-
Electrochemical uranium extraction (EUE) offers a sustainable pathway to support nuclear energy development amid the projected depletion of terrestrial uranium resources within the next 80–120 years. By enabling precisely controllable operation, rapid response, and high selectivity[1], EUE provides an environmentally compatible strategy for recovering uranium from unconventional sources such as seawater, nuclear wastewater, and other environmental media. Recent advances in porous electrode materials, including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and covalent organic polymers (COPs), have further improved EUE efficiency through their high surface areas, tunable pore architectures, and abundant functional groups capable of selectively binding uranium[2,3]. Nevertheless, EUE continues to face key challenges, including electrode passivation, limited selectivity in complex ionic environments, and the high cost associated with scalable electrode fabrication. In a notable recent development, Jin et al.[4] reported a groundbreaking indirect electrochemical strategy employing a self-standing polyarylether-based covalent organic framework (PAE-COF-AO@CC) electrode for uranyl upcycling, offering a promising route toward efficient and sustainable uranium recovery.
The proposed approach features a 'dual-function electrode' design for EUE (Fig. 1a). By directly growing the COF on carbon cloth (CC), the authors fabricated a robust and binder-free electrode with strong catalyst-support integration, thereby enhancing electron transfer efficiency. The PAE backbone catalyzes the oxygen reduction reaction (ORR) to generate H2O2, while the amidoxime groups furnish highly specific chelating sites for uranyl ions, subsequently acting as nucleation centers for controlled precipitation. Beyond the material innovation itself, a major strength of this study lies in its systematic and insightful evaluation of the operational parameters governing uranium extraction. This commentary highlights how factors such as pH, applied voltage, ionic strength (Na+ concentration), and the in-situ generation of H2O2 collectively contribute to the system’s exceptional performance.
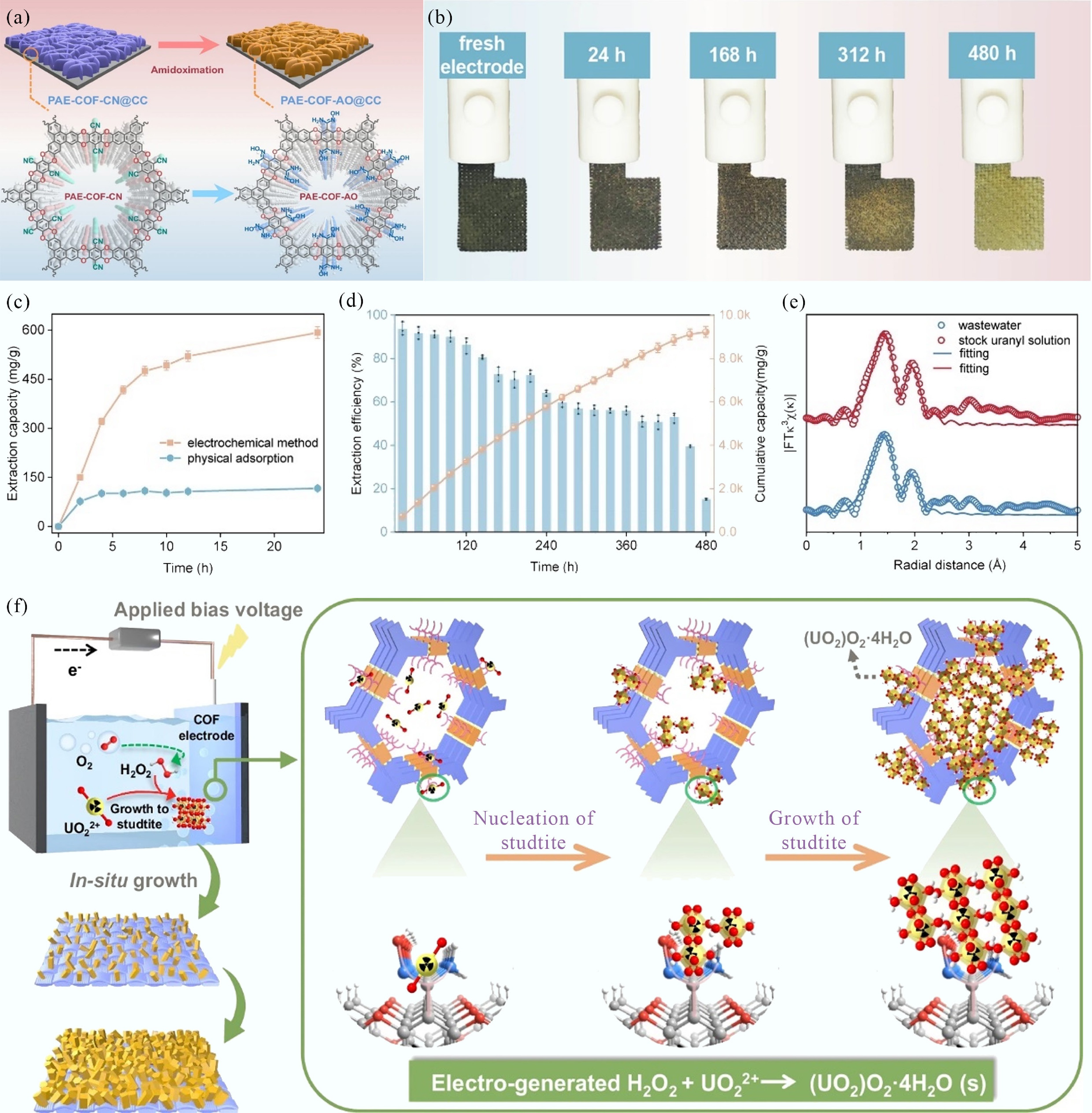
Figure 1.
