-deployable system to produce livestock. It reduces the technical obstacles and extends as far as high-altitude or remote areas with limited resources, where cattle breeding can be advanced with the help of advanced cloning.
-
Our initial experiment was a successful, but a large-scale validation on a large number of animals is essential. Future research will center on developing tests for various Huaxi cattle and other beef breeds and ultimately for dairy breeds. We will examine the genetic and phenotypic characteristics of the resulting offspring, pool the technique with gene-edited embryos and record the outcomes of both production arrangements. Besides extensive validation, further studies should be conducted in the future to determine the relationships among oocyte quality, donor cell type, culture conditions, the progress of vitrification and ET in order to maximize the quality of HMC systems and the batch production of ET[1−5]. This has the potential to decrease the number of high-efficiency breeding pipes in underserved areas by integrating such a system into mobile ET units or farm cooperative models. Later, breeding registration records will be officially integrated into reports by the national cattle biobreeding programs of China.
This study was funded by grants from the National Agricultural Science and Technology Major Project (grant number: 2023ZD040480203, approval date: 2023-12-15) and Hainan Prefecture-level Science and Technology for the Benefit of the People Program of Qinghai Province (2025-KH07-E).
-
All procedures involving animals were reviewed and approved by the Nanjing Agricultural University Laboratory Animal Welfare and Ethics Committee (approval number: NJAULLSC2024002). The research followed the "replacement, reduction and refinement" (3Rs) principles to minimize animal suffering. Details of housing, care, and pain management were addressed to ensure animal welfare throughout the experiment.
-
The authors confirm their contributions to the paper as follows: study conception and design: Cai Y, Li J; sample collection and preparation: Cao D, Wang Z, Saeed HA, Li P; data collection: Guo M, Yan J, Li H; supervision, manuscript writing and revision: Li J. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Cao D, Wang Z, Saeed HA, Li P, Guo M, et al. 2025. Handmade cloning: simplicity meets efficiency in modern livestock reproduction. Animal Advances 2: e032 doi: 10.48130/animadv-0025-0045 |