(a) Schematic diagram for synthesis and structures of PAE-COF-CN@CC and PAE-COF-AO@CC. (b) Visual change of PAE-COF-AO@CC electrode. (c) Comparison of uranyl extraction with the electrochemical method and the physical adsorption, and (d) long-term uranyl extraction performance from low-level uranyl-containing radioactive organic wastewater. (e) FT-EXAFS spectra. (f) Possible reactive pathways of the indirect electrochemical method with self-standing COF electrode[4].
HTML
-
The amidoxime groups undergo protonation under acidic conditions (pH ≤ 3), generating electrostatic repulsion with positively charged uranyl ions, and thereby reducing extraction efficiency. Simultaneously, the equilibrium governing studtite formation,
$ \rm UO_2^{2+} + H_2O_2 + 4H_2O \rightleftharpoons (UO_2)O_2 \cdot 4H_2O + 2H^+$ is shifted unfavorably under acidic conditions, resulting in a low extraction rate. In contrast, alkaline conditions promote deprotonation of amidoxime groups, mitigating electrostatic repulsion, and enabling effective chelation of uranyl ions. Within the optimal pH range of 5–13, both chelation and studtite formation proceed efficiently, yielding uranium extraction efficiencies exceeding 90%.
-
The electrochemical uranium extraction system offers a clear advantage over conventional physicochemical adsorption, particularly at elevated uranium concentrations. Notably, the system achieves an extraction capacity of 660.16 mg g–1 at 50 ppm U(VI) (Fig. 1c). The applied voltage directly governs H2O2 generation by controlling the rate of the 2e– oxygen reduction reaction (ORR). As the voltage increases from –0.193 to –0.493 V (vs Ag/AgCl), uranium recovery improves markedly from 62.35% to 97.96%. This enhancement reflects accelerated 2e– ORR kinetics, which elevate the localized H2O2 concentration at the electrode surface and, in turn, promote studtite precipitation. The pronounced performance gains at higher voltages underscore the critical role of voltage optimization in achieving efficient uranium extraction.
-
The system demonstrates excellent tolerance to ionic strength. Even under conditions with high U/Na ratios, the uranium extraction efficiency remains above 85%. This robust performance arises from the high intrinsic selectivity of amidoxime groups for uranyl ions over competing cations. Such selectivity is particularly critical in real wastewater, where high ionic strength and complex organic additives (e.g., polyvinyl alcohol, urea, tetrahydrofurfuryl alcohol) typically challenge adsorption-based systems. Remarkably, the functionalized electrode maintains strong anti-interference capability and stable extraction performance even in these chemically complex environments.
-
The in-situ production of H2O2 via the electrocatalytic 2e- ORR is central to the extraction mechanism. Aromatic ether groups within the PAE-COF-AO@CC electrode act as active sites for H2O2 formation. The yield of H2O2 is modulated by several parameters—including applied potential, pH, and dissolved oxygen concentration—allowing the system to be fine-tuned for optimal uranyl precipitation. By integrating and optimizing these parameters, the authors achieved an exceptional uranium accumulation capacity of 9,238.9 mg g–1 after 450 h of continuous operation in real organic wastewater (Fig. 1b, d), representing one of the highest reported values for EUE systems.
-
The high extraction efficiency arises from a synergistic two-step mechanism: (1) specific chelation of uranyl ions by amidoxime groups initiates studtite nucleation; and (2) electro-generated H2O2 drives sustained crystal growth until a dense, passivating layer is formed. This dual process—combining molecular-level coordination and electrochemically enhanced precipitation—enables robust uranium extraction even in chemically complex real wastewater, as confirmed by EXAFS analysis (Fig. 1e, f).
Building on the comprehensive analysis presented above, the self-standing COF electrode–based indirect electrochemical upcycling strategy demonstrates exceptional extraction capacity, and long-term operational stability in organic-rich wastewater. Nonetheless, practical deployment is still limited by several challenges, including complex electrode fabrication, sensitivity to pH fluctuations, and partial blockage of active sites during prolonged operation. To accelerate the advancement of this technology toward higher efficiency and practical viability, future research may focus on four key directions:
Inverse material design via machine learning
-
Coupling machine learning (ML) with quantum chemical calculations could enable rational, inverse design of COF architectures, allowing precise tuning of functional groups to enhance uranyl selectivity, binding strength, and resistance to competitive ions.
Advanced electrochemical operating protocols
-
Developing innovative electrochemical strategies—such as pulsed, intermittent, or alternating voltage modes—may mitigate electrode passivation, boost H2O2 generation efficiency, and reduce overall energy consumption.
Operando mechanistic interrogation
-
Integrating operando characterization tools (e.g., Raman spectroscopy, EXAFS) would permit real-time tracking of interfacial processes, including uranyl coordination evolution, studtite/metastudtite nucleation pathways, and structural changes at active sites, thereby providing mechanistic insights to guide further optimization.
Modular flow-system engineering
-
Designing modularized flow electrolyzers will be essential for continuous uranium recovery from wastewater streams with variable compositions, improving scalability, process stability, and adaptability to real-world conditions.
-
Both authors contributed to the study conception and design. Data collection and analysis were performed by Tao Wen and Muhammad Wakeel. The first draft of the manuscript was written by Tao Wen, and both authors commented on previous versions of the manuscript. Both authors reviewed the results and approved the final version of the manuscript.
-
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
-
None.
-
All authors declare that there are no competing interests.
-
Full list of author information is available at the end of the article.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Wen T, Wakeel M. 2025. Synergistic parameter optimization in electrochemical upcycling of uranyl: mechanisms and perspectives of self-standing COF electrodes. Sustainable Carbon Materials 1: e008 doi: 10.48130/scm-0025-0009 |












